Abstract
Thymidylate synthase (TS) is a key enzyme in the biosynthesis of thymidine. TS inhibitors, which are used in cancer chemotherapy, suffer from resistance development in tumors through upregulation of TS expression. Autoregulatory translation control has been implicated with TS overexpression. TS binding at its own mRNA, which leads to sequestration of the start codon, is abolished when the enzyme forms an inhibitor complex, thereby relieving translation suppression. We have used the protein binding site from the TS mRNA in the context of a bicistronic expression system to validate targeting the regulatory motif with stabilizing ligands that prevent ribosomal initiation. Stabilization of the RNA by mutations, which were studied as surrogates of ligand binding, suppresses translation of the TS protein. Compounds that stabilize the TS binding RNA motif and thereby inhibit ribosomal initiation might be used in combination with existing TS enzyme-targeting drugs to overcome resistance development during chemotherapy.
Keywords: bicistronic reporter, peptide inhibitor, RNA secondary structure, translation regulation
Introduction
The enzyme thymidylate synthase (TS) catalyzes the reductive methylation of 2′-deoxyuridine-5′-monophosphate (dUMP) to produce 2′-deoxythymidine-5′-monophosphate (dTMP) which is further phosphorylated to the triphosphate dTTP, an essential building block for cellular DNA synthesis.[1] TS is an attractive target for cancer chemotherapy since it provides the only de novo source for dTTP.[2] Inhibitors of TS are important drugs for cancer therapy including 5-fluorouracil (5-FU) which was one of the earliest anti-cancer agents and is still used in the treatment of colorectal cancer.[3] 5-FU is metabolized to the active TS inhibitor 5-fluoro-2′-deoxyuridine-5′-monophosphate (FdUMP). FdUMP has a dual mechanism of action. It is incorporated into cellular nucleic acids and also directly inhibits TS via the formation of a covalent ternary complex with the methyl donor cofactor N5-N10-methylene-tetrahydrofolate (mTHF).[2]
A major drawback of the clinical use of 5-FU is the development of resistance against the drug in tumors through, among other more complex mechanisms,[4] upregulation of TS expression.[2] TS overexpression emerging during 5FU chemotherapy has been implicated with the autoregulatory mechanism of translation control for the enzyme. In the absence of a substrate ligand, TS associates at two independent binding sites within its own mRNA and thereby represses translation.[5] Complex formation with the cognate substrate dUMP or the inhibitor FdUMP abolishes mRNA binding of TS.[6] The presence of FdUMP during chemotherapy with 5-FU thus leads to increased levels of TS expression despite inactivation of the enzyme, which ultimately results in emergence of tumor resistance. Whereas such feedback regulation of translation is common in bacteria, the TS system represents the first known example of translational autoregulation in human.[7] It has been demonstrated that full translational repression is achieved through TS protein binding at both mRNA sites.[5b] One of the TS binding sequences (site 2) is located in an extended stretch of 200 nucleotides within the mRNA coding region.
The site 1 is predicted to fold into a stem loop structure that spans across the translation initiation site (Figure 1).[5a] It has been suggested that protein binding to the regulatory mRNA site 1 motif stabilizes the hairpin loop which sequesters the start codon unavailable for ribosomal recognition.
Figure 1.
Secondary structure of the human TS mRNA which contains two binding sites for the enzyme. Site 1 is predicted to adopt a stem loop structure that contains several base mismatches and a hairpin sequestering the AUG initiation codon.[5a] Site 2 is located within the reading frame.[5b] Base changes are shown for four stabilized mutant sequences (mt1–mt4) which are used in this work. Numbering is according to the Homo sapiens sequence, record NM_001071 of the NCBI Nucleotide Database.[16]
We propose that small molecule ligands of the TS mRNA may bind and stabilize the site 1 hairpin independent of TS binding and suppress translation sufficiently to overcome 5-FU dependent overexpression of the enzyme. Such hairpin-stabilizing ligands might overcome resistance development during 5-FU chemotherapy. Previously, it has been reported that promiscuous nucleic acid binders including aminoglycosides and a bis-benzimidazole dye used for DNA staining (Hoechst 33258) interact with RNA constructs resembling the upper part of the TS site 1 hairpin.[8] Here, we have used the authentic regulatory element from the TS mRNA to validate the approach of targeting the hairpin motif with stabilizing ligands. As a proof of concept, we aimed to investigate if interaction energies typically provided by the binding of a small molecule ligand would provide sufficient stabilization of the hairpin RNA to impact translation initiation. We have constructed a reporter system carrying mutations within the TS site 1 motif as a surrogate for stabilizing ligands to assess the potential energetic contribution required for translation suppression through sequestration of the start codon. A small exploratory set of compounds was tested for their impact on reporter expression under the control of the TS regulatory motif.
Results and Discussion
The TS site 1 regulatory motif confers TS-dependent repression of reporter translation
To test the function of the TS site 1 motif as a protein binding hairpin that confers TS-dependent regulation of translation we inserted the site 1 motif upstream of a reporter gene to be used in a coupled in vitro transcription-translation (IVT) assay. A bicistronic system was constructed in which a firefly luciferase reporter is translated cap-driven under the control of the TS site 1 motif followed by a Renilla luciferase reporter initiated at a hepatitis C virus (HCV) internal ribosome entry site (IRES) (Figure 2A).[9] Bicistronic expression constructs provide an internal control of translation integrity and allow the exclusion of false positive inhibitors which affect the ribosome independently of the presence of the TS site 1 regulatory motif. In addition, the use of bicistronic transcripts obviates the need for determining absolute mRNA concentrations or screening for polymerase inhibition in the IVT assay.
Figure 2.

Reporter translation under the control of the TS site 1 regulatory element. A) Bicistronic reporter constructs used for experiments in a coupled in vitro transcription-translation assay (IVT). In the TS1 construct a cap-driven firefly reporter gene under the control of the TS site 1 motif is followed by an HCV IRES-driven Renilla luciferase reporter. The ΔTS1 construct lacks the site 1 motif upstream of the firefly reporter. B) Impact of huTS protein addition on cap-driven firefly luciferase reporter expression from the TS1 and ΔTS1 constructs. C) Impact of huTS protein addition on IRES-driven Renilla luciferase reporter expression from the same two constructs. Data are averages of three replicates with error bars showing ±1σ.
The proper function of the TS site 1 regulatory motif in the context of the bicistronic reporter constructs was demonstrated by titration of human TS protein in the IVT assay (Figure 2B). Addition of the enzyme suppressed reporter expression in a dose-dependent fashion and completely inhibited translation at 25μM concentration in the construct carrying the TS site 1 motif, thus reproducing the effect shown for TS protein acting on its authentic mRNA.[7] In the absence of the TS site 1 hairpin (ΔTS1), cap-driven translation was not affected by the TS protein (Figure 2B) and neither was the IRES-driven process in any of the bicistronic constructs (Figure 2C). Compared to the translation of a cap-driven luciferase from the ΔTS1 construct, reporter expression under the TS regulatory motif was about 40% lower (Figure 3A). We assumed that the reduced translation activity was due to TS protein present in the reticulocyte lysate (full cell extract) which was used in the IVT assay. After immunodepletion of TS protein from the lysate, the expression of cap-driven reporter under the TS site 1 motif was restored to the level of the ΔTS1 construct (Figure 3B). This demonstrates that the secondary structure of the wild type TS site 1 hairpin by itself does not provide sufficient stabilization to prevent the ribosome from access to the initiation site. Binding of TS protein to the RNA is required to efficiently sequester the start codon for translation suppression.
Figure 3.
Impact of stabilizing mutations on reporter translation under the control of the TS site 1 regulatory element. A) Expression levels of the cap-driven firefly and IRES-driven Renilla luciferase reporters in bicistronic constructs containing wild type (TS1) and mutant (mt1-mt4) TS site 1 regulatory elements in the presence of full reticulocyte extract. B) Reporter expression from the same two constructs in cell extract that was depleted of residual TS protein. Mutations in mt1-mt4 are shown in Figure 1. The ΔTS1 construct is included as a control. Note that expression data has been normalized to the level of translation from the TS1 construct (=100%) for the separate experiments shown in panels A and B. Absolute expression from the ΔTS1 construct does not change between experiments with full extract (A) and TS-depleted extract (B) while expression levels from TS1 and mutants rise uniformly in TS-depleted extract (B). Data are averages of three replicates with error bars showing ±1σ.
Stabilization of the TS site 1 regulatory motif suppresses mRNA translation
We propose to exploit the site 1 regulatory motif in the TS mRNA as a target for small molecule translation inhibitors that stabilize a hairpin motif in which the initiation codon is sequestered. The feasibility of this approach was tested by investigation of stabilizing mutations in the stem part of the site 1 motif and their impact on translation (Figure 1). In the absence of selective ligands, we planned to use stabilizing mutations as surrogates to correlate stabilization energy with the extent of translation suppression in the IVT assay. We assumed that translation inhibition in stabilized mutant hairpins would result from an overall increased stability of the TS protein-RNA hairpin complexes. However, this holds true only if protein binding is not adversely affected by the mutations. Therefore, we selected three positions for mutations upstream from the initiation codon since preliminary crosslinking studies and truncated TS mutants examined in the IVT assay indicated that the TS protein binds to the site 1 motif downstream from the start site.
Testing in the IVT assay of four mutant constructs (mt1-mt4), which introduce stabilizing base changes in the TS site 1 structure, showed that each additional Watson-Crick base pair formed led to further repression of reporter translation under control of the site 1 element (Figure 3A). Reduction of reporter expression was a specific consequence of the stabilizing mutations in the TS site 1 motif as indicated by the relatively unaffected levels of IRES-driven translation from the bicistronic constructs. Whereas in vitro translation in cell lysate that was depleted of residual TS protein partially restored reporter expression of the stabilized mutant constructs (Figure 3B), only the single mutation mt3 reached an expression level comparable to the wild type sequence. The other three mutants maintained relatively suppressed translation that was correlated to the stabilization introduced by the base exchanges. Marginal stabilization, corresponding to one additional stacked base pair as in mt1, of the wild type TS site 1 hairpin was sufficient to reduce translation efficiency, even in the absence of TS protein binding. In conjunction with our finding that the TS site 1 motif by itself does not provide sufficient stabilization to prevent translation initiation, this suggests that the wild type hairpin is only just labile enough to allow free read through by the ribosome whereas binding with TS protein affords a stable complex that efficiently inhibits translation.
Stabilization energies introduced by the mutations, which were estimated from RNA folding predictions for the TS site 1 motif, suggest a correlation to reporter expression in full cell extract (see Supporting Information). As expected, TS site 1 mutants with higher stability led to lower translation efficiency, presumably through requiring more energy for the ribosome to dissociate the TS complex and disrupt the site 1 motif RNA secondary structure during initiation of protein synthesis. In agreement with this hypothesis was the observation that already the wild type TS site 1, which recruits TS protein present in the reticulocyte lysate, reduced translation efficiency compared to the ΔTS1 construct. The correlation of energy stabilization and reporter expression in TS-depleted extract confirmed the notion of the TS site 1 hairpin by itself as a marginally stable roadblock to ribosomal initiation. Whereas additional contribution of ~3–4 kcal/mol of free energy to the RNA secondary structure, as in the mutant mt3, did not yet reduce translation, stabilization beyond this level reduced reporter expression.
From the correlation of secondary structure stability and translation efficiency, it was estimated that a contribution of ~8 kcal/mol of stabilization free energy was sufficient to reduce translation by ~50%. This suggests that a small molecule ligand engaging in just 2 to 3 intermolecular hydrogen bonds to the RNA target will induce inhibition of TS translation upon binding to the site 1 regulatory element. Thus, compounds selectively binding to the TS mRNA might stabilize the site 1 hairpin independent of protein binding and suppress TS synthesis sufficiently to overcome 5-FU dependent overexpression of the enzyme.
Exploratory screen for compounds that affect translation under control of the TS site 1 motif
To discover compounds that selectively impact protein expression under the control of the TS site 1 regulatory element, we performed a screen of a small exploratory compound set emerging from our efforts to design and synthesize molecules biased for RNA binding. Compounds were tested in the IVT assay using the bicistronic reporter construct which carried the wild type TS site 1 motif. Testing was performed initially at two concentrations of 10μM and 100μM compound concentration. In the primary screen, we did not discover ligands that selectively suppressed expression of the firefly luciferase under the control of the TS site 1 regulatory element. However, two compounds, derivatives of diamino-propionic acid (1) and dihydropyrimidine (2), were identified that increased reporter translation. Subsequent titration in the IVT assay revealed that both molecules selectively stimulated TS1-specific translation in a dose-dependent fashion while not affecting reporter expression in the ΔTS1 construct (Figure 4A).
Figure 4.
Compounds that increase translation of luciferase reporter under control of the TS site 1 regulatory element in an IVT assay using full reticulocyte extract. A) Derivatives of diamino-propionic acid (1) and dihydropyrimidine (2). B) Tetrahydrofolic acid (THF), which is structurally related to the TS enzyme co-substrate 5,10-methylenetetrahydrofolic acid. Graphs show firefly luciferase expression under control of the TS site 1 motif normalized to reporter expression from a construct that lacks the TS site 1 motif (see Figure 2A). Open symbols show reporter expression using TS-depleted extract (“dep”) at the maximum concentrations tested for compounds (1), (2) and THF. Data are averages of three replicates with error bars showing ±1σ.
When TS-depleted lysate was used in the IVT assay, the compounds (1) and (2) did not affect reporter expression, suggesting that these molecules may ablate the TS protein’s ability to repress translation by binding to the site 1 motif. We hypothesized that the compounds perhaps target the co-substrate binding site of the enzyme, which is known to exert allosteric control over RNA binding of the TS protein.[2] As a control, we investigated the impact of tetrahydrofolic acid (THF) whose pteridine moiety shows structural similarity to the heterocyclic scaffold in the dihydropyrimidine (2) (Figure 4B). THF is a precursor of the TS enzyme co-substrate 5,10-methylenetetrahydrofolic acid and a ligand of the co-substrate binding site. We found that THF, like the compounds (1) and (2), increased TS1-specific translation albeit with better potency. At 5μM concentration, THF led to increased reporter expression in the TS1-controlled construct comparable to the level of ΔTS1 mRNA, thus confirming that ligand binding to the enzyme co-substrate site ablates translational repression conferred by TS binding to the site 1 motif.
The hypothesis that compounds (1) and (2) may directly interact with the co-substrate binding site of TS was further explored by testing both molecules for their impact on the protein’s enzymatic activity. Competition with the cognate co-substrate 5,10-methylenetetrahydrofolic acid would lead to inhibition of the enzyme. Addition of (1) or (2) to a TS in vitro activity assay showed that both molecules suppressed enzymatic function in a dose-dependent fashion (Figure 5). The finding of direct enzyme inhibition along with the ability to ablate TS-mediated repression of translation under the control of the site 1 motif suggest that compounds (1) and (2) function as ligands of the TS co-substrate binding site and might provide starting points for the development of more potent TS inhibitors. However, similar to 5FU, such inhibitors will still suffer from resistance development through ablation of enzyme binding to its mRNA and relieving translation suppression.
Figure 5.
Inhibition of TS enzymatic activity by compounds (1) and (2), which likely are competitors of the 5,10-methylenetetrahydrofolic acid co-substrate, and the active site substrate analog 5-FU as a control. Data are averages of three replicates with error bars showing ±1σ.
Discovery of peptides that bind to the TS site 1 motif and suppress translation
Since screening of the small exploratory set of compounds did not yield a stabilizing ligand for the TS site 1 motif, we decided to explore short arginine-rich peptides as potential binders which have a propensity for RNA interaction.[10] Peptides for binding testing were designed following two criteria. Firstly, the length of peptides was limited to 6 amino acids to avoid problems with solubility and permeability in future cell based testing. Secondly, we included sequences that contained dyads of arginines around pairs of neutral polar residues (Figure 6). The selection and distribution of arginines was inspired by observations from a high resolution crystal structure of the TS enzyme in which a cluster of tightly bound phosphate ions is found around two spatially close pairs of arginines at the surface of the protein.[11] It has been suggested that the phosphate cluster indicates the location of an RNA binding site in TS. Based on these considerations we initially explored hexapeptides of the general sequence RRXXRR (X = any amino acid) as potential ligands. A 33mer RNA construct representing the TS site 1 motif was used to analyze the interaction of peptides by native polyacrylamide gel electrophoresis (PAGE). Peptides containing tyrosine or tryptophane pairs (3 and 6) showed nonspecific binding, and in the case of 6 led to aggregation. Two peptides (4 and 5) which had arginine dyads separated by leucine or serine pairs formed specific complexes with the RNA (Figure 6A). Titration of 4 and 5 revealed dose-dependent binding of the peptides to the TS site 1 motif with affinities of ≤ 50μM (Figure 6B).
Figure 6.
Identification of arginine dyad peptides that bind the TS site 1 regulatory element. A) Native PAGE assay of four hexapeptides (3) – (6) interacting with a 33mer TS site 1 RNA construct (residues C80 – G112, Figure 1). “M” indicates a single stranded RNA marker. “R” indicates the free RNA. Peptides were tested at stoichiometric ratios of 1:1 and 4:1 peptide:RNA. B) Dose response titration of peptides (4) and (5) in the PAGE assay. Numbers indicate the stoichiometric ratio of peptide:RNA. C) Quantification of peptide-RNA complex formation in gels shown in panel B. For both peptides binding affinity is KD ≤ 50μM.
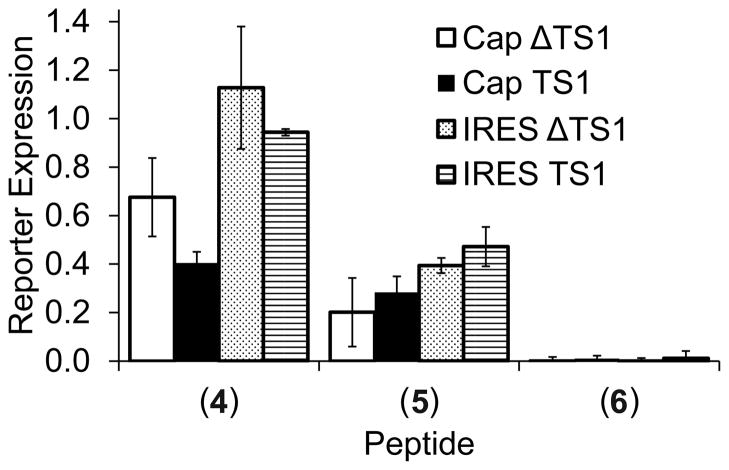
To assess whether peptide binding would occur to the TS site 1 target as part of reporter mRNA, we tested the impact of 4, 5 and, as a control, 6 in the IVT assay. Reporter expression from constructs TS1 and ΔTS1 was measured in the presence of 200μM peptide (Figure 7). Strong general translation inhibition occurred in the presence of peptide 6, consistent with the observation of RNA aggregation in the PAGE analysis (Figure 6A).
Figure 7.
Impact of hexapeptides (4) – (6) on reporter translation from bicistronic constructs TS1 and ΔTS1. Peptides were added at 200μM concentration to the IVT assay. Reporter expression was normalized to levels measured in the absence of peptides. Peptide 6 induces aggregation and likely precipitation of RNA (see Figure 6A), resulting in general translation inhibition. Data are averages of three replicates with error bars showing ±1σ.
The serine containing peptide 5, which formed a defined complex with TS site 1 RNA (Figure 6B), inhibited translation in a non-specific fashion, reducing expression of reporter both in the presence and absence of the TS site 1 motif as well as affecting the IRES-driven process. In contrast, the leucine peptide 4 showed selective stronger (60%) inhibition of cap-initiated translation under the control of the TS site 1 motif. Reporter expression from the IRES element was not diminished in the presence of 4, demonstrating that the leucine peptide does not promiscuously affect the translational machinery.
Comparison of binding behavior (Figure 6) and inhibition potential (Figure 7) of hexapapetides (3) – (6) suggests that target-selective ligands might be developed by optimization of the lead peptide 4. Sequence variation of residues separating the arginine dyads and modification of the number as well as nature of basic amino acids will be used in future studies to explore peptide inhibitors derived from 4.
Conclusions
We have demonstrated that the TS site 1 hairpin constitutes an autonomous regulatory RNA motif that can be transplanted to reporter systems for mechanistic studies and the development of screening assays. The secondary structure of the wild type motif by itself provides only a marginally stable roadblock to ribosomal initiation, whereas binding of the TS protein reduces translation initiation by sequestration of the start codon. Concluding from mutational studies, we determined that stabilization of the RNA secondary structure by the binding of a small molecule ligand may be a viable approach to suppress translation.
Discovery of a peptide (4) that forms a defined complex with the TS site 1 RNA and inhibits translation under the control of this regulatory motif provides validation of the TS mRNA as a target for translation inhibitors that stabilize a hairpin motif in which the initiation codon is sequestered. The finding that among ligands that bind the TS site 1 (peptides 4 and 5) selective inhibition of translation under control of the site 1 RNA motif can be achieved in a sequence specific fashion (peptide 4) suggests that both target affinity and selectivity might be improved in future studies by modification of the lead inhibitor 4.
Reduction of TS expression levels might be beneficial to counter overexpression of the enzyme during cancer therapy with TS inhibitor drugs which ablate binding of the protein to its mRNA. A combination therapy approach of a TS enzyme inhibitor and a site 1 RNA-targeting ligand might overcome emergence of tumor resistance.
Experimental Section
TS protein expression and purification
A DNA construct coding for a codon optimized C-terminal hexahistidine tagged human TS (huTS) was purchased from GenScript (Piscataway, NJ) and inserted into the pET22b+ plasmid (Invitrogen, Carlsbad, CA) which was transformed into Neb5α competent cells. Hexahistidine tagged huTS protein was expressed and purified as described previously[12] with the following modifications. The enzyme was expressed in T7 Express E.coli (Neb) and purified on a Ni2+ affinity column. An additinal wash step utilizing 10 column volumes of 1% 3-[(3-cholamidopropyl)-dimethylammonio] 1-propane sulfonate (CHAPS) buffer was performed prior to elution to remove any bacterial TS from the solution. The huTS was eluted on a linear gradient of 40mM-100mM imidazole. Fractions were pooled, concentrated, and dialyzed into 20mM TRIS pH 8, 1mM DTT.
Construction of the bicistronic reporter constructs
Cloning of bicistronic reporter constructs (Figure 2A) was performed as outlined in the Supporting Information.
In vitro translation assay and luciferase detection
The in vitro transcription-translation assay (IVT) was conducted using the TNT Quick coupled reticulocyte lysate system (Promega, Madison, WI). The TNT Quick system was chosen since it works efficiently with templates that lack an optimal Kozak sequence such as the TS1 construct which had to contain the native TS site 1 sequence upstream of the initiation codon. IVT reactions were run in 7.5μl volume according to manufacturer recommendations. Briefly, each reaction (7.5μl) contained 100ng/ul plasmid DNA (1.5μl), H2O or compound solution (1.5μl), and reaction buffer containing reticulocyte lysate (5μl), SP6 polymerase, and RNase inhibitor. Incubation was at 30° C.
Detection of firefly and Renilla luciferase levels was done using the Dual-Glo Luciferase Assay System (Promega) according to manufactures recommendations. Briefly, 5μl of the reaction mixture was mixed with Dual-Glo luciferase substrate (25μl) on a 96 well plate. After incubation for 60 seconds at RT the firefly luminescence was measured on a SpectraMax Gemini XS by Molecular Devices (Sunnyvale, CA). Then, Dual-Glo Stop & Glo substrate (25μl) was added to each well, incubated for 60 seconds, and the luminescence of the Renilla luciferase was quantified.
IVT TS enzyme titration
Previously frozen TS enzyme was diluted in buffer to a 5-fold concentrated sample (i.e. 5, 25, and 125μM), and 1.5μl was added to the transcription-translation reaction in order to achieve a final concentration of 1, 5, or 25μM. No other conditions were modified.
IVT Compound screening
Aqueous compound stock (1.5ul) was added to the assay mixture to a final concentration of 10μM or 100μM, respectively. Controls were H2O or 1% DMSO.
TS immunodepletion
To deplete the TNT extract of cellular TS, mouse antibody (MAb-TS(106) at 200μg/ml; Thermo Fisher, Waltham, MA) was mixed with the extract at a 1/250 dilution. This mixture was gently agitated at 4° C for 45 minutes, at which point Red agarose conjugated goat-anti-mouse antibody was mixed 1/250 with the antibody/reticulocyte lysate mixture and allowed to gently agitate for 30 minutes at 4°C. The agarose conjugated complex was removed from the mixture by centrifugation at 1000× g for 1 minute.
TS enzyme assay
The TS enzyme activity assay was conducted as described in the literature [13]. Briefly, the enzymatic conversion of 5,10-methylenetetrahydrofolate (5,10-mTHF) to dihydrofolate results in an increased absorbance at 340nm. The reaction conditions were 50mM TRIS pH 7.0, 125mM KCl, 5μM dUMP, and 5μM 5,10-mTHF. TS protein was added to a final concentration of 5μM. Enzymatic reactions were carried out at 37° C for 15 minutes and quenched by the addition of 0.36N HCl. Absorbance was measured on a Shimadzu UV-2401PC spectrophotometer.
Compound synthesis
Compound (1), methyl 3-amino-2-(((benzyloxy)carbonyl)(methyl)amino) propanoate, was synthesized in five steps from benzylooxycarbonyl (Cbz)-protected L-asparagine following published procedures.[14] Spectroscopic data: 1H NMR (400 MHz, DMSO-d6) δ 8.30 (bs, 2H), 7.35–7.29 (m, 5H), 5.10–5.03 (m, 2H) 4.73 (t, 1H, J=6.89Hz), 3.63 (s, 3H) 3.32–3.26 (m, 2H), 2.91 (s, 3H); 13C NMR (400 MHz, DMSO-d6) δ 169.74, 156.68, 137.17, 129.09, 128.41, 128.10, 67.55, 59.22, 53.15, 38.11, 34.85; MS (ESI positive ion mode) exact mass calculated for C13H18N2O4: 266.13, found (M+H)+: 267.09.
Compound (2), 2-amino-5-(benzyl(methyl) amino)pyrimidine-4,6(1H,5H)-dione, was obtained from condensation of 2-(benzylmethyl-amino)malonate and guanidine following established procedures.[15] Spectroscopic data: 1H NMR (400 MHz, CD3OD) δ 7.57–7.35 (m, 5H), 4.65–4.57 (d, 1H, J=11.73Hz), 4.20–4.12 (m, 1H), 3.96–3.79 (m, 1H), 2.92 (s, 3H); 13C NMR (400 MHz, CD3OD) δ 167.02, 157.35, 151.66, 131.22, 130.18, 129.78, 129.23, 129.06, 60.06, 54.77, 52.36, 42.84; HRMS (HR-ESI TOFMS) exact mass calculated for C12H14N4O2: 246.1117, found (M+H)+: 247.1190, Delta (ppm) −0.19.
Peptide RNA binding gel shift assay
RNA representing the TS site 1 sequence (nt C80 – G112, Figure 1) was obtained as HPLC-purified oligonucleotide from IDT (Coralville, IA). Peptides were purchased from Genscript and dissolved in water to a final concentration of 5mM. Individual reaction mixtures consisted of 2x reaction buffer (5μl; 12% glycerol, 25mM TRIS pH 7.0, 5mM DTT, 2mM MgCl2), 50μM of RNA, an appropriate aliquot of the desired peptide, and brought to a final volume of 10μl with dH2O. Mixtures were allowed to incubate at room temperature for 5 minutes, then placed on ice for another 5 minutes.
Native polyacrylamide gels (6%) were prepared using 40% acrylamide bis-acrylamide solution (1.5ml), (19:1), 10x MOPS buffer (1μl; 200mM MOPS pH 7.0, 80mM sodium acetate) diluted with dH2O (7.3ml), and polymerized by addition TEMED (20μl) and 10% ammonium persulfate (0.2ml). The gel was pre-run in 1x MOPS running buffer (20mM MOPS pH 7.0, 8mM sodium acetate) at 4°C for 30 minutes at 100V, then loaded with the reaction mixtures and allowed to run for another 40 minutes. Visualization of RNA was done using SYBR Gold Nucleic Acid Gel Stain (Invitrogen) according to manufacturer protocol.
Supplementary Material
Acknowledgments
We thank Jaime McLean for help with construct cloning as well as Maria Alvarado and Andrea Potocny for help with compound synthesis. This work was supported by the National Institutes of Health (grant No. CA132753). Support of the NMR facility by the National Science Foundation is acknowledged (CRIF grant CHE-0741968).
References
- 1.Carreras CW, Santi DV. Ann Rev of Biochem. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 2.Chu E, Callender MA, Farrell MP, Schmitz JC. Cancer Chemother Pharmacol. 2003;52(Suppl 1):S80–89. doi: 10.1007/s00280-003-0625-9. [DOI] [PubMed] [Google Scholar]
- 3.a) Heidelberger C. Cancer Treatment Reports. 1981;65(Suppl 3):3–9. [PubMed] [Google Scholar]; b) Davies JM, Goldberg RM. Seminars in Oncology. 2011;38:552–560. doi: 10.1053/j.seminoncol.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Scartozzi M, Maccaroni E, Giampieri R, Pistelli M, Bittoni A, Del Prete M, Berardi R, Cascinu S. Pharmacogenomics. 2011;12:251–265. doi: 10.2217/pgs.10.167. [DOI] [PubMed] [Google Scholar]
- 5.a) Chu E, Voeller D, Koeller DM, Drake JC, Takimoto CH, Maley GF, Maley F, Allegra CJ. Proc Nat Acad Sci USA. 1993;90:517–521. doi: 10.1073/pnas.90.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lin X, Parsels LA, Voeller DM, Allegra CJ, Maley GF, Maley F, Chu E. Nucleic Acids Res. 2000;28:1381–1389. doi: 10.1093/nar/28.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai N, Schmitz JC, Liu J, Lin X, Bailly M, Chen TM, Chu E. Front Biosci. 2004;9:2521–2526. doi: 10.2741/1413. [DOI] [PubMed] [Google Scholar]
- 7.Chu E, Koeller DM, Casey JL, Drake JC, Chabner BA, Elwood PC, Zinn S, Allegra CJ. Proc Nat Acad Sci USA. 1991;88:8977–8981. doi: 10.1073/pnas.88.20.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Tok JB, Cho J, Rando RR. Biochemistry. 1999;38:199–206. doi: 10.1021/bi9819428. [DOI] [PubMed] [Google Scholar]; b) Cho J, Rando RR. Nucleic Acids Res. 2000;28:2158–2163. doi: 10.1093/nar/28.10.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellen CU, Pestova TV. J Viral Hepatitis. 1999;6:79–87. doi: 10.1046/j.1365-2893.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 10.Tan R, Frankel AD. Proc Nat Acad Sci USA. 1995;92:5282–5286. doi: 10.1073/pnas.92.12.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan J, Steadman DJ, Koli S, Ding WC, Minor W, Dunlap RB, Berger SH, Lebioda L. J Biol Chem. 2001;276:14170–14177. doi: 10.1074/jbc.M009493200. [DOI] [PubMed] [Google Scholar]
- 12.Steadman DJ, Zhao PS, Spencer HT, Dunlap RB, Berger SH. Biochemistry. 1998;37:7089–7095. doi: 10.1021/bi9725428. [DOI] [PubMed] [Google Scholar]
- 13.Wahba AJ, Friedkin M. J Biol Chem. 1961;236:PC11–12. [PubMed] [Google Scholar]
- 14.a) Aurelio L, Box JS, Brownlee RT, Hughes AB, Sleebs MM. J Org Chem. 2003;68:2652–2667. doi: 10.1021/jo026722l. [DOI] [PubMed] [Google Scholar]; b) Sokolov VV, Kozhushkov SI, Nikolskaya S, Belov VN, Es-Sayed M, De Meijere A. Eur J Org Chem. 1998:777–783. [Google Scholar]
- 15.a) Borowitz IJ, Bloom SM, Rothschild J, Sprinson DB. Biochemistry. 1965;4:650–655. doi: 10.1021/bi00880a006. [DOI] [PubMed] [Google Scholar]; b) Legraverend M, Boumchita H, Bisgani E. Synthesis - Stuttgart. 1990:597–589. doi: 10.1021/jm00171a022. [DOI] [PubMed] [Google Scholar]
- 16.Takeishi K, Kaneda S, Ayusawa D, Shimizu K, Gotoh O, Seno T. Nucleic Acids Res. 1985;13:2035–2043. doi: 10.1093/nar/13.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.