The thrombotic microangiopathies (TMA) are a group of disorders defined by the presence of microangiopathic hemolytic anemia and thrombocytopenia. The most common of these is thrombotic thrombocytopenic purpura (TTP), which is a systemic disorder of microvascular thromboses due to deficiency of ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). A less common TMA is the atypical hemolytic uremic syndrome (aHUS), which is a renal vascular TMA caused by complement dysregulation.
Despite overlapping clinical and pathologic manifestations, TTP and aHUS have distinct etiologies. TTP is often caused by a deficiency of ADAMTS-13 that is the result of gene mutations or acquired autoantibodies (Tsai, 2006). Atypical HUS is caused by defects of regulation and/or excessive activation of the alternative complement pathway (Kavanagh & Goodship, 2010).
The mechanism by which complement dysregulation contributes to aHUS is not precisely defined, although complement-mediated glomerular endothelial injury and enhanced complement-mediated platelet activation are probably involved (Stahl et al, 2008). Similarly, triggers and co-factors directing systemic platelet deposition in TTP are not completely understood. Evidence that complement activation might play a role in TTP (Noris et al, 1999; Ruiz-Torres et al, 2005; Reti et al, 2012) raises the possibility of a cross-talk between ADAMTS-13/ultra-large von Willebrand factor (ULVWF) and the complement system.
We studied plasma samples of 81 patients diagnosed with TMA according to clinical criteria for functional abnormalities in both ADAMTS-13 and complement regulation. Citrated platelet-poor plasma samples were obtained for testing before the initial plasma infusion or exchange procedures. All patients had microangiopathic hemolytic anemia and thrombocytopenia without an alternative cause, and treated with either plasma infusion or plasma exchanges. None of our patients had acute renal failure. Samples for analysis of DNA were not obtained/stored from this group of patients. All human subject studies were conducted according to the approved institutional review board protocols in the Rice University and University of Texas M.D. Anderson Cancer Center.
ADAMTS-13 activity was measured by: (1) the rate of cleavage of a substrate that contains 73 amino acids of the A2 domain of VWF with fluorescence resonance energy transfer (FRET) tags on either side of the cleavage site for ADAMTS-13 (FRETS-VWF73), according to the manufacturer’s protocol (GTi Diagnostics); and (2) cleavage of urea-treated ULVWF multimers (obtained from human umbilical vein endothelial cell supernatant) by citrated patient plasma, followed by VWF multimeric analysis using SDS-1% agarose electrophoresis and Western-blotting with anti-VWF antibody. This is a modification of the method described by Furlan, et al. (Furlan et al, 1998). The presence or absence of ADAMTS-13 inhibitors was determined by measuring cleavage of urea-treated ULVWF multimers before and after mixing normal citrated plasma with an equal volume of patient citrated plasma (Furlan et al, 1998).
Complement activity was measured by the hemolysis of sheep erythrocytes after incubation with human serum or plasma according to modified techniques from Sanchez-Corral, et al. (Sanchez-Corral et al, 2004).
Factor H-depleted plasma causes complement-induced lysis of sheep erythrocytes with the visually apparent release of hemoglobin. Pooled normal plasma or serum caused 7% and 8% hemolysis of sheep erythrocytes, respectively. Optimal dilution of plasma or serum for the assay was determined to be between 4/100 to 6/100, and optimal incubation time was 10 min.
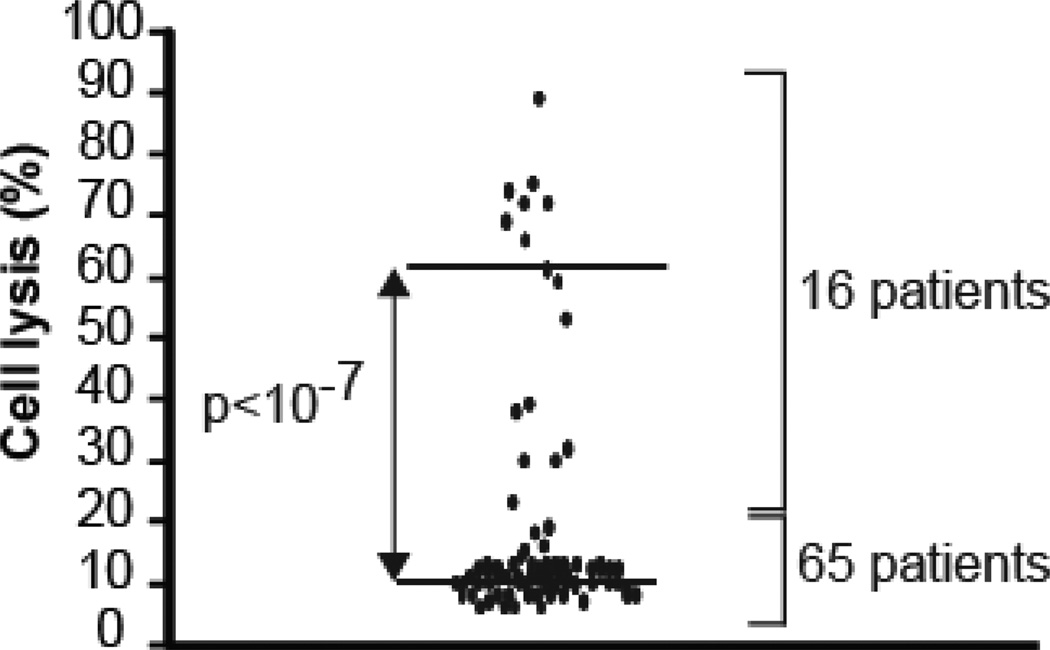
Sixty percent (49/81) of TMA patients had severe ADAMTS-13 deficiency (less than 10% activity). Eighty percent (65/81) of our patients’ plasma samples caused little to no hemolysis of sheep erythrocytes (median of 10%; range of 0–15%). In contrast, 20% (16/81) of the patients’ samples showed significant hemolysis (median of 60% hemolysis; range of 23–89%) (Figure 1). Sixteen percent (8/49) of plasma samples from TTP patients with severe ADAMTS-13 deficiency caused increased hemolysis. Only one of the 8 patients with concurrent excessive complement-induced hemolysis and severe ADAMTS-13 deficiency had detectable antibody (in low titer) against ADAMTS-13 (Table 1). Twenty-five percent (8/32) of plasma samples from patients who did not have severe ADAMTS-13 deficiency also caused increased hemolysis.
Figure 1. Sheep erythrocyte hemolysis assay in plasma samples from patients with thrombotic microangiopathy.
Fifty µl of freshly washed sheep erythrocytes (5×108 cells/ml, Complement Tech.) were mixed with 10 µl of plasma samples from patients with TMA, and the total volume brought up to 200 µl using Gelatin Veronal Buffered saline plus calcium and magnesium (GVB++: 0.1 % gelatin, 5 mM Veronal, 141 mM NaCl, 1.8 mM sodium barbital, 3.1 mM barbituric acid, pH 7.3, 0.15 mM calcium chloride, 0.5 mM magnesium chloride), and incubated at 37°C for 10 min. Background controls were measured by mixing the same amount of plasma samples and sheep erythrocytes with GVB plus 10 mM EDTA (GVBE). The supernatant was separated and its optical density (OD) absorbance was detected at 414 nm wave length. Hemolysis was measured by the change in the OD as a result of released hemoglobin. The percentage of hemolysis in each sample was calculated by dividing its OD value by that of factor H-depleted serum multiplied by 100. Factor H-depleted serum (100% hemolysis) and pooled normal plasma (7% hemolysis) or serum (8% hemolysis) served as controls. Each patient’s plasma sample was studied in three independent experiments. The results are shown as a dot plot (p<10−7, n=3, two-tailed t-test).
Table 1.
Patients with a clinical diagnosis of TTP and decreased complement regulation.
| ADAMTS-13 function (%) | ||||||
|---|---|---|---|---|---|---|
| Patient | Gender | Familal/Recurrent TTP |
VWF multimer cleavage |
FRETS VWF73 |
inhibitor | Hemolysis (%) |
| 1 | F | + | 0 | 0 | ND | 23 |
| 2 | M | 100 | 38 | NP | 38 | |
| 3 | F | 50 | 45 | ND | 69 | |
| 4 | M | + | 0 | 8 | ND | 39 |
| 5 | M | + | 0 | 7 | LOW TITER | 72 |
| 6 | M | + | 0 | 7 | ND | 59 |
| 7 | M | 45 | 98 | ND | 30 | |
| 8 | F | 12.5–25 | 7 | ND | 30 | |
| 9 | F | 12.5–25 | 13 | ND | 74 | |
| 10 | F | 0 | 6 | ND | 66 | |
| 11 | M | 12.5–25 | 10 | ND | 75 | |
| 12 | M | + | 25 | 90 | ND | 53 |
| 13 | F | + | 100 | 109 | ND | 32 |
| 14 | M | 100 | 71 | ND | 89 | |
| 15 | M | 50 | 39 | ND | 72 | |
| 16 | M | + | 0 | 5 | ND | 61 |
ND: Not detected
NP: Not Performed
Severe deficiency of functional ADAMTS-13 is associated with TTP; however, many patients with a TTP-like syndrome have normal ADAMTS-13 levels, as did 40% (32/81) of the patients in our study. There are several reports of patients with reduced ADAMTS-13 function who either did not develop TTP, or did so later in life (Noris et al, 2005). These observations raise the possibility of the presence of additional factors besides ADAMTS-13 deficiency involved in the pathophysiology of TTP (Ruiz-Torres et al, 2005; Reti et al, 2012; Noris et al, 2005; Chapin et al, 2012). Activation of the complement system in both familial (Noris et al, 1999) and acquired TTP (Reti et al, 2012) has been reported, based on the lower concentration of C3 and elevated levels of complement activation products (C3a and sC5b-9) in the sera of patients with acute TTP, and deposition of C3 and C5b-9 on endothelial cell exposed to TTP sera (Ruiz-Torres et al, 2005). We studied activity of the alternative complement pathway in 81 patients with the clinical diagnosis of TTP-like TMA requiring plasma infusion and/or plasma exchange. Some patients with severe ADAMTS-13 deficiency (8/49; 16%) or TTP-like TMA (8/32; 25%), had elevated plasma complement activity. We did not detect an increased titer of ADAMTS-13 inhibitor in ADAMTS-13 deficient TTP patients with complement dysregulation, and the majority of these patients (5/8; 68%) had a history of familial or recurrent TTP (Table 1).
Our data suggest that the complement system may be an important co-factor involved in the pathogenesis of TMA. Excessive alternative pathway activity occurred in a significant number of TTP patients indicating that concurrent defects in ADAMTS-13 and complement regulation may occur more frequently than previously reported (Noris et al, 2005; Chapin et al, 2012). In addition, our findings indicate that excessive alternative pathway activity can be associated with a TTP-like TMA in some patients who do not have severe deficiencies of ADAMTS-13. Further genetic studies of patients with the clinical diagnosis of TTP may be informative.
Acknowledgements
S.F performed the research; M.H.K designed the research study and interpreted the data; L.N performed the research, J.M designed the research study and interpreted the data, and V.A-K designed the research study, interpreted the data, and wrote the paper.
References
- Chapin J, Weksler B, Magro C, Laurence J. Eculizumab in the treatment of refractory idiopathic thrombotic thrombocytopenic purpura. Br.J.Haematol. 2012;157:772–774. doi: 10.1111/j.1365-2141.2012.09084.x. [DOI] [PubMed] [Google Scholar]
- Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome [see comments] N.Engl.J.Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- Kavanagh D, Goodship TH. Atypical hemolytic uremic syndrome. Curr.Opin.Hematol. 2010;17:432–438. doi: 10.1097/MOH.0b013e32833cae86. [DOI] [PubMed] [Google Scholar]
- Noris M, Bucchioni S, Galbusera M, Donadelli R, Bresin E, Castelletti F, Caprioli J, Brioschi S, Scheiflinger F, Remuzzi G. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J.Am.Soc.Nephrol. 2005;16:1177–1183. doi: 10.1681/ASN.2005010086. [DOI] [PubMed] [Google Scholar]
- Noris M, Ruggenenti P, Perna A, Orisio S, Caprioli J, Skerka C, Vasile B, Zipfel PF, Remuzzi G. Hypocomplementemia discloses genetic predisposition to hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: role of factor H abnormalities. Italian Registry of Familial and Recurrent Hemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura. J.Am.Soc.Nephrol. 1999;10:281–293. doi: 10.1681/ASN.V102281. [DOI] [PubMed] [Google Scholar]
- Reti M, Farkas P, Csuka D, Razso K, Schlammadinger A, Udvardy ML, Madach K, Domjan G, Bereczki C, Reusz GS, Szabo AJ, Prohaszka Z. Complement activation in thrombotic thrombocytopenic purpura. J.Thromb.Haemost. 2012;10:791–798. doi: 10.1111/j.1538-7836.2012.04674.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Torres MP, Casiraghi F, Galbusera M, Macconi D, Gastoldi S, Todeschini M, Porrati F, Belotti D, Pogliani EM, Noris M, Remuzzi G. Complement activation: the missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb.Haemost. 2005;93:443–452. doi: 10.1160/TH04-07-0450. [DOI] [PubMed] [Google Scholar]
- Sanchez-Corral P, Gonzalez-Rubio C, Rodriguez de CS, Lopez-Trascasa M. Functional analysis in serum from atypical Hemolytic Uremic Syndrome patients reveals impaired protection of host cells associated with mutations in factor H. Mol.Immunol. 2004;41:81–84. doi: 10.1016/j.molimm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Stahl AL, Vaziri-Sani F, Heinen S, Kristoffersson AC, Gydell KH, Raafat R, Gutierrez A, Beringer O, Zipfel PF, Karpman D. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111:5307–5315. doi: 10.1182/blood-2007-08-106153. [DOI] [PubMed] [Google Scholar]
- Tsai HM. The molecular biology of thrombotic microangiopathy. Kidney Int. 2006;70:16–23. doi: 10.1038/sj.ki.5001535. [DOI] [PMC free article] [PubMed] [Google Scholar]