Abstract
INTRODUCTION:
Menarche or first menstrual period is a landmark in reproductive life span and it is the most prominent change of puberty. The timing of menarche can be under the influence of genes as well as individual environmental factors interacting with genetic factors.
OBJECTIVE:
Our study objectives were (a) to investigate the heritability of age of menarche in twins, (b) to obtain the association between age of menarche and childhood factors, and reproductive events/behavior, (c) to examine whether or not having a male co-twin affects early/late menarche.
METHODOLOGY:
A group of female-female identical (n = 108, 54 pairs), non-identical twins (n = 68, 34 pairs) and 17 females from opposite-sex twin sets were identified from twin registries of Malaysia and Iran. Genetic analysis was performed via two methods of Falconers’ formula and maximum likelihood.
RESULTS:
Heritability was found to be 66% using Falconers’ formula and 15% using univariate twin analysis. Model analysis revealed that shared environmental factors have a major contribution in determining the age of menarche (82%) followed by non-shared environment (18%).
DISCUSSION:
Result of this study is consistent with that of the literature. Timing of menarche could be under the influence of shared and non-shared environmental effects. Hirsutism was found to have a higher frequency among subjects with late menarche. There was no significant difference in age of menarche between females of opposite-sex twins and females of same-sex twins.
CONCLUSION:
It is concluded that twin models provide a powerful means of examining the total genetic contribution to age of menarche. Longitudinal studies of twins may clarify the type of environmental effects that determine the age of menarche.
Keywords: Menarche, reproductive health, twin
Introduction
Age at menarche, the time of first menstrual period, is an important biological and social event as well as a developmental milestone in females. Age at menarche has been identified as a risk factor for several traits including, depression,[1] eating disorders,[2] breast cancer,[3] body image,[4] osteoporotic fracture, risk of coronary heart disease, and conduct disorder.[5] The latter is a common example of age of menarche being a complex trait, which is determined by an array of genetic and environmental factors. Early age of menarche has been associated with conduct disorders.[6–9]
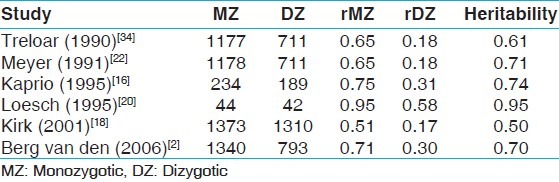
Several studies have been conducted to partition inter-individual trait variation in age at menarche into genetic and environmental components and their findings are summarized in Table 1. These studies suggest that genetic factors play a strong role in determining age at menarche as correlation coefficients of the trait range from 0.51 to 0.95 (for monozygotic twins [MZ]) and 0.17-0.58 (for dizygotic twins [DZ]). The large variation reported in the literature could be due to measurement errors and bias or the effect of environmental factors. In addition, family studies support the high heritability values for age of menarche.[10–14] The current study examines the effect of genetics and environment on age of menarche among Iranian and Malaysian twins. The possible link between age of menarche and childhood factors, reproductive events, and behavior are investigated.
Table 1.
A comparison between heritability value for age of menarche estimated by various studies
Methodology
The sample consisted of 193 female identical and non-identical Asian twins (which included 17 females from opposite-sex twin sets) who participated in the volunteer reproductive health study. Samples were drawn from two twin registries of Iran and Malaysia.
The Iran Twin Registry (ITR) was established at the Avicenna Research Institute, Shahid-Behashti University using a World Health Organization grant in 2000. The procedures and other details of ITR are published elsewhere.[15] For the purpose of this study, three trained research assistants contacted the registered married twins born between 1945 and 1988 (n = 122).
The remaining samples were drawn from the National Malaysian Twin Registry (NMTR) database. NMTR was established at the Royal College of Medicine, Perak, the University Kuala Lumpur in June 2008. Samples (n = 71) were inclusive of twins born between 1951 and 1993 inclusive of Chinese (45.1%), Indian (32.4%) and Malay (22.5%) ethnic subpopulations.
Twin zygosity was determined from twins’ (or their parents’) responses to a zygosity questionnaire that included questions on physical similarities and frequency of confusion of the twins by family members and friends. Accuracy of this method has been estimated around 90% as compared with deoxyribonucleic acid analyses.[16]
A standard questionnaire was utilized inclusive of questions on socio-demographic characteristics of subjects, reproductive events, and reproductive behavior. Both twins were interviewed. Menstrual history included age of menarche (year); duration (day), interval (day), and irregular menstrual bleeding. Clinical symptoms of premenstrual symptoms (PMS) were also asked comprising of several questions pertaining to physiological and psychological symptoms a week prior to menstrual flow (e.g., abdominal cramps, breast tenderness, emotions). Presence of two or more symptoms would classify PMS as existent. Regular menstrual cycles were defined as having regular periods every 21-35 days. The diagnosis of polycystic ovary syndrome was recorded as affirmative if both hyperandrogenemia (hirsutism, acne), and chronic anovulation (amenorrhea, oligomenorrhea or irregular menstruation) were present.
Reproductive behavior was assessed by asking the following questions: Age of first pregnancy, number of pregnancies and deliveries and number of children. Twin pairs were living together during the childhood.
Because data were obtained from various Asian ethnic groups, tests of homogeneity were employed to determine if significant variation between different ethnicities (Chinese, Indian and Malay) or countries (Iran and Malaysia) exist. Raw data were fed to the MX script for "Testing Homogeneity of Parameters Across Countries".[17] Test of homogeneity was done for grand mean, sex deviation, age, additive genetic standard deviation, shared and non-shared standard deviation (absolute), and no significant variation was observed between “ethnicity” and “countries” using Chi-square test.
Descriptive analysis and computation of variance and co-variance of variables was carried out using SPSS version 12. Basic genetic analysis was completed using the Falconer’s formula for estimation of heritability (h2) where h2= 2(rMZ− rDZ). The shared environment is estimated by deducting heritability value from MZ correlation: C = (rMZ− h2). Non-shared environment or E2is a reflection of the degree to which identical twins raised together are dissimilar, E = (1 − rMZ).
Then, a more comprehensive genetic analysis was conducted using the Mx 2.0 software[17] designed for maximum likelihood analysis (MLA) of twin data. The hypothesis was whether additive or dominance genetic factors or common environmental factors influence the age of menarche. Univariate twin model[18] was applied to the variance and co-variance matrices derived from MZ and DZ twin data. The methodological description is described elsewhere.[19] The full model included additive genetic (A), shared environment (C), and non-shared environmental variances (E). M × 2.0 compares Model 1 (Y = a2+ c2+ e2) and model 2 (Y = a2+ e2) investigating the effect of common environment. Another comparison was carried out (Model 1 and 3 where Y = a2+ c2) to investigate the role of non-shared environment. Finally, the best fit model was introduced and the proportion of genetic factor or environmental factor was given in percentages.
Results
Genetic analysis
Using Falconer’s formula,[20] heritability value was estimated at 0.66 where the shared environment influence was estimated at 0.22 and non-shared environment was 0.13. Both Figures 1 and 2 show the correlation between twin 1 and twin 2 for age of menarche. As shown by scatter plots, co-efficient of correlation for MZ (r = 0.875) was higher than that of the DZ (r = 0.657). Univariate twin analysis using Mx 2.0, however, found an estimate of 15 for heritability and the best fitting model for age of menarche was CE model where C stands for shared environment with the contribution of 82% and E stands for non-shared environment with 18% influence.
Figure 1.
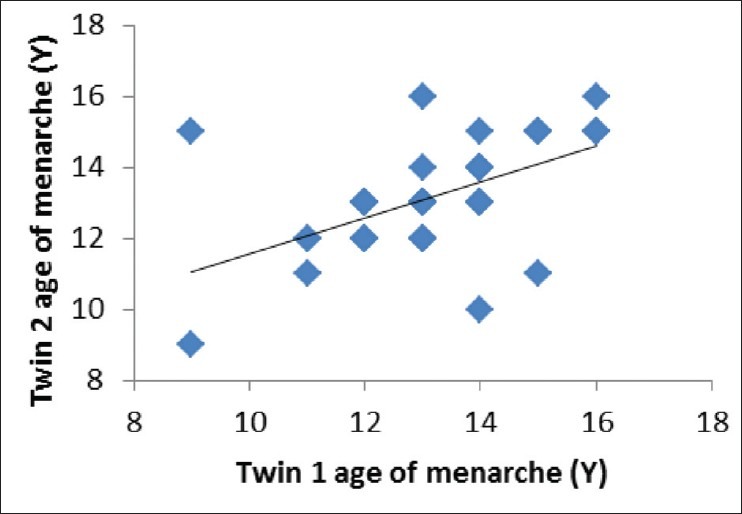
Scatter plot of age of menarche (Years) twin 1 and twin 2-monozygotic twins
Figure 2.
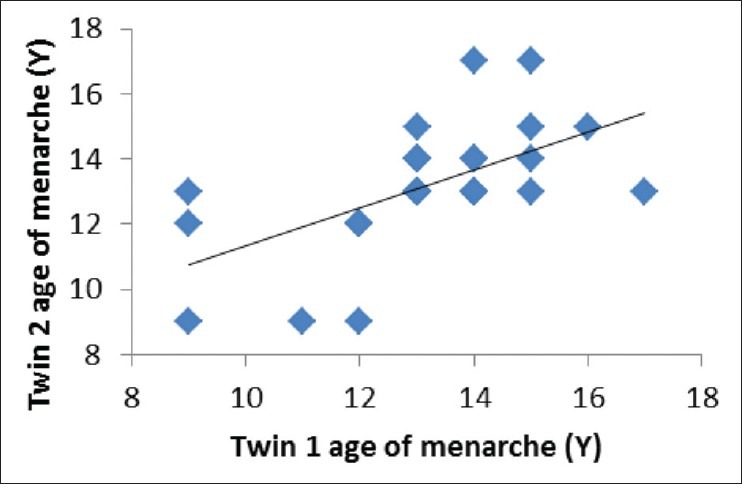
Scatter plot of age of menarche (Years) twin 1 and twin 2-dizygotic twins
Non-genetic analysis
Descriptive analysis
Mean age was 31.52 ± 9.53 years old with median of 30 years, minimum of 16 and maximum of 63. Average age of menarche was 13.18 ± 1.60 years with the minimum of 9, maximum of 17 and median of 13 years of age.
Tests of association
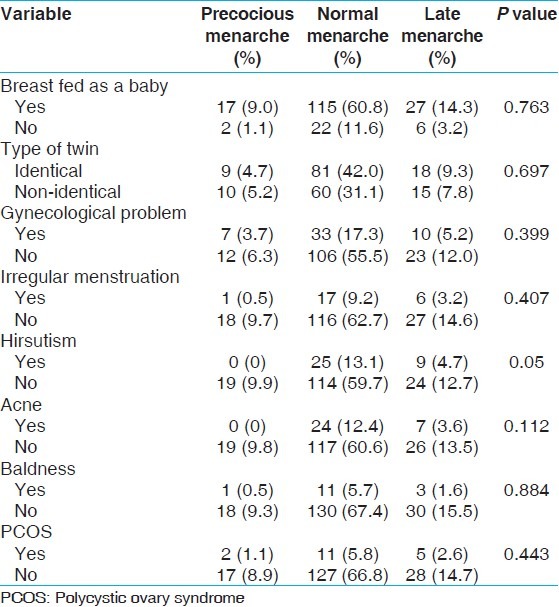
There was no significant difference in age of menarche between MZ and DZ twins (13.15 ± 1.43 vs. 13.21 ± 1.81, P = 0.785). In addition, age of menarche was not associated with being breastfed during neonatal phase (P = 0.763), and type of zygosity (P = 0.697), birth weight (P = 0.830), and gestational age (P = 0.936). In addition, age of menarche was not found to be associated with variables categorized as reproductive behavior or reproductive events during adulthood except for hirsutism where proportion of hirsutism was found to be higher among those who had late menarche (9/24) compared with those with normal menarche (25/114) [Table 2].
Table 2.
Association of reproductive events with early, normal and late age of menarche
Reproductive behavior was further analyzed and compared between Malaysian and Iranian participants. Although the age of marriage (25.89 ± 3.29 vs. 19.47 ± 4.45; P = 0.001) and age of first pregnancy (27.00 ± 2.95 vs. 20.38 ± 4.21; P = 0.001) were higher among Malaysians the number of pregnancies, deliveries and children were not statistically significantly different (P = 0.348, 0.423 and 0.423, respectively).
Sub-analysis: Precocious and late menarche
We conducted a sub-analysis comparing two categories of early and late menarche. About 10 of subjects (n = 19) had precocious menarche (below 11.58 years of age minus one standard deviation below the mean) and 17 (n = 33) had late menarche (above 14.78 years of age plus one standard deviation above mean). Early or late menarche was not associated with birth weight (P = 0.925), duration of menarche (P = 0.425), menstrual interval (P = 0.887), weight (P = 0.327), height (P = 0.569) or any of the variables categorized as reproductive events or reproductive behavior e.g., (age of marriage P = 0.210; age of first pregnancy P = 0.614). Moreover, a comparison between identical and non-identical twins showed no significant difference in the proportion of subjects with precocious and late menarche (P = 0.399).
Opposite-sex twins
A comparison between age of menarche among females of opposite-sex twins (male-female, n = 17) and same-sex twins (n = 176) showed no significant difference between the two groups (P = 0.635).
Discussion
Menarche or first menstrual period is a landmark in reproductive life span and it is the most prominent change of puberty which shows successful reactivation of hypothalamic-pituitary-gonadal axis, leading to sexual maturation.[21] Several studies have indicated that the time of menarche can be under the influence of genes as well as individual environmental factors interacting with genetic factors.[19,22,23] These studies introduce a range of heritability values from 50% to 80%. This variation is mostly due to measurement errors, recall bias or additive gene-environment interaction.
Measurement and bias errors are not accounted for in retrospective studies. Our study is also susceptible to both of these errors. Evidence suggests that there is a relatively strong correlation between the true age of menarche and that reported by adults after 30 years.[24] More than 50% of our participants were 33 years of age or less. And the difference between age of menarche and current age was not greater than 20 years for each individual (at 50thpercentile) reducing the chance of recall bias.
Pickles et al.[25] claimed that nearly all variations in age of menarche are due to additive genetic effects. He, however, used a method of analysis where gene-environment interaction was not taken into consideration. If there are interactions between gene and environmental factors (shared or not-shared), the heritability coefficient is biased when the interactions are ignored. MLA modifies the older methods of twin analysis and introduces three proportions of genetic, shared and not-shared environment in determination of measured trait, taking the interaction between gene and environmental variation into account. Using the Falconer’s method, heritability was calculated for our data as 0.66% or 66%. This value fits well with the reported range of heritability value in the literature. However, conducting MLA (with interaction terms) suggested that only 15% of this variable is determined by genetic deviation and the remaining variation was due to environmental factors. Based on the quantitative genetic modeling, significant evidence of environmental contribution to individual differences in age at menarche was found. The best fitted model was CE translating that 82% of the trait is determined by common environment (C) and 18% by no-shared environment (E).
Difference between these two estimations (Falconer’s method and MLA) might also be due to differences amongst the variances and co-variances of MZ and DZ twins in the matrix analyses. The high concordance between twins might be due to the fact that the twins in this study shared their childhood and adolescence together and grew up in the same environment. Although MZ twins are genetically identical, if a trait is influenced by environmental factors (inclusive of intra-uterine, neonatal, childhood, and adolescent environment), high concordance is an expected observation. Given that DZ twins also have the opportunity to share the same environment, a high correlation was observed between DZ twins (r = 0.675). Variation between co-variance of DZ and MZ twins have been therefore, smaller than expected. The maximum likelihood method of twin analysis ignores the co-variance and thus is a more accurate twin analysis.[17] Our MLA heritability estimates are consistent with evidence from the literature.[18,26–28]
Next, we examined the association between age of menarche and reproductive event/behavior. Until this date, no study has investigated such association among twins.
The conceptual frame-work was based on evidence from the literature that precocious menarche leads to “early exposure to reproductive hormones”, which can then increase the chance for exposure to risk factors for various diseases.[29,30] Early age of menarche is therefore introduced as a life predictor of breast cancer risk.[14] We postulated that other reproductive events during adulthood could also be under the influence of age of menarche. None of the measured variables (reproductive events or reproductive behavior) was found to be associated with age of menarche. Frequency of hirsutism, however, was significantly higher among subjects with late menarche (P = 0.05).
Reason for higher hirsutism level among those with late menarche is discussed in the literature.[31,32] If free testosterone in the serum is high, it could both delay the trigger needed to stimulate HPO-axis causing late menarche and causes clinical symptoms of hyperandrogenism such as hirsutism. On the other hand, Zukauskaite et al.[33] found earlier age of menarche in hirsute adolescent girls.
Late or early menarche can be due to intrauterine exposure to testosterone. Animal studies on rodents have shown that male twins can influence the hormonal balance of female fetus and cause a male dominant phenotype.[33] For instance, female fetuses located between male fetuses have higher testosterone concentrations than do female fetuses located between female fetuses. Hormonal imbalance can cause behavioral as well as morphological differences in adult rodents.[34] Human studies show similar phenomena occurring in twins with opposite gender. The hypothesis supported by these studies is called “intrauterine position effect” and is about diffusion of steroid hormones from one fetus to another through direct transfusion or through fetal membrane, placenta or amniotic fluid that could cause similarities in terms of hormonal balance. Our study postulated that opposite sex twins could have earlier or late menarche rather than normal menarche however, findings showed otherwise.
We also hypothesized that early or late menarche could have an effect on decision making related to reproductive life events such as age of marriage, age of first pregnancy, number of pregnancies and deliveries, and number of children. Our sub-analysis, however, failed to show any link between age of menarche and these reproductive behaviors. Cultural influence on reproductive behavior is undeniable. Age of marriage and age of first pregnancy was lower among Iranian twins. However, this did not influence subjects’ behavior in terms of number of pregnancies and/or number of children.
Conclusion
Age of menarche is under both influences of genetic and environmental factors. Our findings support a greater role for shared and non-shared environment. Birth weight as well as childhood events such as being breastfed, and vaccination were not related to age of menarche. Reproductive events in life were not related to age of menarche except for hirsutism, which was found to be higher among those with late menarche. Reproductive behavior was not linked with age of menarche. Age of menarche was also not associated with gender of co-twin. It is suggested that future studies consider longitudinal surveys of twins to measure pre-menarche anthropometric milestones as well as environmental factors such as nutrition, exercise, and stress in determining the age of menarche.
Footnotes
Source of Support: This study was done using financial support of University Kuala Lumpur and Avicenna Research Institute. We would like to thank the twins who participated in the study and research assistants who collected data. Special thanks go to colleagues from Avicenna Research Institute Dr. M.M. Akhondi, Dr. Mohammad Reza Sadeghi and Ms. Haleh Maleki, Beki Hamzezadeh, Baik, Sadighiniya.
Conflict of Interest: None declared.
References
- 1.Kaltiala-Heino R, Kosunen E, Rimpelä M. Pubertal timing, sexual behaviour and self-reported depression in middle adolescence. J Adolesc. 2003;26:531–45. doi: 10.1016/s0140-1971(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 2.Kaltiala-Heino R, Rimpelä M, Rissanen A, Rantanen P. Early puberty and early sexual activity are associated with bulimic-type eating pathology in middle adolescence. J Adolesc Health. 2001;28:346–52. doi: 10.1016/s1054-139x(01)00195-1. [DOI] [PubMed] [Google Scholar]
- 3.Velie EM, Nechuta S, Osuch JR. Lifetime reproductive and anthropometric risk factors for breast cancer in postmenopausal women. Breast Dis. 2005;24:17–35. doi: 10.3233/bd-2006-24103. [DOI] [PubMed] [Google Scholar]
- 4.Blyth DA, Simmons JK, Zakin DF. Satisfaction with body image for early adolescent females: The impact of pubertal timing within different school environments. J Youth Adoles. 1985;14:207–25. doi: 10.1007/BF02090319. [DOI] [PubMed] [Google Scholar]
- 5.Mueller NT, Odegaard AO, Gross MD, Koh WP, Yyan JM, Pereira MA. Age at menarche and cardiovascular disease mortality in Singaporean Chinese women: The Singapore Chinese Health Study. Ann Epidemiol. 2012;22:717–22. doi: 10.1016/j.annepidem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspi A, Lynam D, Moffitt TE, Silva PA. Unraveling girls’ delinquency: Biological, dispositional, and contextual contributions to adolescent misbehavior. Dev Psychol. 1993;29:19–30. [Google Scholar]
- 7.Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood. J Am Acad Child Adolesc Psychiatry. 2004;43:718–26. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- 8.Obeidallah D, Brennan RT, Brooks-Gunn J, Earls F. Links between pubertal timing and neighborhood contexts: Implications for girls’ violent behavior. J Am Acad Child Adolesc Psychiatry. 2004;43:1460–8. doi: 10.1097/01.chi.0000142667.52062.1e. [DOI] [PubMed] [Google Scholar]
- 9.Ge X, Brody GH, Conger RD, Simons RL, Murry VM. Contextual amplification of pubertal transition effects on deviant peer affiliation and externalizing behavior among African American children. Dev Psychol. 2002;38:42–54. doi: 10.1037//0012-1649.38.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: Associations between and within families across late adolescence. Dev Psychol. 2000;36:180–9. [PubMed] [Google Scholar]
- 11.Treloar SA, Martin NG. Age at menarche as a fitness trait: Nonadditive genetic variance detected in a large twin sample. Am J Hum Genet. 1990;47:137–48. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaprio J, Rimpelä A, Winter T, Viken RJ, Rimpelä M, Rose RJ. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67:739–53. [PubMed] [Google Scholar]
- 13.Kirk KM, Blomberg SP, Duffy DL, Heath AC, Owens IP, Martin NG. Natural selection and quantitative genetics of life-history traits in Western women: A twin study. Evolution. 2001;55:423–35. doi: 10.1111/j.0014-3820.2001.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 14.Towne B, Czerwinski SA, Demerath EW, Blangero J, Roche AF, Siervogel RM. Heritability of age at menarche in girls from the Fels Longitudinal Study. Am J Phys Anthropol. 2005;128:210–9. doi: 10.1002/ajpa.20106. [DOI] [PubMed] [Google Scholar]
- 15.Jahanfar S, Maleki H, Mosavi AR, Jahanfar M. Leptin and its association with polycystic ovary syndrome: A twin study. Gynecol Endocrinol. 2004;18:327–34. doi: 10.1080/09513590410001667256. [DOI] [PubMed] [Google Scholar]
- 16.Rietveld MJ, van Der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin Res. 2000;3:134–41. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- 17.Neale MC, Cardon LR. Ch. 1 Dordrecht. The Nethrelands: Kuluwer Academic; 1992. Model fitting functions and optimization methodology for genetic studies of twins and families; pp. 56–76. [Google Scholar]
- 18.Ku SY, Kang JW, Kim H, Kim YD, Jee BC, Suh CS, et al. Age at menarche and its influencing factors in North Korean female refugees. Hum Reprod. 2006;21:833–6. doi: 10.1093/humrep/dei271. [DOI] [PubMed] [Google Scholar]
- 19.Loesch DZ, Hopper JL, Rogucka E, Huggins RM. Timing and genetic rapport between growth in skeletal maturity and height around puberty: Similarities and differences between girls and boys. Am J Hum Genet. 1995;56:753–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Jahanfar S, Lye MS, Krishnarajah IS. The heritability of premenstrual syndrome. Twin Res Hum Genet. 2011;14:433–6. doi: 10.1375/twin.14.5.433. [DOI] [PubMed] [Google Scholar]
- 21.Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–83. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- 22.Palmert MR, Hirschhorn JN. Genetic approaches to stature, pubertal timing, and other complex traits. Mol Genet Metab. 2003;80:1–10. doi: 10.1016/s1096-7192(03)00107-0. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JM, Eaves LJ, Heath AC, Martin NG. Estimating genetic influences on the age-at-menarche: A survival analysis approach. Am J Med Genet. 1991;39:148–54. doi: 10.1002/ajmg.1320390207. [DOI] [PubMed] [Google Scholar]
- 24.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: After 30 years, how well do women remember. Am J Epidemiol. 2002;155:672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 25.Pickles A, Pickering K, Simonoff E, Silberg J, Meyer J, Maes H. Genetic clocks and soft events: A twin model for pubertal development and other recalled sequences of developmental milestones, transitions, or ages at onset. Behav Genet. 1998;28:243–53. doi: 10.1023/a:1021615228995. [DOI] [PubMed] [Google Scholar]
- 26.Khan AD, Schroeder DG, Martorell R, Haas JD, Rivera J. Early childhood determinants of age at menarche in rural Guatemala. Am J Hum Biol. 1996;8:7171–23. doi: 10.1002/(SICI)1520-6300(1996)8:6<717::AID-AJHB3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Serjeant GR, Singhal A, Hambleton IR. Sickle cell disease and age at menarche in Jamaican girls: Observations from a cohort study. Arch Dis Child. 2001;85:375–8. doi: 10.1136/adc.85.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onland-Moret NC, Peeters PH, van Gils CH, Clavel-Chapelon F, Key T, Tjønneland A, et al. Age at menarche in relation to adult height: The EPIC study. Am J Epidemiol. 2005;162:623–32. doi: 10.1093/aje/kwi260. [DOI] [PubMed] [Google Scholar]
- 29.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 30.Petridou E, Syrigou E, Toupadaki N, Zavitsanos X, Willett W, Trichopoulos D. Determinants of age at menarche as early life predictors of breast cancer risk. Int J Cancer. 1996;68:193–8. doi: 10.1002/(SICI)1097-0215(19961009)68:2<193::AID-IJC9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Mahesh VB, Brann DW. Regulation of the preovulatory gonadotropin surge by endogenous steroids. Steroids. 1998;63:616–29. doi: 10.1016/s0039-128x(98)00075-0. [DOI] [PubMed] [Google Scholar]
- 32.Xita N, Tsatsoulis A, Stavrou I, Georgiou I. Association of SHBG gene polymorphism with menarche. Mol Hum Reprod. 2005;11:459–62. doi: 10.1093/molehr/gah178. [DOI] [PubMed] [Google Scholar]
- 33.Zukauskaite S, Seibokaite A, Lasas L, Lasiene D, Urbonaite B, Kiesylyte J. Serum hormone levels and anthropometric characteristics in girls with hyperandrogenism. Medicina (Kaunas) 2005;41:305–12. [PubMed] [Google Scholar]
- 34.Gandelman R, vom Saal FS, Reinisch JM. Contiguity to male foetuses affects morphology and behaviour of female mice. Nature. 1977;266:722–4. doi: 10.1038/266722a0. [DOI] [PubMed] [Google Scholar]


