Abstract
Many inborn errors of metabolism (IEMs) are associated with irreversible brain injury. For many, it is unclear how metabolite intoxication or substrate depletion accounts for the specific neurologic findings observed. IEM-associated brain injury patterns are characterized by whether the process involves gray matter, white matter, or both, and beyond that, whether subcortical or cortical gray matter nuclei are involved. Despite global insults, IEMs may result in selective injury to deep gray matter nuclei or white matter. This manuscript reviews the neuro-imaging patterns of neural injury in selected disorders of metabolism involving small molecule and macromolecular disorders (ie, Phenylketonuria, urea cycle disorders, and maple syrup urine disease) and discusses the contribution of diet and nutrition to the prevention or exacerbation of injury in selected inborn metabolic disorders. Where known, a review of the roles of individual differences in blood–brain permeability and transport mechanisms in the etiology of these disorders will be discussed.
Introduction
The majority of the inborn errors of metabolism (IEMs) are associated with a potential for injury to the developing central nervous system (CNS), resulting in chronic encephalopathy. The etiologies of neurologic injury have not been fully established in many of these disorders but may share mechanisms of injury such as disrupted astrocyte function, excitotoxic energy, and energy failure. There is a striking anatomical pattern of vulnerability in many of the disorders that cannot be easily understood, given that the entire brain is subjected to the global metabolic insult. Neuro-imaging has emerged as a powerful clinical and research tool to study the brain in a noninvasive manner. Several platforms exist to study neural networks underlying cognitive processes, white matter or myelin microstructure, and cerebral metabolism in vivo, providing tools to evaluate the extent and potential mechanisms of neurological damage.
Selectivity for particular brain regions or even cell types based on morphology or neurotransmitter systems (astrocytes or neurons: glutamatergic and gamma-aminobutyric acid [GABA]-ergic) is poorly understood. The presenting neurologic features reflect gray or white matter involvement. Patients with cortical gray matter involvement may present with seizures, encephalopathy, or dementia, whereas deep gray matter injury may result in extrapyramidal movement disorders (dystonia, chorea, or athetosis). White matter disorders present with pyramidal signs and visual findings (spasticity or hyperreflexia). Involvement of the cerebellum or its connecting tracts may lead to ataxia or dysmetria.
Patterns of brain injury in various IEMs may be explained based on various metabolic pathways. For example, some IEMs lead to brain injury due to a substrate intoxication model of injury. This pattern encompasses aminoacidopathies, organic acidurias, urea cycle disorders (UCDs), sugar intolerances, metal disorders, and porphyrias. Clinical expression can be acute and can present at any stage of life from the neonatal period to adulthood or can be intermittent in a partial form, from infancy to late adulthood. Most of these disorders are treatable but require the emergency removal of the neurotoxin by dietary intervention, extracorporeal procedures such as dialysis, scavenging drugs or vitamins serving as cofactors for enzymes.1 Another pattern of injury may be due to a substrate-depletion model of injury, such as observed in creatine deficiencies. These disorders affect the cytoplasmic and mitochondrial energetic processes. In addition to creatine deficiencies this group also includes disorders of glycolysis, glycogenosis, gluconeogenesis, hyperinsulinisms, and creatine and pentose phosphate pathways.2
Additionally, anatomical patterns of brain injury may be discerned based on location. Although the entire brain is exposed to the insult, there are typically areas of vulnerability. The insult may be focal or more diffuse having a predilection for neurons and impact the gray matter vs the white matter. Additionally, gray matter injury may be cortical only, involve cortical and deep structures, or alternatively involve only the deep gray structures such as the thalamus and basal ganglia. Small molecule disorders and lysosomal disorders have a predilection for white matter involvement, but the location within the white matter often differs based on the individual disorder and may be restricted to deep centrum semiovale white matter, periventricular white matter, and the U fibers.
The Blood–Brain Barrier and Neurometabolic Disorders
One must understand the properties of the blood–brain barrier (BBB) in order to start to understand the unique environment of the CNS and how IEMs may lead to subsequent neurologic injury.2 The BBB is a network of closely packed endothelial cells in brain capillaries that restrict passage of certain substances from the bloodstream into the capillary system.3 Within the BBB, individual neurons are rarely more than 8–20 mm from a brain capillary. In addition, the cells lining the capillary walls are more selective than endothelial cells in capillaries elsewhere in the body.
The BBB exerts control over the immediate microenvironment of brain cells forming a true physical barrier consisting of tight junctions between adjacent endothelial cells, which force most molecular traffic to take a transcellular route rather than moving paracellularly through the junctions, leading to a selective transport barrier permitting or facilitating entry of required nutrients, and excluding or leading to efflux of harmful compounds. The details of the BBB in IEMs remain incompletely understood, but are an area of active research. Heterogeneity in the neurologic manifestations of IEMs suggests that additional factors, such as interindividual variations in the capacity of BBB transporters, may underlie clinical variability.
Substrate Intoxication Models
UCDs
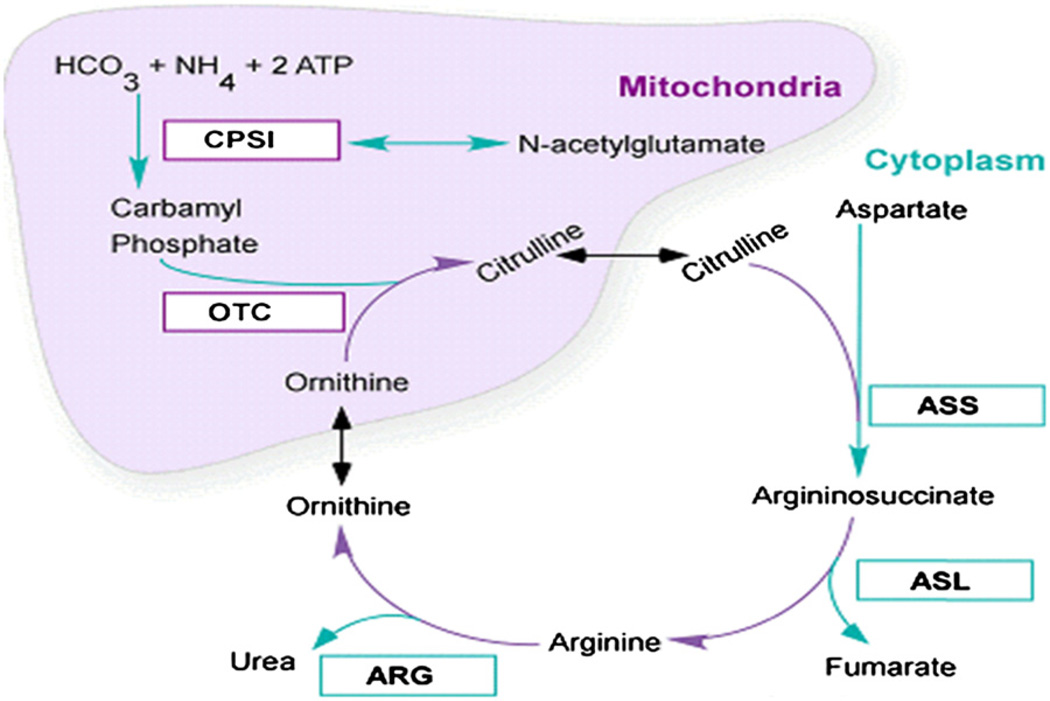
UCDs are rare but devastating IEMs with high mortality and morbidity. UCDs include deficiencies in any of the 6 enzymes and 2 membrane transporters involved in urea biosynthesis4 (Fig 1). All the UCDs are inherited as autosomal recessive disorders, except for Ornithine transcarbamylase deficiency (OTCD), which is X-linked. Infants with complete blocks in this enzyme commonly present in the first week of life with hyperammonemic (HA) coma. Despite aggressive treatment with hemodialysis, the mortality in the newborn period approaches 50%.5 Survivors sustain intellectual disabilities (IDs) that correlate with the number and severity of HA episodes.6 Patients with partial defects can present at any age with HA episodes that carry approximately 10% mortality rate and a significant risk for ID.7,8 Our research as part of a National Institutes of Health (NIH) funded UCD consortium found that even asymptomatic OTCD heterozygotes have cognitive deficits that place them at increased risk for ID and attention deficit hyperactivity disorder.9 Some patients with OTCD, who have been reported to be asymptomatic for decades may become symptomatic or have a lethal HA episode later in life.
Figure 1.
UCD pathway.
The urea cycle functions to eliminate 40%–45% of food protein nitrogen that is not needed for growth. It also provides important intermediates for other biochemical pathways (arginine). Net synthesis of intermediates is derived from the gut and kidneys. Surplus nitrogen cannot be stored and has to be excreted. In children on a protein intake of 1.25 g/kg, 50% of the urinary nitrogen is excreted as urea.4 Quantitatively, 1 g of protein contains approximately 0.16 g of nitrogen which, if catabolized completely, is converted to 5.7 mmol of urea.
Neuro-imaging Markers of OTC
There is a specific pattern of brain injury in UCDs. Acute hyperammonemia selectively affects the white matter of the brain, and initially may be seen as reversible changes involving the deep sulci of the insular and peri-rolandic region watershed territories, and hypoperfusion may play a role. Uptake of ammonia in the astrocyte and its conversion to glutamine is believed to lead to astrocytic swelling that occurs during HA coma.10 Astrocytes are responsible for regulation of water volumes and electrolyte concentrations in the extracellular fluid spaces of the nervous system. Glutamine is osmotically active and can lead to astrocytic swelling leading to cytotoxic edema. Evidence exists linking astrocytic swelling as the cause of cerebral edema in HA states in humans and experimental models. Studies in which the glutamine synthetase inhibitor, methionine sulfoximine, is administered demonstrate reduced ammonia-induced brain edema in vivo, and in vitro models.11 The astrocyte, therefore, is an important intermediate in the interactions of glutamine and ammonia via the glutamine-glutamate cycle.
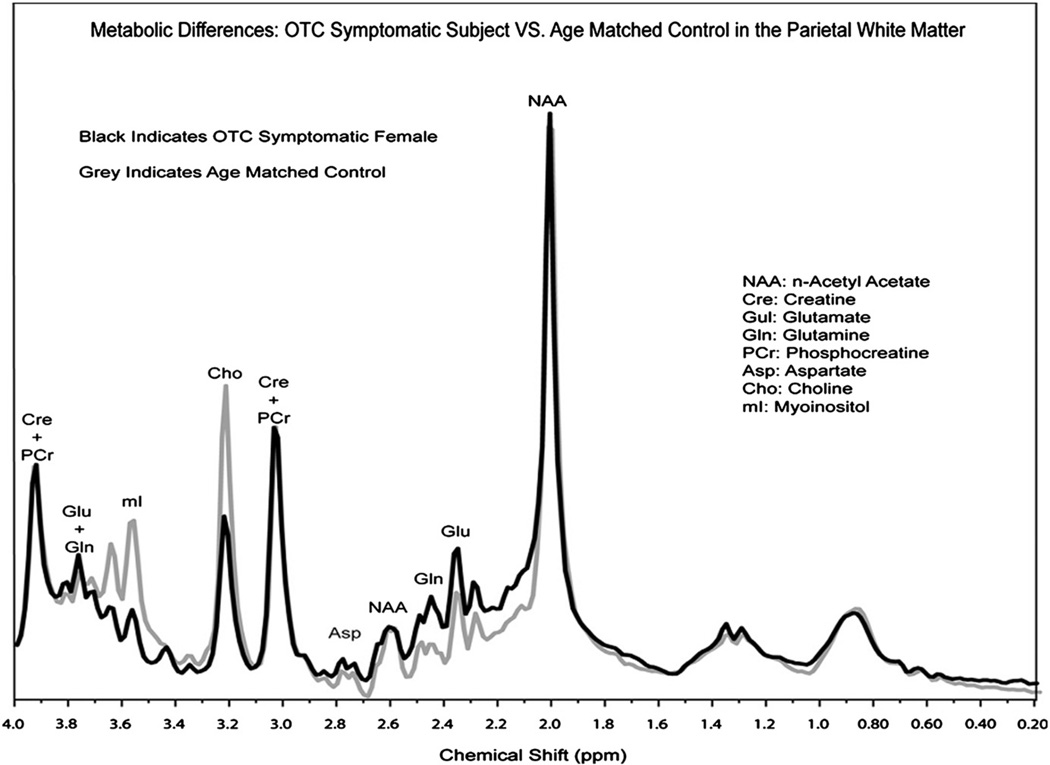
As part of an imaging project under the Urea Cycle Rare Disorders Consortium,12 20 subjects with partial OTC and 20 controls have been studied and evidence of disrupted osmoregulation was found. Significant findings included decreases in myoinositol (mI), an osmotic marker that shows an inverse correlation with disease severity. Elevations in brain glutamine were also observed(Gln), often in the presence of normal peripheral levels of glutamine and ammonia (Fig 2).13
Figure 2.
1H MRS showing elevations of glutamine and lowered myoinositol in a patient with OTCD relative to a control subject.
Maple syrup Urine Disease (MSUD)
MSUD is an autosomal recessive disorder caused by deficiency in a subunit of the branched-chain alpha ketoacid dehydrogenase complex.14 This enzyme complex is required for the oxidative decarboxylation of branched-chain ketoacids. First described in 4 siblings with progressive infantile cerebral dysfunction and urine with odor of maple syrup, the elevated plasma amino acids that define the condition are isoleucine, leucine, and valine, with leucine being the neurotoxin.15,16 This is a rare condition in Caucasians, with an incidence of 1:185,000–1:290,000; however, it is much more common in Mennonites where it is 1:176 due to a common founder effect.
During the acute stages of MSUD, neurologic consequences are due to the accumulation of branched-chain amino acids, which leads to CNS injury. Alpha-ketoisocaproic acid derived from leucine is neurotoxic leading to acute brain edema. If untreated, MSUD can lead to coma and death (by herniation). Clinical evidence suggests that improvement and deterioration may be predicted by changes in serum sodium and osmolarity rather than by branched-chain amino acid levels.17
Risk factors for critical brain edema include recurrent vomiting, prolonged and persistent ketonuria after intravenous therapy is started, serum sodium less than 135 mEq/L, serum osmolarity less than 275 mOsm/L, rapid increases of blood glucose to levels greater than 200 mg/dL, and the use of intravenous solutions that contain less than 140 mEq/L of sodium (hypotonic).
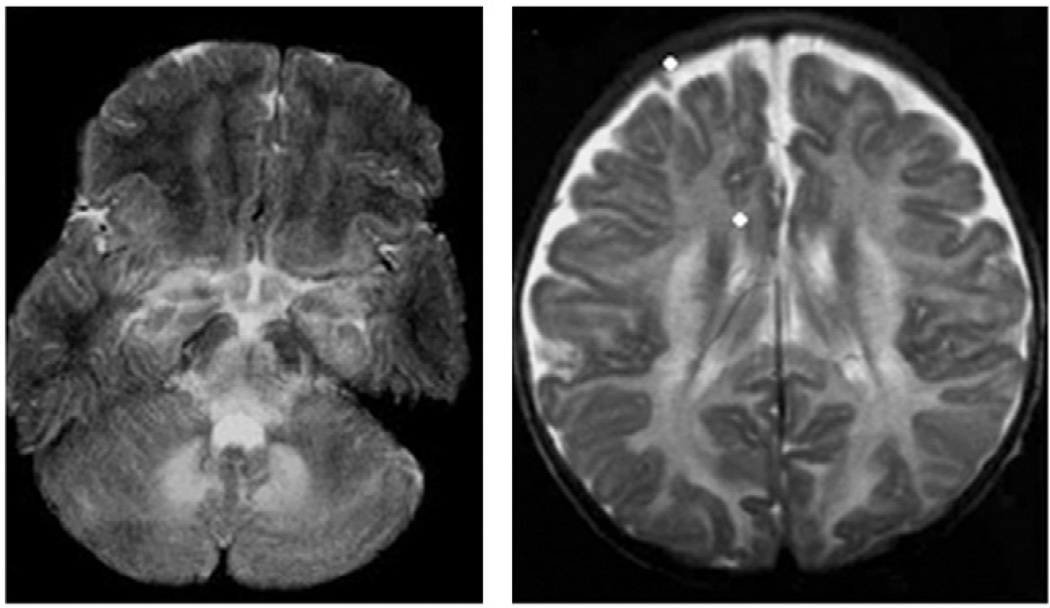
Critical edema can be visualized on magnetic resonance imaging (MRI) scans of the brain as an increase in T2 signal throughout the cerebral and cerebellar hemispheres, and is especially prominent in the basal ganglia and brainstem15,18,19 (Fig 3). Water is pulled from the vascular and extracellular space into the intracellular spaces of the brain by intracellular metabolites with osmotic activity. This is similar to the mechanism of neural injury and edema in OTCD. To balance this osmotic imbalance, mI, an osmolyte, is depleted from the brain in both conditions.
Figure 3.
Brain swelling in MSUD.
The amount of water that enters the brain under the influence of such metabolites is controlled by the sodium concentration in the extracellular space. The balance of extracellular sodium and pathologic intracellular osmolytes determines whether critical edema develops. Therefore, the use of hypotonic fluids is discouraged as it causes rapid expansion of intravascular fluids.
MSUD-associated metabolites initiate a process leading to the proteolytic degradation of myelin proteins, thereby producing abnormal myelin sheaths.16 With increased signal of myelin, predominantly affecting the mesencephalon, brain stem, thalamus, and globus pallidus, supratentorial lesions can be observed. Jan et al. assessed the utility of diffusion-weighted imaging and spectroscopy findings during metabolic decompensation to assess the value of these findings in the prediction of patient outcome.20 Diffusion-weighted imaging demonstrated marked restriction of proton diffusion compatible with cytotoxic or intramyelinic sheath edema in the brainstem, basal ganglia, thalami, cerebellar and periventricular white matter, and the cerebral cortex. This was accompanied by the presence of an abnormal branched-chain amino acid, and branched-chain alpha-keto acid peaks at 0.9 ppm as well as elevated lactate on 1H magnetic resonance spectroscopy (MRS).21 The changes seen in the patients were reversed with treatment without evidence of volume loss or persistent tissue damage. These results demonstrate that MRI can monitor therapeutic effects, and suggest that proton spectroscopy can detect cerebral accumulation of branched-chain amino acids and oxoacids in MSUD.
Oxidative Stress Model of Neuronal Injury in MSUD
The effect of toxic levels of branched-chain keto acid accumulation has been studied in cell culture models as a surrogate for MSUD.22 Results strongly indicate that oxidative stress might be involved in the cell morphological alterations and death, as well as in the cytoskeletal reorganization elicited by the branched-chain ketoacids.
Glutaric Acidemia Type 1
Glutaric acidemia type 1 (GA-1) is an autosomal disorder of lysine and tryptophan metabolism due to a deficiency of glutaryl-CoA dehydrogenase (GCDH). The incidence is about 1:30,000. Biochemical markers included elevations of serum and urine glutaric acid, 3-OH glutaric acid, and glutaconic acid. Neurologic involvement may be subtle at first. Initial symptoms may be missed or are nonspecific for this disorder. The earliest symptoms may be macrocephaly with rapid head growth in a child with hypotonia, gross motor delays, tremors, or jitteriness. Acute neurologic crisis may be precipitated by fever, dehydration, or other stressors. Early neuro-imaging findings after crisis include fronto-temporal atrophy, a delayed myelination pattern and increased signal intensity on T2-weighted and fluid-attenuated inversion recovery–weighted images localized mainly within the periventricular white matter subcortical U fibers. It is uncertain whether these are demyelinated areas or represent patchy hypomyelination. Other consistent findings include subdural hygromas, ventriculomegaly, and basal ganglia lesions.23 Very little is known about the morphological changes in long-term serial MRI seen after treatment.
The basal ganglia injuries in GA-1 that lead to the movement disorder include acute striatal necrosis and neuro-anatomically, the acute event is referred to as acute striatal necrosis. The putamen, either alone or in combination with the caudate nucleus, or globus pallidus is involved. MRI may demonstrate hyperintensities in the thalamus and dentate nuclei.24 The severity of clinical presentation depends upon the various combinations of basal ganglia involvement, the most important neurodegenerative manifestation in GA-1. The majority of untreated children present with an acute stroke-like neurologic crisis during the first years of life. Comparable to changes in ischemic stroke, diffusion is restricted, with a hyperintense signal on diffusion-weighted images and decreased signal on apparent diffusion coefficient.25
GA-1, while a systemic and lifelong disorder, has developmental predilection with anatomically restricted injury to the brain occurring within a particular developmental period, frequently between the ages of 2 months and 3 years. After age 5, it is rare to see crises. Some cases will not have typical features but present as dystonic cerebral palsy, which is progressive. Developmental delay may be present from the first few weeks or months of life. Patients with the Amish GA-1 are high excretors of GA and 3-OH glutaric acid. The majority of patients present with dystonic cerebral palsy with basal ganglia injury.
Mechanisms of Neuronal Degeneration
Current concepts regarding pathologic mechanisms of GA-1 focus on investigations of excitotoxic effects of 3-OH-GA. Recent studies using microarray analysis using brain material from GCDH-deficient (GCDH (−/−) and control mice also reveal alterations in genes involved in vascular biology.26 N-methyl-d-aspartate (NMDA) receptor antagonism may be a potential mechanism of injury as GA and 3-OH-GA may act as glutamate analogs at the NMDA receptor, which is linked to the calcium and sodium ion channels. Activation leads to opening of channels with influx of Ca2+ and sodium into the astrocyte, which leads to osmotic consequences, particularly cell swelling and volume overload. Energy depletion in GA-1 is also expected to play a role. 3-OH glutaric acid is associated with a decrease in cellular Adenosine triphosphate, probably mediated by NMDA receptor mediated excitotoxicity.
GA-1 Patterns of Vulnerability
Mouse models of GA-1 are biochemically similar to human disease; however, they do not share the same brain pathology: no striatal necrosis. Instead, there is a pattern of vacuolating white matter frontal predominant spongiform changes. Additionally, the mice have no dystonia, but rather manifest a mild motor impairment. Unanswered questions in GA-1 include “What is the relationship between metabolite intoxication and neurological damage and can it be prevented by early dietary therapy?”
Propionic Academia
Propionic acidemia (PA) is an organic academia, which classically presents in the neonatal period with vomiting, lethargy, refusal to feed, hypotonia, and less frequently with dehydration and seizures.27 It is due to a deficiency of propionyl coenzyme A (CoA). Acute neurologic events such as metabolic stroke-like episodes and seizures may or may not be associated with systemic metabolic acidosis in patients with PA. Long-term chronic neurologic complications are common despite optimal therapy. These include intellectual disability, spastic quadriplegia, and athetosis.28 Since its original description in 1961, patients with PA were identified to have a variety of neurologic signs and symptoms. Characteristic neurologic findings include ataxia and basal ganglia changes.
It was first described in 1961 by Childs et al. in a patient with severe ketoacidotic episodes precipitated by protein ingestion (specifically, methionine and threonine administration) but manifested by marked elevations in plasma and urinary glycine.29 The resultant toxic build-up of organic acid metabolites, namely propionate, β-hydroxypropionate, β-hydroxybutyrate, and methylcitrate, is presumed to be responsible for the observed hyperammonemia, metabolic acidosis, and bone marrow suppression. Incidence is estimated at approximately 1 per 100,000 live births.
A common clinical finding is mild-to-moderate hyperammonemia, which may lead to additional neurotoxicity and lead to impaired cognitive status. The underlying cause of the hyperammonemia is due to inhibition of N-acetylglutamate synthase activity by free propionic acid. N-acetylglutamate is the allosteric activator of carbamoylphosphate synthase, which is the entry step into the urea cycle. As a result, decreased ureagenesis occurs with accumulation of free ammonia. The free organic acid inhibits bone marrow production of leukocytes, red cells, and platelets, and pancytopenia is commonly seen 2–3 days after the acute presentation.
Acute metabolic crisis is associated with anorexia, feeding refusal, and vomiting leading to dehydration, lethargy, coma, seizures, metabolic strokes, metabolic acidosis, and neutropenia. Neurologic features include subsequent intellectual disability, mixed tone with spasticity and dystonia, ataxia, seizure, disorder, and metabolic strokes. Another competing hypothesis states that hyperammonemia, which is often associated with propionic acidemia, leads to an accumulation of glutamine or glutamate, or both in astrocytes. This excess glutamate may be excitotoxic to neuronal cells selectively in the basal ganglia. Thiamine (vitamin B1) is a cofactor for various biochemical reactions such as the oxidative decarboxylation of the alpha-keto acids pyruvate and alpha-l-ketoglutarate. It has been linked to lactic acidosis and encephalopathy.1 Deficiency inhibits the entry of pyruvate into the citric acid cycle. Alternatively, accumulating pyruvate is dehydrogenated to lactic acid, which results in hyperlactacidemia.30
In PA, hyperlactacidemia is assumed to be caused by competitive inhibition of the pyruvate dehydrogenase complex by propionyl-CoA.
The role for neuro-imaging in PA management has not been well established. Advanced neuro-imaging is not available in all centers, and one has to weigh the risk of sedation in some cases with potential benefits of the information gained. In PA, generalized atrophy, which develops during the first year of life, is the chronic change most commonly noted on neuro-imaging.31–34 MRS has been performed in PA and has shown decreased N-acetylaspartate (NAA), a reflection of neuronal loss, along with elevated myoinositol and elevated glutamine or glutamate in the basal ganglia of patients even under stable metabolic conditions.35 These MRS changes reflect the impact of hyperammonemia on cerebral brain water with elevations of glutamine leading to disruption of osmolytes resulting in a decrease of mI. The decrease in NAA is accounted for by neuronal cell loss, as NAA is a marker of neuronal integrity. Elevated lactate may also be present.
Methylmalonic Acidemia (MMA)
Similar to PA, MMA is a heterogeneous group of disorders characterized by accumulation of methylmalonic acid and its by-products in biological fluids. It is due to a defect in intracellular cobalamin metabolism (coenzyme deficiency). It exhibits autosomal recessive inheritance. The approximate frequency for MMA is 1 per 48,000 infants. It is due to deficiency of the adenosylcobalamin-dependent enzyme methylmalonyl-CoA mutase (apoenzyme deficiency). A subset of children with defects of intracellular cobalamin metabolism also may have simultaneous homocystinuria. Complementation studies reveal 8 different complementation groups (mut0, mut-, cblA, cblB, cblC, cblD, cblF, and cblH) causing MMA, with mut0 showing undetectable mutase activity fibroblasts. Fibroblasts of the mut-group show some residual mutase activity. Additionally, cblA, cblB, and cblH are defects in the pathway of adenosylcobalamin synthesis.36
MMA typically presents in either a newborn who was healthy for the first days to weeks of life (mut0 or MMA mut-) or in infants having a history of poor feeding, vomiting, progressive lethargy, floppiness, and muscular. Older children with 1 of the other forms of MMA or mild mut- may present for the first time during an episode of decompensation with lethargy, seizures, and hypoglycemia. Metabolic changes in these patients include urine organic acids demonstrating large amounts of methylmalonic acid, methylcitrate, propionic acid, and 3-OH propionic acid. Plasma amino acids typically show elevation of glycine but may be normal. Glycine may not be used as a metabolic marker. Acylcarnitine profile (dry blood spot or plasma) shows an elevation of propionylcarnitine (C3) and may show decreased free carnitine and total carnitine levels.
MMA-Patterns of Injury
Brain Computed tomography or MRI scans typically demonstrate involvement of basal ganglia and white matter with the globus pallidus being selectively affected. One also sees demyelination of corticospinal tracts in a pattern that resembles B12 deficiency of combined systems degeneration. Using advanced imaging technology, diffusion-weighted abnormalities have been seen in patients with MMA during acute metabolic acidosis.37,38
Phenylketonuria
First identified in 1934 by Norwegian physician Asbjørn Følling, phenylketonuria (PKU) is an autosomal recessive genetic disorder characterized by deficiency in the phenylalanine hydroxylase enzyme. Phenylalanine hydroxylase is necessary for the metabolism of the amino acid phenylalanine (Phe) to tyrosine (Tyr), an essential precursor to an array of neurotransmitters, notably dopamine (DA).39 DA is critically involved in higher order cognitive operations subserved by the prefrontal cortex of the brain.40 Early studies in the 1930s suggested that dietary restriction of protein intake reduced Phe levels in PKU patients, and in the following decade, Phe-free protein supplements were developed. Shortly thereafter, the implementation of Phe-free diet was shown to improve behavioral outcomes in PKU. Debate persisted regarding the age at which dietary restriction could be safely terminated. Studies in the later decades of the 20th century cited precipitous drops in IQ following termination of Phe restriction, and today, lifelong dietary treatment is the clinical standard.41 DA is essential for prefrontal pyramidal neurons involved in executive processes such as working memory, cognitive updating, and inhibitory control. Histopathology and neuro-imaging studies in humans have overwhelmingly indicated extensive white matter damage, in both untreated and early-treated PKU cases. It has been estimated that over 90% of patients show white matter pathology on structural MRI scans,41 and diffusion tensor imaging, an index of white matter integrity, has shown microstructural white matter damage, even in cases where no pathology was evident on structural MRI.42
Brain Injury Due to Substrate Depletion
Creatine Deficiency
The cerebral creatine deficiency syndromes are inborn errors of creatine metabolism that include the 2 creatine biosynthesis disorders, guanidinoacetate methyltransferase (GAMT) deficiency and l-arginine:glycine amidinotransferase (AGAT or GATM) deficiency, as well as the creatine transporter (SLC6A8) deficiency. In all 3 conditions, seizures and intellectual disability are the most common symptoms, although behavioral difficulties such as autism spectrum and an extrapyramidal movement disorder may be seen. Onset is between ages 3 months and 3 years. The phenotype of SLC6A8 deficiency in affected males ranges from mild intellectual disability and speech delay to significant intellectual disability, seizures, and behavioral disorder. The disorder is recognized across the life span with the age at diagnosis ranging from 2 to 66 years. Females heterozygous for SLC6A8 deficiency may have learning and behavior problems. GAMT and AGAT deficiency are autosomal recessive conditions, whereas the transport defect is an X-linked disorder.43,44
The common feature of all creatine deficiency syndromes is the severe depletion of creatine or phosphocreatine in the brain. GAMT deficiency is characterized by accumulation of guanidinoacetic acid in brain and body fluids. Guanidinoacetic acid seems to be responsible for intractable seizures and the movement disorder, both exclusively found in GAMT deficiency. Treatment with oral creatine supplementation is in part successful in GAMT and AGAT deficiencies, whereas in CrT1 defect, it is not able to replenish creatine in the brain.45
Creatine Deficiency Disorders
The creatine (Cr)-phosphocreatine (PCr) system plays an important role in energy storage and transmission. Synthesis and transport of Cr are integral parts of cellular energy metabolism Neuro-imaging findings in creatine deficiency disorder are marked by the diminution or lack of the creatine-phosphocreatine peak in the patient’s brain on in vivo proton MRS. This serves as the diagnostic clue in all 3 diseases.46
Treatment attempts to restore depleted creatine in the brain by supplementation of creatine in pharmacological doses but is not effective in the transporter defect. Oral supplementation with 0.35–2.0 g/kg/d of Cr slowly increases the Cr-PCr concentration in the brain. After several months, Cr-PCr in the brain remains significantly below the normal range as measured by 1H MRS. In addition, treatment of combined arginine restriction and ornithine substitution in GAMT deficiency is capable of decreasing guanidinoacetic acid permanently and improves the clinical outcome. Because the precursors of Cr are the non–essential amino acids glycine (Gly) and arginine (Arg), both of which have their own transporters at the BBB level, supplementation with arginine has been attempted in 5 Italian males as an attempt at overcoming the block of Cr transport within the brain.47 The study reported clinical improvement in motor skills and to a lesser extent in communication and attention. The 5 patients all had a reduction in the frequency of seizures. Total Cr and phosphocreatine evaluated by 1H MRS and 31P MRS showed a mild increase in brain creatine, although still below the normal range. The study was small and no long-term conclusions can be drawn. Dietary arginine restriction (15 mg/kg/d) in combination with ornithine supplementation (100 mg/kg/d) led to a substantial and permanent decrease of GAA in body fluids and reduced epileptogenic activities. GAA may exert an important epileptogenic potential in man.48
Conclusions
The majority of IEMs cause brain injury. Precursors or metabolites may exert direct toxicity on the brain, such as ammonia derived from protein breakdown, leucine in MSUD, and metabolites of GA. Dietary recommendations are based on following peripheral blood measures of metabolism but neuro-imaging may guide the CNS effects and may be needed to follow patients long-term and modify therapy. As neurologists, the authors are interested in the impact on the developing brain and whether the damage is modifiable. BBB considerations may explain clinical variability, despite similar dietary control. The barrier may enhance or inhibit uptake of substances into brain, whose concentrations may not correlate with peripheral measures in the blood. The BBB may also interfere with medication therapies.
References
- 1.Saudubray JM, Sedel F, Walter JH. Clinical approach to treatable inborn metabolic diseases: An introduction. J Inherit Metab Dis. 2006;29:261–274. doi: 10.1007/s10545-006-0358-0. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 4.Brusilow SW, Maestri NE. Urea cycle disorders: Diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127–170. [PubMed] [Google Scholar]
- 5.Batshaw ML, Monahan PS. Treatment of urea cycle disorders. Enzyme. 1987;38:242–250. doi: 10.1159/000469211. [DOI] [PubMed] [Google Scholar]
- 6.Msall M, Batshaw ML, Suss R, et al. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;310:1500–1505. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- 7.Batshaw ML, Msall M, Beaudet AL, et al. Risk of serious illness in heterozygotes for ornithine transcarbamylase deficiency. J Pediatr. 1986;108:236–241. doi: 10.1016/s0022-3476(86)80989-1. [DOI] [PubMed] [Google Scholar]
- 8.Maestri NE, Lord C, Glynn M, et al. The phenotype of ostensibly healthy women who are carriers for ornithine transcarbamylase deficiency. Medicine (Baltimore) 1998;77:389–397. [PubMed] [Google Scholar]
- 9.Gyato K, Wray J, Huang ZJ, et al. Metabolic and neuropsychological phenotype in women heterozygous for ornithine transcarbamylase deficiency. Ann Neurol. 2004;55:80–86. doi: 10.1002/ana.10794. [DOI] [PubMed] [Google Scholar]
- 10.Norenberg MD. Astrocytic-ammonia interactions in hepatic encephalopathy. Semin Liver Dis. 1996;16:245–253. doi: 10.1055/s-2007-1007237. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Koehler RC, Brusilow SW, et al. Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am J Physiol. 1991;261:H825–H829. doi: 10.1152/ajpheart.1991.261.3.H825. [DOI] [PubMed] [Google Scholar]
- 12.Seminara J, Tuchman M, Krivitzky L, et al. Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab. 2010;100(suppl 1):S97–S105. doi: 10.1016/j.ymgme.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gropman AL, Fricke ST, Seltzer RR, et al. 1H MRS identifies symptomatic and asymptomatic subjects with partial ornithine transcarbamylase deficiency. Mol Genet Metab. 2008;95:21–30. doi: 10.1016/j.ymgme.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton DH, Strauss KA, Robinson DL, et al. Diagnosis and treatment of maple syrup disease: A study of 36 patients. Pediatrics. 2002;109:999–1008. doi: 10.1542/peds.109.6.999. [DOI] [PubMed] [Google Scholar]
- 15.Riviello JJ, Rezvani I, DiGeorge AM, et al. Cerebral edema causing death in children with maple syrup urine disease. J Pediatr. 1991;119:42–45. doi: 10.1016/s0022-3476(05)81036-4. [DOI] [PubMed] [Google Scholar]
- 16.Schonberger S, Schweiger B, Schwahn B, et al. Dysmyelination in the brain of adolescents and young adults with maple syrup urine disease. Mol Genet Metab. 2004;82:69–75. doi: 10.1016/j.ymgme.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Zinnanti WJ, Lazovic J. Interrupting the mechanisms of brain injury in a model of maple syrup urine disease encephalopathy. J Inherit Metab Dis. 2012;35:71–79. doi: 10.1007/s10545-011-9333-5. [DOI] [PubMed] [Google Scholar]
- 18.Sutter R, Killer HE, Bilz S, et al. Cerebral edema and intracranial hypertension in an adult with maple syrup urine disease. Eur J Neurol. 2009;16:e45–e46. doi: 10.1111/j.1468-1331.2008.02425.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferraz-Filho JR, Floriano VH, Quirici MB, et al. Contribution of the diffusion-weighted MRI in the diagnosis and follow-up of encephalopathy caused by maple syrup urine disease in a full-term newborn. Arq Neuropsiquiatr. 2009;67:719–723. doi: 10.1590/s0004-282x2009000400033. [DOI] [PubMed] [Google Scholar]
- 20.Jan W, Zimmerman RA, Wang ZJ, et al. MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology. 2003;45:393–399. doi: 10.1007/s00234-003-0955-7. [DOI] [PubMed] [Google Scholar]
- 21.Felber SR, Sperl W, Chemelli A, et al. Maple syrup urine disease: Metabolic decompensation monitored by proton magnetic resonance imaging and spectroscopy. Ann Neurol. 1993;33:396–401. doi: 10.1002/ana.410330412. [DOI] [PubMed] [Google Scholar]
- 22.Funchal C, Gottfried C, De Almeida LM, et al. Evidence that the branched-chain alpha-keto acids accumulating in maple syrup urine disease induce morphological alterations and death in cultured astrocytes from rat cerebral cortex. Glia. 2004;48:230–240. doi: 10.1002/glia.20072. [DOI] [PubMed] [Google Scholar]
- 23.Brismar J, Ozand PT. CT and MR of the brain in glutaric acidemia type I: A review of 59 published cases and a report of 5 new patients. American Journal of Neuroradiology. 1995;16:675–683. [PMC free article] [PubMed] [Google Scholar]
- 24.Twomey EL, Naughten ER, Donoghue VB, et al. Neuroimaging findings in glutaric aciduria type 1. Pediatr Radiol. 2003;33:823–830. doi: 10.1007/s00247-003-0956-z. [DOI] [PubMed] [Google Scholar]
- 25.Eastwood JD, Lev MH, Wintermark M, et al. Correlation of early dynamic CT perfusion imaging with whole-brain MR diffusion and perfusion imaging in acute hemispheric stroke. AJNR Am J Neuroradiol. 2003;24:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 26.Rosa RB, Schwarzbold C, Dalcin KB, et al. Evidence that 3-hydroxyglutaric acid interacts with NMDA receptors in synaptic plasma membranes from cerebral cortex of young rats. Neurochem Int. 2004;45:1087–1094. doi: 10.1016/j.neuint.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Wolf B, Hsia YE, Sweetman L, et al. Propionic acidemia: A clinical update. J Pediatr. 1981;99:835–846. doi: 10.1016/s0022-3476(81)80004-2. [DOI] [PubMed] [Google Scholar]
- 28.Nyhan WL, Bay C, Beyer EW, et al. Neurologic nonmetabolic presentation of propionic acidemia. Arch Neurol. 1999;56:1143–1147. doi: 10.1001/archneur.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 29.Childs B, Nyhan WL, Borden M, et al. Idiopathic hyperglycinemia and hyperglycinuria: A new disorder of amino acid metabolism I. Pediatrics. 1961;27:522–538. [PubMed] [Google Scholar]
- 30.Matern D, Seydewitz HH, Lehnert W, et al. Primary treatment of propionic acidemia complicated by acute thiamine deficiency. J Pediatr. 1996;129:758–760. doi: 10.1016/s0022-3476(96)70162-2. [DOI] [PubMed] [Google Scholar]
- 31.Wolf B, Hsia YE, Sweetman L, et al. Propionic acidemia: A clinical update. J.Pediatr. 1981;99:835–846. doi: 10.1016/s0022-3476(81)80004-2. [DOI] [PubMed] [Google Scholar]
- 32.Surtees RA, Matthews EE, Leonard JV. Neurologic outcome of propionic acidemia. Pediatr.Neurol. 1992;8:333–337. doi: 10.1016/0887-8994(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 33.North KN, Korson MS, Gopal YR, et al. Neonatal-onset propionic acidemia: Neurologic and developmental profiles, and implications for management. J.Pediatr. 1995;126:916–922. doi: 10.1016/s0022-3476(95)70208-3. [DOI] [PubMed] [Google Scholar]
- 34.Brismar J, Ozand PT. CT and MR of the brain in disorders of the propionate and methylmalonate metabolism. AJNR. 1994;15:1459–1473. [PMC free article] [PubMed] [Google Scholar]
- 35.Chemelli AP, Schocke M, Sperl W, et al. Magnetic resonance spectroscopy (MRS) in five patients with treated propionic acidemia. J.Magn Reson.Imaging. 2000;11:596–600. doi: 10.1002/1522-2586(200006)11:6<596::aid-jmri4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Tanpaiboon P. Methylmalonic acidemia (MMA) Mol Genet Metab. 2005;85:2–6. doi: 10.1016/j.ymgme.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Radmanesh A, Zaman T, Ghanaati H, et al. Methylmalonic acidemia: Brain imaging findings in 52 children and a review of the literature. Pediatr Radiol. 2008;38:1054–1061. doi: 10.1007/s00247-008-0940-8. [DOI] [PubMed] [Google Scholar]
- 38.Gao Y, Guan WY, Wang J, et al. Fractional anisotropy for assessment of white matter tracts injury in methylmalonic acidemia. Chin Med J (Engl) 2009;122:945–949. [PubMed] [Google Scholar]
- 39.Matalon R, Michals K. Phenylketonuria: Screening, treatment and maternal PKU. Clin Biochem. 1991;24:337–342. doi: 10.1016/0009-9120(91)80008-q. [DOI] [PubMed] [Google Scholar]
- 40.White DA, Connor LT, Nardos B, et al. Age-related decline in the microstructural integrity of white matter in children with early- and continuously-treated PKU: A DTI study of the corpus callosum. Mol Genet Metab. 2010;99(suppl 1):S41–S46. doi: 10.1016/j.ymgme.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson PJ, Leuzzi V. White matter pathology in phenylketonuria. Mol Genet Metab. 2010;99(suppl 1):S3–S9. doi: 10.1016/j.ymgme.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Ding XQ, Fiehler J, Kohlschutter B, et al. MRI abnormalities in normal-appearing brain tissue of treated adult PKU patients. J Magn Reson Imaging. 2008;27:998–1004. doi: 10.1002/jmri.21289. [DOI] [PubMed] [Google Scholar]
- 43.Braissant O, Henry H. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: A review. J Inherit Metab Dis. 2008 doi: 10.1007/s10545-008-0826-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Braissant O, Henry H, Be ard E, et al. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids. 2011;40:1315–1324. doi: 10.1007/s00726-011-0852-z. [DOI] [PubMed] [Google Scholar]
- 45.Cheillan D, Cognat S, Vandenberghe N, et al. Creatine deficiency syndromes. Rev Neurol (Paris) 2005;161:284–289. doi: 10.1016/s0035-3787(05)85034-9. [DOI] [PubMed] [Google Scholar]
- 46.Renema WK, Schmidt A, van Asten JJ, et al. MR spectroscopy of muscle and brain in guanidinoacetate methyltransferase (GAMT)-deficient mice: Validation of an animal model to study creatine deficiency. Magn Reson Med. 2003;50:936–943. doi: 10.1002/mrm.10627. [DOI] [PubMed] [Google Scholar]
- 47.Chilosi A, Leuzzi V, Battini R, et al. Treatment with L-arginine improves neuropsychological disorders in a child with creatine transporter defect. Neurocase. 2008;14:151–161. doi: 10.1080/13554790802060821. [DOI] [PubMed] [Google Scholar]
- 48.Schulze A, Ebinger F, Rating D, et al. Improving treatment of guanidinoacetate methyltransferase deficiency: Reduction of guanidinoacetic acid in body fluids by arginine restriction and ornithine supplementation. Mol Genet Metab. 2001;74:413–419. doi: 10.1006/mgme.2001.3257. [DOI] [PubMed] [Google Scholar]