Abstract
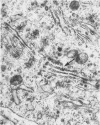
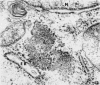
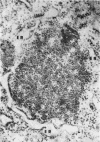
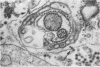
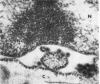
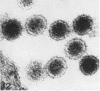
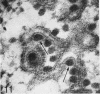
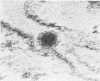
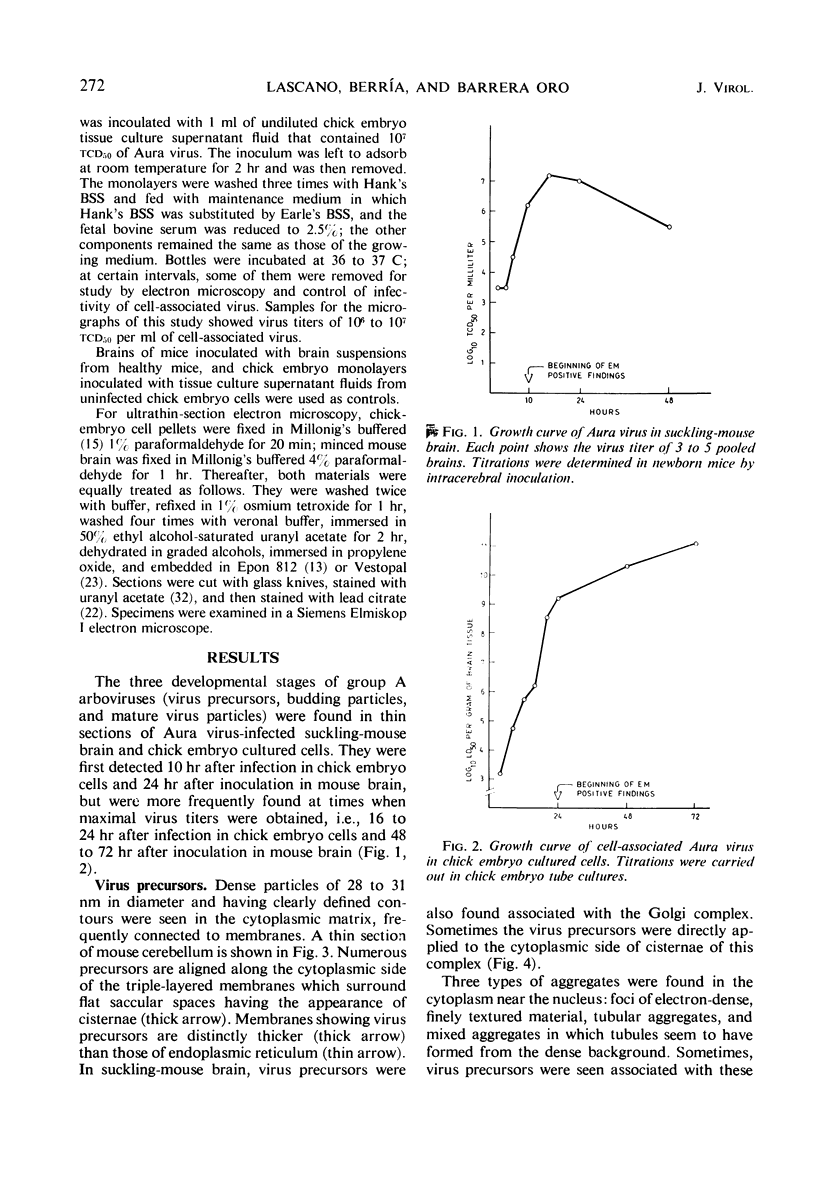
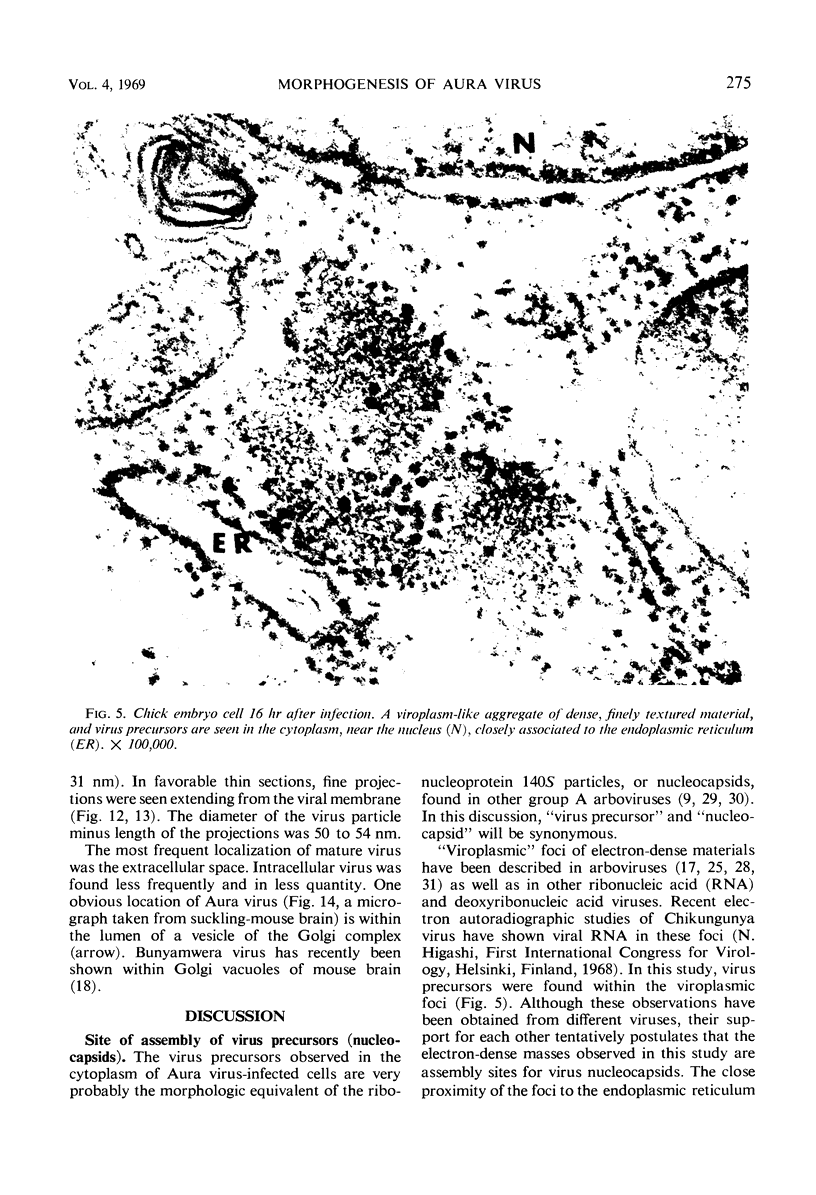
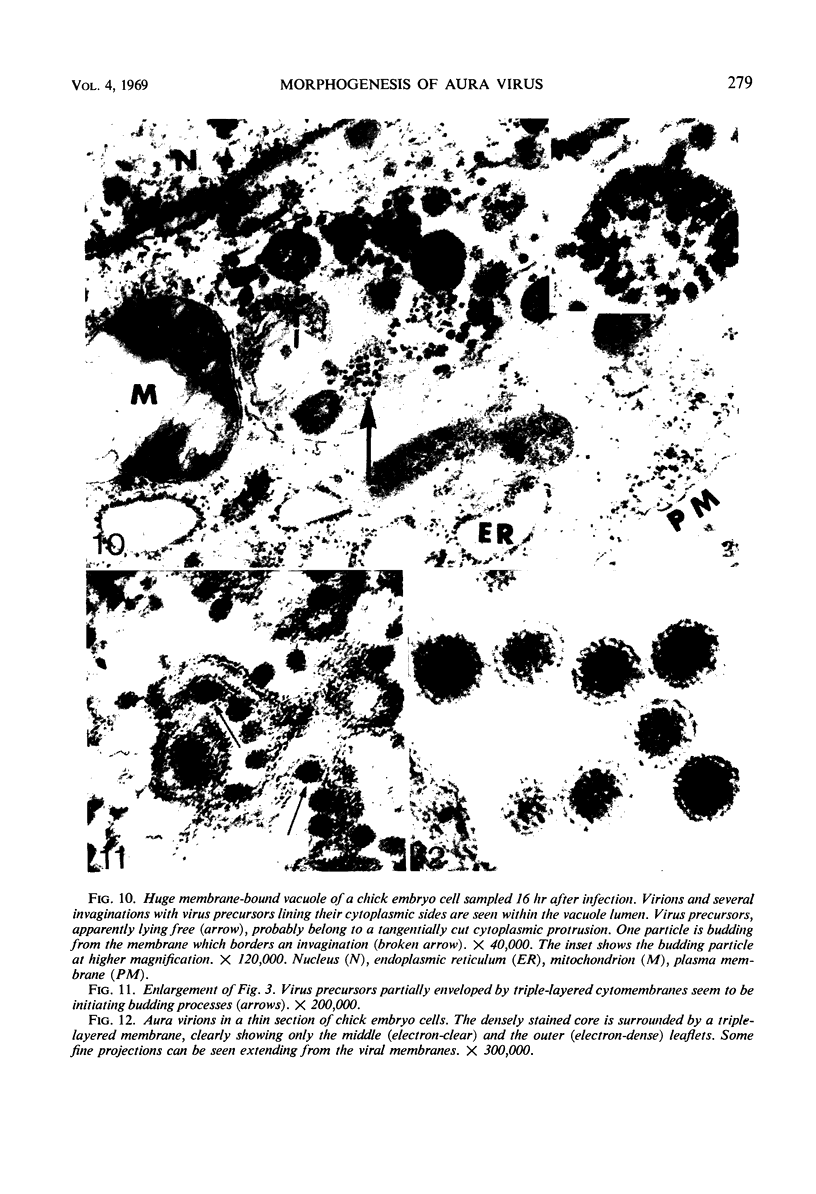
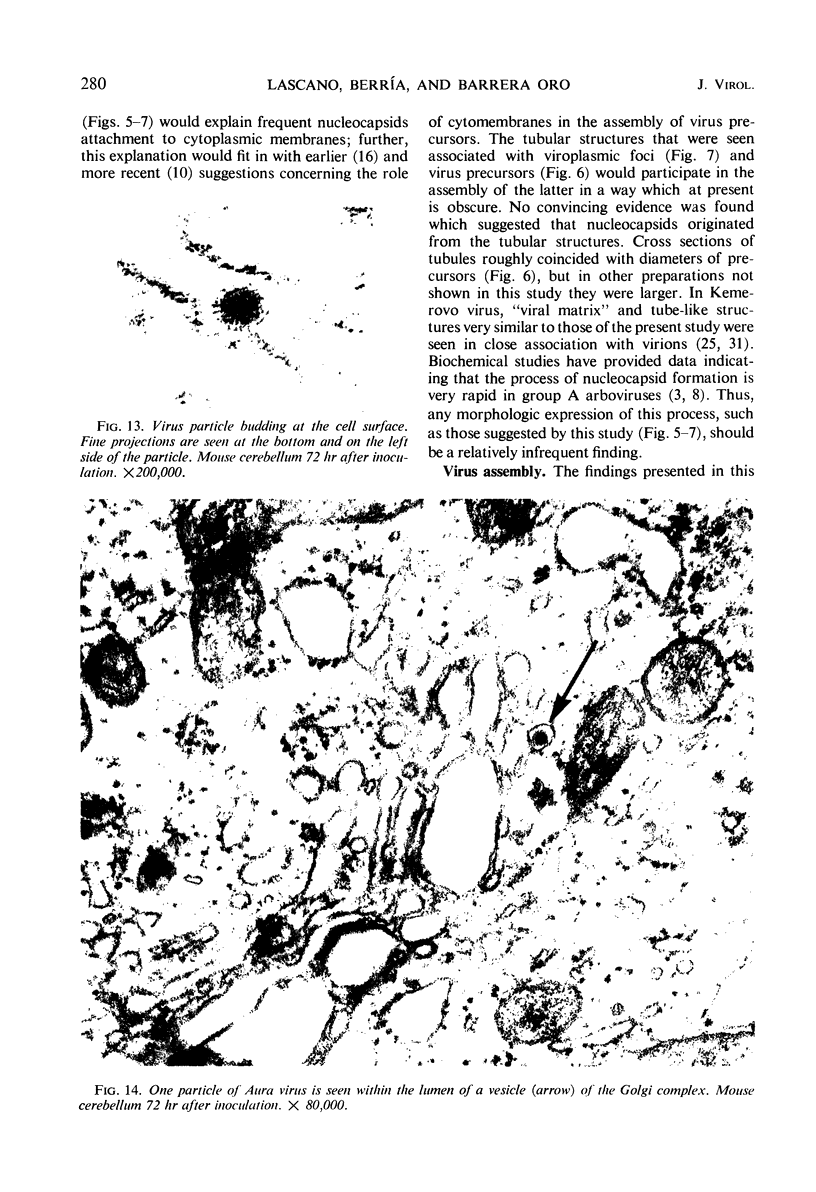
Aura virus, a member of the Western equine-encephalitis-Whataroa subgroup of group A arboviruses, was studied by electron microscopy in suckling mouse brain and chick embryo cultured cells. Virus precursors, budding particles, and complete virus particles were first detected 10 hr after infection in chick embryo cells and 24 hr after inoculation in mouse brain. Virus precursors were generally seen aligned along cytomembranes, and were less frequently seen closely associated with viroplasm-like foci, tubular aggregates, or scattered in the cytoplasmic matrix without an apparent connection to any other structure. The assembly of mature virus was observed to take place by a budding process of the virus precursor from the plasma membrane into the extracellular space, and from the cytoplasmic membranes into the lumina of vacuoles and cisternae. It was demonstrated that the endoplasmic reticulum participates in the assembly of intracellular virions. Indirect evidence was found to indicate that the Golgi complex may also form mature virus. Aura virions had a size, shape, and structure similar to those of the previously described group A arboviruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Ben-Ishai Z., Goldblum N., Becker Y. The intracellular site and sequence of Sindbis virus replication. J Gen Virol. 1968 May;2(3):365–375. doi: 10.1099/0022-1317-2-3-365. [DOI] [PubMed] [Google Scholar]
- CAUSEY O. R., CASALS J., SHOPE R. E., UDOMSAKDI S. AURA AND UNA, TWO NEW GROUP A ARTHROPOD-BORNE VIRUSES. Am J Trop Med Hyg. 1963 Sep;12:777–781. doi: 10.4269/ajtmh.1963.12.777. [DOI] [PubMed] [Google Scholar]
- Chain M. M., Doane F. W., McLean D. M. Morphological development of Chikungunya virus. Can J Microbiol. 1966 Oct;12(5):895–900. doi: 10.1139/m66-122. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., Babcock V. I., Southam C. M., Bailey R. B., Shipkey F. H. Semliki Forest virus in HEp-2 cell cultures. J Virol. 1967 Oct;1(5):996–1009. doi: 10.1128/jvi.1.5.996-1009.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Berezesky I. K. Cytoplasmic fractions associated with Semliki Forest virus ribonucleic acid replication. J Virol. 1967 Apr;1(2):374–383. doi: 10.1128/jvi.1.2.374-383.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Protein synthesis directed by an arbovirus. J Virol. 1968 Jan;2(1):26–32. doi: 10.1128/jvi.2.1.26-32.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
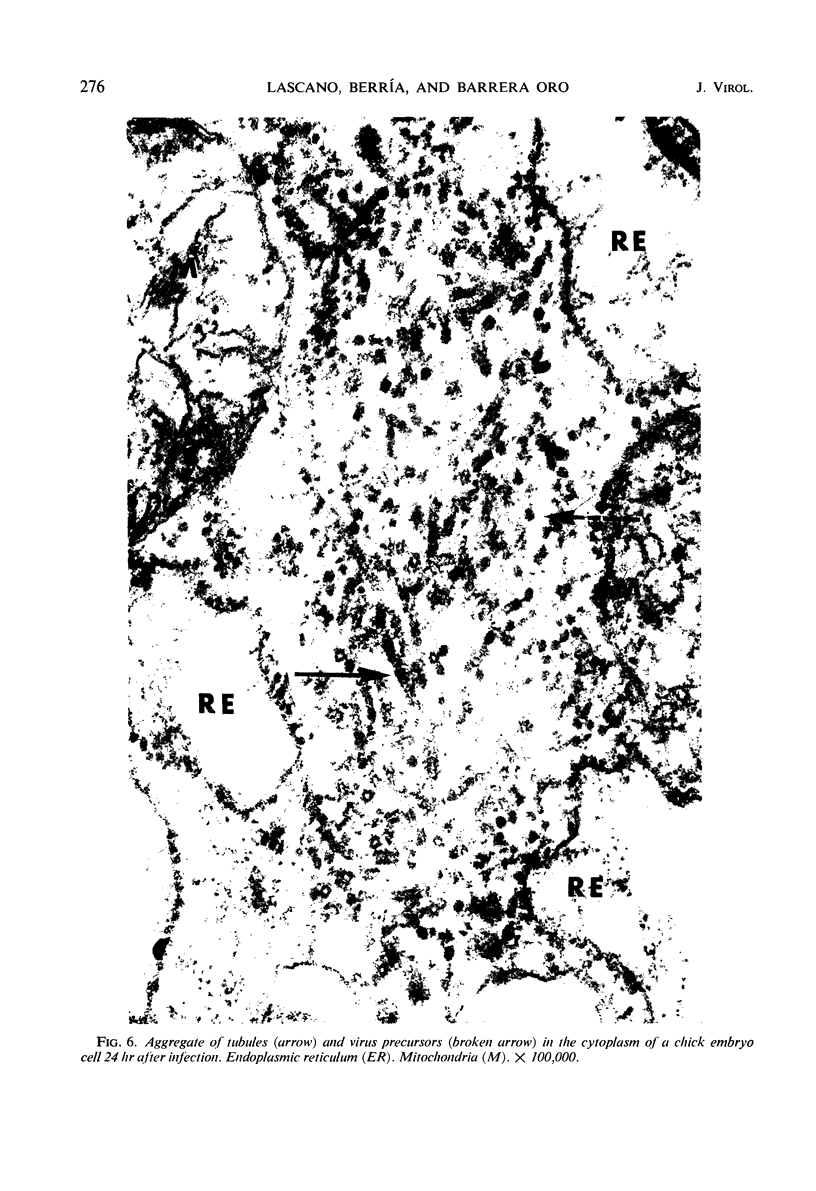
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi N., Matsumoto A., Tabata K., Nagatomo Y. Electron microscope study of development of Chikungunya virus in green monkey kidney stable (VERO) cells. Virology. 1967 Sep;33(1):55–69. doi: 10.1016/0042-6822(67)90093-1. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., HOWE C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. V. Western equine encephalomyelitis virus. J Exp Med. 1961 Jan 1;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSGAY M. STUDIES ON THE STRUCTURE OF A HEMAGGLUTINATING COMPONENT OF A GROUP A ARBO VIRUS (SINDBIS). Virology. 1964 Aug;23:573–581. doi: 10.1016/0042-6822(64)90241-7. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Electron microscopic and biological studies on the growth of Venezuelan equine encephalitis virus in KB cells. Virology. 1962 Jan;16:52–62. doi: 10.1016/0042-6822(62)90201-5. [DOI] [PubMed] [Google Scholar]
- McGee-Russell S. M., Gosztonyi G. Assembly of Semliki forest virus in brain. Nature. 1967 Jun 17;214(5094):1204–1206. doi: 10.1038/2141204a0. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Coleman P. H., Harrison A. K., Gary G. W., Jr Colorado tick fever virus: an electron microscopic study. Virology. 1968 May;35(1):28–40. doi: 10.1016/0042-6822(68)90302-4. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Tzianabos T. Electron microscopic observations of mouse brain infected with Bunyamwera group arboviruses. J Virol. 1968 Nov;2(11):1315–1325. doi: 10.1128/jvi.2.11.1315-1325.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth P. M., Calberg-Bacq C. M. Changes in morphology, infectivity and haemagglutinating activity of Semliki Forest virus produced by the treatment with caseinase C from Streptomyces albus G. J Gen Microbiol. 1966 Apr;43(1):19–30. doi: 10.1099/00221287-43-1-19. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- SATURNO A. THE MORPHOLOGY OF MAYARO VIRUS. Virology. 1963 Sep;21:131–133. doi: 10.1016/0042-6822(63)90313-1. [DOI] [PubMed] [Google Scholar]
- SOUTHAM C. M., SHIPKEY F. H., BABCOCK V. I., BAILEY R., ERLANDSON R. A. VIRUS BIOGRAPHIES. I. GROWTH OF WEST NILE AND GUAROA VIRUSES IN TISSUE CULTURE. J Bacteriol. 1964 Jul;88:187–199. doi: 10.1128/jb.88.1.187-199.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Basic structure of group A arbovirus strains Middelburg, Sindbis, and Semliki Forest examined by negative staining. Virology. 1968 Feb;34(2):358–361. doi: 10.1016/0042-6822(68)90248-1. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural differentiation of group A arboviruses based on nucleoid morphology in ultrathin sections. Virology. 1968 Mar;34(3):568–570. doi: 10.1016/0042-6822(68)90077-9. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Allen P. T. Replication of Western equine encephalomyelitis virus. II. Cytoplasmic structure involved in the synthesis and development of the virions. J Virol. 1968 Oct;2(10):1038–1046. doi: 10.1128/jvi.2.10.1038-1046.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]