Abstract
At least two lineages of Mesozoic birds are known to have possessed a distinct feather morphotype for which there is no neornithine (modern) equivalent. The early stepwise evolution of apparently modern feathers occurred within Maniraptora, basal to the avian transition, with asymmetrical pennaceous feathers suited for flight present in the most basal recognized avian, Archaeopteryx lithographica. The number of extinct primitive feather morphotypes recognized among non-avian dinosaurs continues to increase with new discoveries; some of these resemble feathers present in basal birds. As a result, feathers between phylogenetically widely separated taxa have been described as homologous. Here we examine the extinct feather morphotypes recognized within Aves and compare these structures with those found in non-avian dinosaurs. We conclude that the “rachis dominated” tail feathers of Confuciusornis sanctus and some enantiornithines are not equivalent to the “proximally ribbon-like” pennaceous feathers of the juvenile oviraptorosaur Similicaudipteryx yixianensis. Close morphological analysis of these unusual rectrices in basal birds supports the interpretation that they are modified pennaceous feathers. Because this feather morphotype is not seen in living birds, we build on current understanding of modern feather molecular morphogenesis to suggest a hypothetical molecular developmental model for the formation of the rachis dominated feathers of extinct basal birds.
Keywords: dinosaur integument, feathers, Mesozoic birds, Similicaudipteryx, rectrix, molecular development, Confuciusornis
1. Introduction
Scientific understanding of the evolution of modern feathers has increased exponentially over the past three decades thanks as much to the numerous feathered Jurassic and Cretaceous dinosaurs uncovered from northeastern China [1–7] as to the results of laboratory experiments on the molecular morphogenesis of feathers in living birds [8–15]. It can now be hypothesized that the integumentary structures preserved in some non-avian dinosaurs represent primitive stages in the step-wise evolution of modern feathers and confirm independently generated experimental data that suggests feathers first evolved from simple filamentous tubular structures (barbs) that later developed a rachis (through barb fusion), followed by barbule development and finally symmetrical and asymmetrical vanes [9,10,13–15]. Although some recent studies disagree with this view, alternative hypotheses are so far unsupported [16].
Simple integumentary structures, perhaps marking early stages in this developmental sequence, are known to have an increasingly wide distribution within Dinosauria (Figure 1) [5,7,17,18], although the known complexity of feather-like integument structures is highest within Maniraptora (Theropoda: Coelurosauria), the derived group of theropod dinosaurs inferred to include Aves [19]. Indeed, several taxa have been found with complex integumentary patterns that include multiple feather morphologies spatially arranged over their bodies including pennaceous feathers (e.g., Caudipteryx zoui, Protoarchaeopteryx robusta). Pennaceous feathers have even been found on the hindlimbs of some taxa (e.g., Microraptor gui, Pedopenna daohugouensis, Anchiornis huxleyi, Xiaotingia zhengi) [20–23]. Asymmetrical pennaceous feathers, however, are only known so far in the dromaeosaurid Microraptor gui, which possesses long remiges on its forelimbs as well as shorter asymmetrical pennaceous feathers on its hindlimbs [19,22].
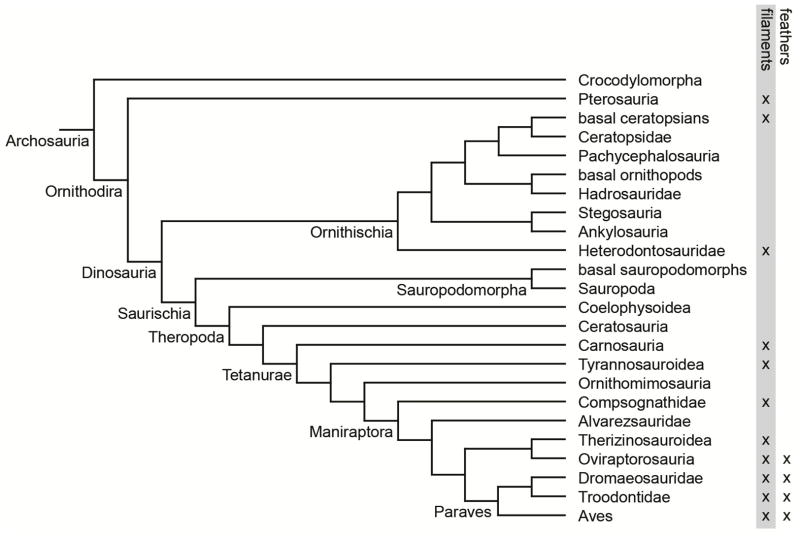
Figure 1.
A simplified tree of archosaurian relationships [28–31] showing the known distribution of “proto-feathers” and feathers within Archosauria. Feathers (as opposed to filaments) are defined by the presence of a rachis.
Although some non-avian maniraptoran theropods clearly did possess feathers of essentially modern morphology, it appears most known taxa also retain apparently primitive morphotypes, such as large single filaments [24], multiple filaments joined basally [20,25,26], distally branched filaments [18,26], and pennaceous feathers with proximally undifferentiated vanes [4], variably distributed throughout the clade [4,19]; integumentary structures lacking a rachis are here referred to informally as “proto-feathers.” As new discoveries have come to light, the degree of morphological variation that characterizes early feather evolution continues to increase [3,4]. The recent discovery of two specimens comprising two different developmental stages of a single taxon reveals that, just like modern birds, some non-avian dinosaurs apparently experienced considerable ontogenetic variation in their plumage [4]: adults were marked by different feather types than juveniles of the same species. However, limited preservation and overlap of fossil feathers prevents unequivocal interpretation of integumentary structures in most specimens; as a result, many recently described extinct feather morphotypes are very poorly understood and interpretations regarding their morphology and function vary [3,4,6,27].
The preservation of advanced feather structures in the earliest diverging birds and some non-avian theropods suggests that basal avians would have had comparable if not more derived integument with respect to their dinosaurian predecessors. Archaeopteryx lithographica was first identified as a bird mainly on the basis of its large, asymmetric and pennaceous remiges; the morphology and distribution of the wing feathers in this taxon and other primitive birds (e.g., Confuciusornis sanctus) is considered essentially modern [32–36]. However, some recent studies suggest that although superficially modern in appearance, the remiges of basal birds may have been structurally weaker, raising questions about the flight capabilities of these taxa [37,38]. Furthermore, the identification of at least two feather types not present in modern birds [38], and the wide and variable distribution of different feather morphologies among non-avian maniraptorans [4], suggests that the evolution of modern avian integument is more complicated than suggested by just Archaeopteryx lithographica and warrants further investigation.
The first unique feather type recognized within Mesozoic birds was the elongate and paired “streamer” rectrices (tail feathers) preserved in some specimens of Confuciusornis sanctus [32,39]. Similar paired feathers were later reported in some specimens of enantiornithines [40,41] although at first interpretation of these structures was controversial [34]. While some workers regarded these paired rectrices as a primitive stage in the evolution of feathers from elongated scales [40], others inferred them to be more advanced structures based on inferences that the rachis itself is a derived feather feature [9,42]. Most recent interpretations have concurred with the latter hypothesis: these paired rectrices are modified pennaceous feathers [27,35,42] not present in living birds—they represent an extinct morphotype.
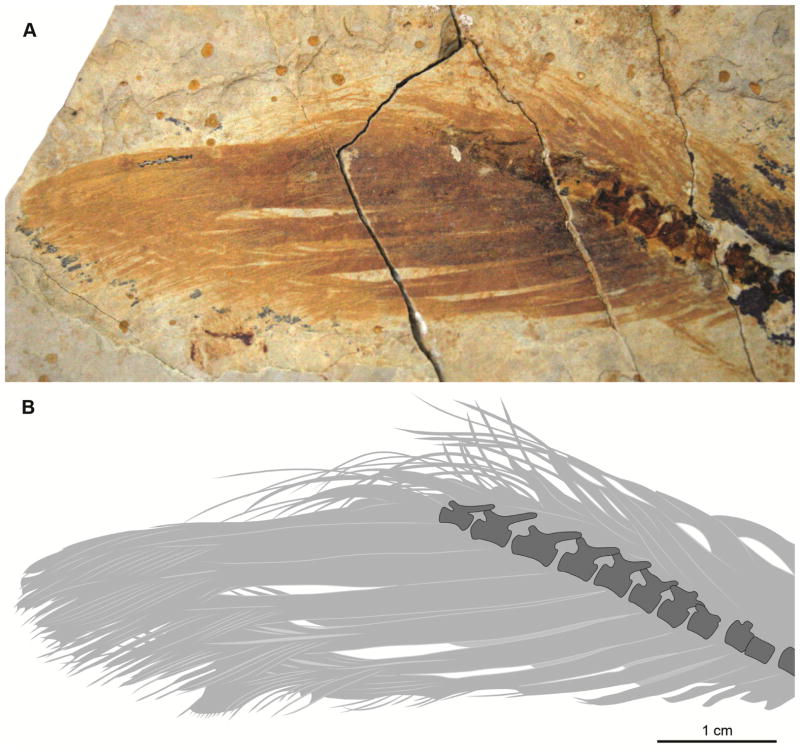
Recently, a similar feather type was described in a non-avian maniraptoran, the basal oviraptorosaur Similicaudipteryx yixianensis [43]. The youngest of the known specimens of this species (STM4-1) preserves proximally ribbon-like pennaceous feathers (PRPFs) [4]. The ribbon-like portion of the feather is interpreted as an undifferentiated vane, while distally the feather is reported to have normal pennaceous morphology (Figure 2) [4], with vanes separated by a rachis. These feathers are replaced in the adult Similicaudipteryx yixianensis (STM22-6) with longer, normal pennaceous feathers [4] in which the rachis and vanes extend throughout the length of the feather. This feather morphology was described as the same morphotype seen preserved in the tail of the basal scansoriopterygid Epidexipteryx hui [6], another non-avian maniraptoran, and some basal avians (e.g., some confuciusornithiforms and enantiornithines), suggesting that these rectrices are retained in birds from a much earlier stage in feather evolution [4]. Alternatively, the PRPFs in juvenile Similicaudipteryx STM4-1 have been interpreted as pin-feathers, with the protective keratinous sheath still attached [27,44].
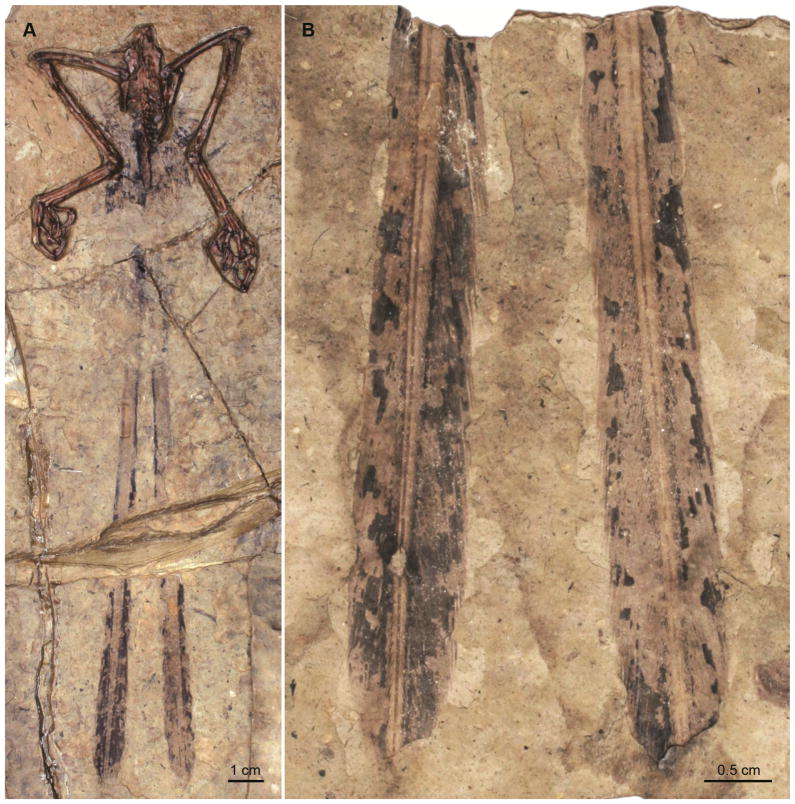
Figure 2.
Proximally ribbon-like pennaceous feathers (PRPFs) in the juvenile specimen of Similicaudipteryx yixianensis STM4-1: (A) close up photograph; (B) interpretive drawing.
Here we describe the morphology and variation of known extinct feather morphotypes in non-avian dinosaurs and basal birds. A close comparison of these feathers together with information from new enantiornithine specimens support previous interpretations that these rectrices, in basal birds, represent modified pennaceous feathers, and are in fact distinct from the feather morphotype preserved in juvenile Similicaudipteryx yixianensis (STM4-1). We also apply current knowledge on the cellular and molecular process of feather development to discuss the key morphogenetic process that may have produced this extinct morphology.
2. Morphological Description
2.1. Non-avian Maniraptoran Theropods
2.1.1. Similicaudipteryx yixianensis
A recently described juvenile specimen of the oviraptorosaur Similicaudipteryx yixianensis (STM4-1) preserves remiges and rectrices described as proximally ribbon-like and distally pennaceous [4]. The proximal two thirds of each feather is composed of unbranched plane (i.e., a plane in which rachis cannot be differentiated); the distal third is pennaceous, with a narrow, centered rachis (Figure 2). The referred adult specimen of this species (STM22-6) bears longer, normal pennaceous feathers on the forelimb and tail [4].
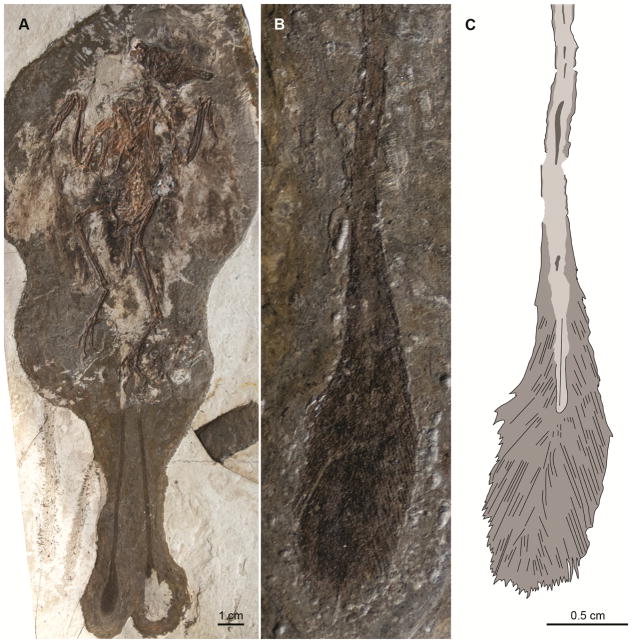
2.1.2. Epidexipteryx hui
This scansoriopterygid preserves four incomplete rectrices [6]; these tail feathers are very long and superficially resemble those of the enantiornithine bird Protopteryx fengningensis, which are also distally incomplete. Although the feathers of Epidexipteryx hui are only preserved as impressions, each bears a centered longitudinal striation that has been interpreted as the rachis; the region on either side is interpreted as undifferentiated vane lacking barb individualization (Figure 3) [6,35].
Figure 3.
(A) Holotype of Epidexipteryx hui IVPP V15471; (B) close up of the tail feathers.
2.2. Basal Birds
2.2.1. Confuciusornithiformes
Approximately twenty percent of all the known specimens of Confuciusornis sanctus preserve a pair of elongate rectrices [45]; paired feathers are also preserved in the holotype specimens of Changchengornis hengdaoziensis [46] and Eoconfuciusornis zhengi [47], although in both the distal ends are missing. Even though preservation produces a false diversity of morphologies, we interpret confuciusornithiforms as possessing a single morphology. The feathers in these birds are also very long, exceeding skeletal body length in specimens where complete (i.e., GMV 2150, DNHM D2454) [32]. The proximal 3/4 of the feather is narrow and lacks any clear pennaceous vaning (Figure 4B,C). The narrow, elongate portion of the feather in confuciusornithiforms also bears a longitudinal dark medial stripe that extends nearly its entire length. The lateral margins are dark in color (thus together with the medial stripe delimiting two longitudinal lighter stripes); over the distal quarter the dark lateral margin expands laterally and differentiates into distinct pennaceous barbs organized in symmetrical vanes (Figure 4C). The pennaceous portion typically has a rounded distal margin; the rachis tapers distally, extending to the distal margin of the feather (Figure 4B,C). In agreement with some previous studies, we interpret the proximal three-quarters of the feather as primarily rachis [34,40], with minimal to absent undifferentiated vane laterally bordering it. An alternative interpretation is that the dark medial stripe of the feather is the rachis and the lighter regions on either side are unbranched vanes [47]. However, our preferred interpretation (the former) is supported by observations of well-preserved specimens in which the dark margin expands distally and differentiates into vane (Figure 4C). In addition, the lighter portion of the feather continues into the pennaceous portion supporting an interpretation of this as the rachis; the presence of barbs on the lateral margins (albeit undifferentiated proximally) limits the calamus to only the proximal most portion of the feather. The medial stripe, much fainter than preserved barbs, is interpreted as a preservational artifact resultant from the ventral groove present on the calamus and rachis in living birds [48]. This interpretation is further supported by recent studies on fossil feather microstructure; what typically preserves are the carbonized remains of the melanosomes in the barbs responsible for giving the feather dark color [49,50]. Therefore, the rachis (which does not possess melanosomes) typically does not preserve [50]. In the elongate tail feathers of Confuciusornis, the rachis can only be inferred, delimited laterally by the undifferentiated barbs proximally, and by the vaned portion of the feather distally.
Figure 4.
Detail photographs of elongate tail feathers in Confuciusornis sanctus:(A) holotype of C. feducciai DNHM D2454 (this specimen is considered a junior synonym of C. sanctus [45,51]); (B) close up of rectrices in DNHM D2454; (C) close up of rectrix in DNHM D2859.
2.2.2. Enantiornithes
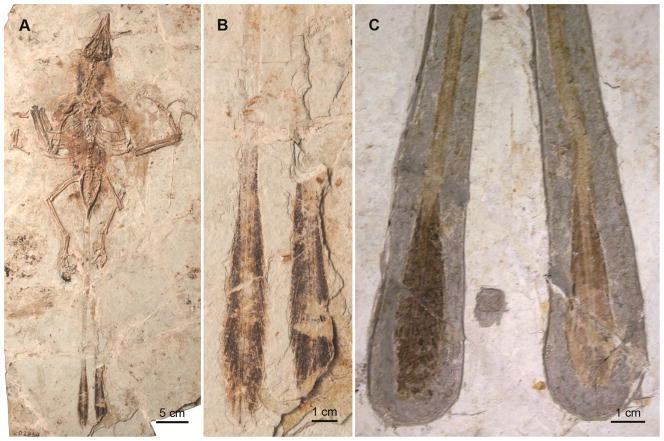
The presence of a pair of elongate rectrices is the second most common tail morphology (the first being an absence of elongate rectrices all together; Figure 5) encountered within Enantiornithes (e.g., Dapingfangornis sentisorhinus, Protopteryx fengningensis, DNHM D2884, GSGM-07-CM-001). Reflecting the diversity of this clade, the elongate rectrices seen in enantiornithines comprise a range of shapes and relative lengths (Figures 6 and 7). The first taxon described with elongate tail feathers was Protopteryx fengningensis [40]. The paired feathers in the holotype specimen of this bird (IVPP V11665) are incomplete; only the proximal ends of the two straight rectrices are preserved, extending nearly parallel to each other (slightly splayed) from the distal end of the pygostyle (for a length of 82 mm). The preserved portion of the feather was interpreted to be a wide and flattened rachis [40].
Figure 5.
(A) Photograph of the holotype of Protopteryx fengningensis IVPP V11665 with elongate tail feathers; (B) photograph of the holotype of Eoenantiornis buhleri IVPP 11537 with no elongate tail feathers; (C) close up of two isolated contour feathers in IVPP V11665.
Figure 6.
Elongate tail feathers in enantiornithine Dapingfangornis sentisorhinus LPM 000039: (A) full feathers; (B) distal left rectrix; (C) interpretative drawing showing transition from unbranched to branched vanes.
Figure 7.
Enantiornithes indet. GSGM-07-CM-001: (A) full slab; (B) detail photograph of paired elongate tail feathers.
Additional complete discoveries (e.g., Dapingfangornis sentisorhinus, Paraprotopteryx gracilis, DNHM D2884, GSGM-07-CM-001) have confirmed that the paired rectrices in enantiornithines have essentially the same morphology as those of Confuciusornis sanctus. As in the latter, the rectrices in these specimens are narrow and rachis dominated for most of their length; the thick rachis stays approximately the same width for the entire length of the feather, tapering slightly at the distal end (Figure 4). Visible in Dapingfangornis sentisorhinus and GSGM-07-CM-001, a narrow dark region borders the lateral margins of the wide rachis for most of its length (Figures 6 and 7). As in Confuciusornis sanctus, we interpret this as representing undifferentiated vane; in a contiguous lateral section visible in the unnamed enantiornithine GSGM-07-CM-001, individual barbs cannot be identified proximally although distally, spaces clearly separate each barb in support of this interpretation (Figure 7). Although the rectrices of GSGM-07-CM-001 are not preserved well enough for a definitive statement, the pennaceous portion of them may have been close vaned proximal on the barb and open vaned distally. If correct, this implies the presence of branched barbules (i.e., differentiated into hooklets and cilia) that have spatially arranged themselves only on the proximal half of the barb, a specialization seen in some modern bird feathers [48].
As in Confuciusornis sanctus, the vane of enantiornithines increases in width laterally and form barbs over the distal portion of the feather so that the feather is distally expanded and vaned, showing herringbone structure in some specimens (e.g., Dapingfangornis sentisorhinus, GSGM-07-CM-001). Among enantiornithines, the point at which the vane region expands (increases in width) and forms barbs occurs at different points along the rachis (along distal 15% of feather in Dapingfangornis sentisorhinus, 50% in GSGM-07-CM-001) and to varying degrees (abrupt in Dapingfangornis sentisorhinus, gradual in GSGM-07-CM-001), revealing intraclade diversity for this morphotype. Feathers also show some variation in their overall lengths with respect to body-size (e.g., femur: rectrix ratios for Dapingfangornis sentisorhinus: 0.145, Paraprotopteryx gracilis: 0.17)—however, the extent to which variation is affected by molting is unknown. The enantiornithine, Paraprotopteryx gracilis preserves four rectrices of this morphotype [52], suggesting further diversity. However, the different color of the laterally preserved two feathers (compared to the medial pair) may indicate that these structures have been artificially added to the slab.
2.3. Comparison
Currently, there are a number of different interpretations for the morphology of the elongate rectrices in basal birds, although most concur that proximally these have an undifferentiated vane, which becomes pennaceous distally [4,35]. Differences in interpretation are largely split over where to define the rachis and where to define the vane; confusing the two in basal birds may have led to the claim that this feather type was similar to that of the juvenile oviraptorosaur Similicaudipteryx yixianensis (STM4-1) [4]. The feathers in both groups are reported to be pennaceous only distally, a morphology unknown for living birds [32]; however, between groups they differ in all other respects suggesting that these are in fact two different feather morphotypes (contra Xu and colleagues [4]). The proximal “ribbon-like” portion of the feather in birds and Similicaudipteryx yixianensis is quite different; in the latter it lacks all structure (including an identifiable rachis). Furthermore, the pennaceous distal end of Similicaudipteryx yixianensis forms an abrupt, wedge-like contact into the ribbon-like proximal end (Figure 2B); in basal birds, however, the proximally ribbon-like portion of the feather is a large rachis that continues almost to the end of the feather, distally bounded laterally by the vane, as in modern pennaceous rectrices (i.e., opposite to that observed in Similicaudipteryx yixianensis, the rachis of the distally pennaceous portion is continuous through the unbranched vane portion of the feather in Mesozoic birds) (Figures 2B and 6C).
Comparison with the incomplete feathers of Epidexipteryx hui, however, remains equivocal; although clearly different from the feathers in Similicaudipteryx STM4-1, because these feathers do not possess their distal ends or any evidence of structures that can be interpreted as vanes (differentiated or otherwise) on their lateral margins, we cannot determine if these tail feathers are truly comparable to those preserved in some basal birds. However, the preserved portion of the feathers in Epidexipteryx hui is more reminiscent of basal birds than Similicaudipteryx yixianensis; these feathers also appear to have the same medial stripe on the rachis, a feature that is absent in the juvenile Similicaudipteryx yixianensis.
3. Results and Discussion
3.1. Rachis Dominated Feathers
The rachis-dominated feathers of some basal birds have been suggested to represent (I) an intermediate morphology providing evidence that pennaceous feathers evolved from elongated scales [40,52], (II) a type of modified pennaceous feather [42], or (III) a completely new type of primitive feather [4]. The presence of modern flight feathers in the non-avian dinosaur Microraptor gui and the basal most bird Archaeopteryx lithographica, and the absence of a specimen with elongate scale-like integument largely covering the body makes the first hypothesis weak given that these supposedly intermediate structures are absent in taxa that are more basal than those that possess them. Instead, proto-feathers in the fossil record are known to be simple, hollow filament-like structures [53–55]; structures with variable branching morphologies (including pennaceous types) are known throughout Theropoda in varying combinations and distributions over the body [2,25,26,56]. Current knowledge of feather morphogenesis also does not support this hypothesis. In one model, barbs are inferred to have evolved before the rachis, which is formed by barb fusion [42]. Since the rectrices of basal birds appear to be rachis-dominated and pennaceous (indicating barbules were also likely present) and it is unparsimonious to assume that all these features evolved convergently, they are more likely to represent modified pennaceous feathers (hypothesis II) than primitive holdovers from a very early stage in feather evolution (hypothesis I) [42]. Although, these long rectricial feathers superficially resemble the filoplumes of modern birds in that they have a broad rachis relative to the “vaned” portion of the feather (simply branched in filoplumes), modern filoplumes are typically shorter than the coverts they are associated with and have a very slender, hair-like rachis [48]. Filoplumes are highly specialized structures morphologically distinct from these feathers and cannot be considered homologous. Indeed, the paired racket-tail feathers seen in motmots (Motmotidae) also superficially resemble the feathers of Confuciusornis sanctus; the bare rachis in these feathers, however, results from natural weakness of the associated barbs and wear and tear over time [57]. Neither filoplumes nor the tail feathers in motmots possess any proximally undifferentiated vane along the rachis. Some birds of paradise possess tail feathers of undifferentiated vane; these specialized display features differ from that of Mesozoic birds in that the entire feather is undifferentiated forming a “plastic strip-like” morphology [58].
3.2. Potential Cellular and Molecular Basis for Evolution Novelty in Feather Morphogenesis
No living bird possesses a feather that is only pennaceous distally [32] and as a result therachis-dominated feathers of basal birds can be considered an extinct morphotype. Further, the isolated nature of this feather morphology on the skeleton (rather than distributed over the entire body) suggests these are derived features [34,42]. Recent experimental work has expanded our understanding of feather morphogenesis [9,15,42], allowing us to infer the molecular pathways that may have produced the bizarre rectricial morphology preserved in these fossil birds.
Here we will discuss the cellular and molecular mechanisms during the formation of diverse feather branches in four aspects:
(1). Opening up epithelial cylinder via localized apoptosis
In this process, the apoptosis (i.e., programmed cell death) of the feather sheath and pulp epithelium allows the feather cylinder to open and to become a two-dimensional epithelial plane. The apoptosis of marginal and barbule plate epithelia leads to the formation of space between barbs and barbules, respectively [11]. In extant birds, these two processes usually happen together [11]. In the Mesozoic birds, these processes may be uncoupled, i.e., apoptosis occurs in feather sheath and pulp epithelium without barb ridge formation, and thus form the potential undivided sheet like structure observed in the proximal region of the rectrices in some enantiornithines and confuciusornithiforms (Figure 8A).
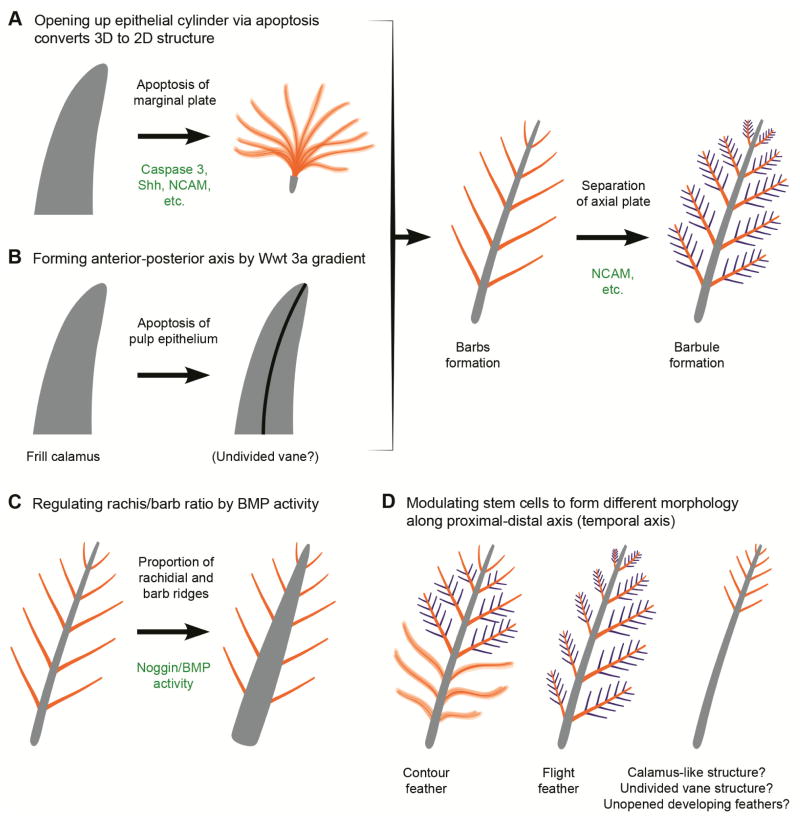
Figure 8.
Illustration depicting important stages in the formation of the modern pennaceous feather: (A) opening up of the epithelial cylinder via localized apoptosis produces branches; (B) anterior-posterior axis forms through a Wnt3a gradient—combination of (A) and (B) converts a three-dimensional appendage into a two-dimensional, planar structure;(C) regulating rachis/barb ratio through BMP activity; (D) modulation of stem cells to form different morphology along the distal-proximal-distal axis, which is made temporally from the distal to the proximal end.
(2). Forming anterior (where rachis is)—posterior axis by Wnt3a gradient
In modern feathers, stem cells are horizontally placed in the base of the downy feather follicle but tilted toward the anterior within the follicle of flight feathers [15]. Further, the protein Wnt3a has an anterior-posterior gradient in flight, but not in downy feathers. Global inhibition of the Wnt3a gradient transforms bilaterally symmetric feathers into radially symmetric feathers [15]. Experimental studies have shown that production of an ectopic local Wnt3a gradient results in re-orientation of the barb ridges toward the source and the origination of an ectopic rachis. Thus, these studies have shown that superimposing a molecular gradient on the periodic pattern of barb-ridge formation can break the symmetry and make each module (in this case, each barb ridge) unequal (Figure 8B) [15].
Combination of the last two processes (shown in Figures 8A,B) will make the radially arranged barbs in downy feathers become bilateral symmetrically inserted into a rachis, thus converting a three-dimensional appendage into a two-dimensional plane.
(3). Regulating rachis/barb ratio by BMP activity
Once the periodic barb ridging process is triggered, cells in the epithelial cylinder will have to become either rachis, barbs, or the space (following apoptosis) between barb branches [11]. We know that feather morphogenesis is the result of activator-inhibitor interactions between the bone morphogenetic proteins BMP2 and BMP4, noggin, sonic hedgehog (Shh), and other molecules, which result in a hierarchical pattern of branching in the epithelial cells leading to the diversity of feather morphologies seen within living birds [9–14,34,59].
The following scenario has been proposed for the morphogenesis of the avian feather: (1) BMP4 exceeds the level of noggin, a broad segment of the feather cylinder becomes rachis; (2) epithelial cells form more barb ridge branches where noggin and other BMP antagonist expression exceeds BMP4 in the ramogenic zone; (3) cells differentiate into a periodic arrangement of alternating Shh and BMP2 positive and Shh negative zones, the former representing where marginal plate cells die creating barb ridge spaces and the latter representing the zone of growth of the barb ridge; (4) barb ridge cells express BMP2 and BMP4, become orderly arranged, and differentiate into barbules; and (5) noggin levels return to low levels, as in stage (1) and the calamus forms (Figure 9) [9]. Experiments show that modifying the expression of these molecules can produce primitive or novel morphologies; for example, changes in BMP activities can result in numerous barb ridges or an enlarged shaft, as seen in the elongate rectrices of enantiornithines and confuciusornithiforms (Figure 8C) [9]. This possibility is even more versatile when we consider the temporal changes (see next point).
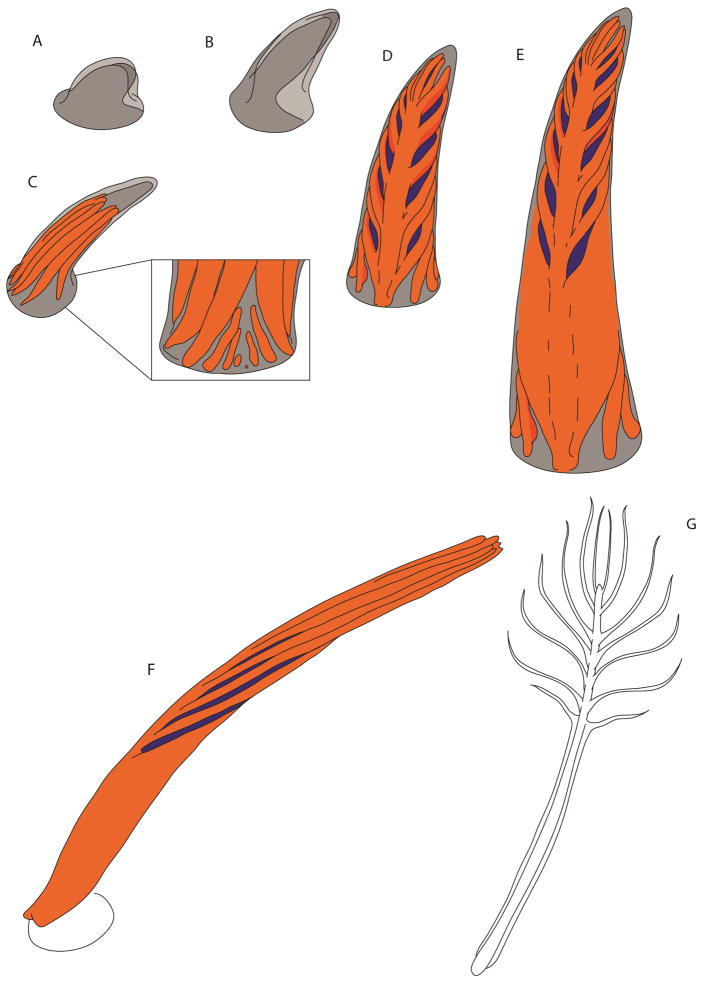
Figure 9.
Illustration of the formation of the rachis-dominated modified pennaceous feather of basal birds. (A,B) formation and initial growth of the feather bud; (C) barb ridge formation; (D) barbs fuse to form a rachis; (E) as feather continues to form, apoptosis ceases, vane becomes undifferentiated; (F,G) illustrate the conversion of a three-dimensional structure into the two-dimensional feather.
(4). Modulating stem cells to form different morphologies along the proximal-distal axis
Stem cells were found to be located in the proximal follicle. They proliferate to generate new cells, which are pushed upward, and differentiate as they move toward the distal end [15]. Thus, the distal end represents more differentiated structures that were formed earlier. Since the micro-environment for stem cells can change at different times, it is possible to generate different structures during the developmental period of the feather. This provides greater possibility to generate new feather morphologies through changes in the signaling environment at different times (Figure 8D).
With the above understanding, it is possible to envisage that the unique morphology of arachis-dominated rectrix could thus be produced by gradual reduction in noggin as the feather grows resulting in decreasing length and in the absence of differentiation between barbs proximally, similar to the early onset of the last stage of feather morphogenesis (Figure 9). A suppression of apoptosis in Shh positive marginal plates during the later part of stage (3) would then result in a sheet-like, undifferentiated keratinous vane (as suggested by Xu and colleagues [4]). BMP2 over-expression has been shown to result in feather phenotypes with enlarged rachises and barb fusion [9], both features present in enantiornithine and confuciusornithiform tail feathers. This may also suggest that the modulation of BMP2 and noggin have played an important role in the evolution of this unique feather morphology [34]. Within this feather type, the enantiornithines Dapingfangornis sentisorhinus and GSGM-07-CM-001, although similar, possess morphological differences suggesting diversity within the phenotype and the modification of a similar molecular pathway. We suggest that these main differences in appearance can be produced by an earlier and more abrupt return to stage (1) during the morphogenesis of the tail feather of Dapingfangornis sentisorhinus.
3.3. Function
Protopteryx fengningensis and most other enantiornithines are thought to have been arboreal. This has led to the interpretation that their elongate tail feathers may have helped them balance in the densely wooded environment in which they lived, similar to the way in which a squirrel uses its tail [34]. Interpretations of the similar feathers present in confuciusornithiforms have instead been dominated by suggestions that they were used for sexual display—their variable presence in the known specimens suggestive of sexual dimorphism [39,51,60,61]. However, recent morphometric studies have not been able to support or reject this hypothesis [45,51] and the function of these feathers remains controversial [62]. The variation in length and morphology observed among the tail feathers of enantiornithines may suggest they are also related to display, species recognition [63], or other forms of visual communication [64]. The tail feathers in another group of basal birds, the Jeholornithiformes, also show a morphology which suggests that visual communication may have been the primary function [65]; the wide range of non-aerodynamic tail morphologies among basal birds suggests that this may have been a trend during the early evolution of Aves.
3.4. Proximally Ribbon-like Pennaceous Feathers
The unusual feathers preserved in the juvenile specimen of Similicaudipteryx yixianensis STM4-1 are more difficult to interpret because of the large amount of overlap between the numerous remiges and rectrices of this specimen (Figure 2). While throughout this paper we have followed morphological interpretations by Xu et al. [4] of the feather structures in STM4-1, alternative interpretations of these feathers exist, primarily that the proximally sheet-like portion of the feather represents the keratin sheath that protects the feather as it grows (pin-feathers), consistent with the interpretation of the specimen as a juvenile [27,44]. This has been refuted based on the considerable length of the feathers in Similicaudipteryx STM4-1 (pin-feathers are short) [66]. We agree that the feathers preserved in STM4-1 are not pin-feathers and offer an alternative interpretation of these structures based on our observations. The proximally ribbon-like portion of the feather is preserved in the same manner as the distally “pennaceous” portion of the feather, suggesting both parts are the same material, and that the proximal structure cannot be a keratinous sheath or enlarged rachis [49,50]. No clear rachis can be identified in the proximally ribbon-like portion of the feather. In fact, from the preservation, it cannot be determined if these feathers were truly distally pennaceous or not (Figure 2). The feathers resemble the strange body feathers of Epidexipteryx hui, described as parallel barbs originating from a single point [6,19], which appear to be undifferentiated proximally; this is also consistent with the uniform preservation of the feather proximodistally in STM4-1. These feathers also superficially resemble the body contour feathers of basal birds, which unlike in modern birds, are not pennaceous [35].
In enantiornithines and confuciusornithiforms, poorly defined feathers are often preserved associated with most of the skeleton, primarily the neck and caudal region (e.g., preserved in the holotype specimens of the enantiornithines Longirostravis hani, Eoenantiornis buhleri, Protopteryx fengningensis, Dapingfangornis sentisorhinus, Shanweiniao cooperorum, Longipteryx chaoyangenesis and DNHM D2884 1/2) (Figures 5–7). These are notoriously difficult to interpret [27]. The feathers appear uniform with only some variation in length in different regions of the body (e.g., Longipteryx chaoyangensis; based on differences in length and morphology, feathers on the tibiotarsus appear to represent a distinct tract, representing crural feathers rather than generalized body coverts). The body feathers in Confuciusornis sanctus and enantiornithines such as Longipteryx chaoyangensis and Protopteryx fengningensis are non-shafted [35]; isolated body feathers preserved in Protopteryx fengningensis IVPP V11665 show the absence of a distinct medially placed rachis or centralized shaft (Figure 5C) [35]. These feathers resemble modern downy feathers, and functionally may have served as such, but differ from in that the barbs do not radiate from a short rachis but rather the feather consists of proximally undifferentiated vane that frays into individual barbs distally, a morphology unknown among living birds (Figure 5C). In modern birds, coverts are found on the unspecialized regions of the body surface [48]. Body coverts are modified pennaceous feathers; their barbs do not form interlocking vanes but possess a stiffened base that maintains their alignment [48]. No herringbone structure is apparent among the coverts of basal birds, as one would expect if they were pennaceous. “Non-shafted” feathers, forming an organic halo of filaments radiating out from the body contour are commonly preserved among Mesozoic birds as well as on parts of some non-avian theropods (e.g., Sinosauropteryx prima, Sinornithosaurus millenii) [35].
The morphology reported in a juvenile Similicaudipteryx yixianensis is similar to the unshafted coverts of basal birds and some non-avian dinosaurs, proximally bearing no structure (e.g., barbs, rachis), except with the distal portion reported to be pennaceous; however given the preservation and overlap of these feathers, clear evidence for pennaceous vane is here considered absent. What has been interpreted as herringbone structure may in fact be two feathers preserved with the open vaned distal ends oriented in opposing directions (Figure 2). We suggest the remiges and rectrices of juvenile Similicaudipteryx yixianensis were a generalized form of covert, similar to feathers found across the body, that were replaced with pennaceous feathers later in ontogeny. Reinterpretation of the feathers as simple structures similar to those that cover the bodies of basal birds and some non-avian theropods (e.g., Sinornithosaurus millenii) that are then replaced with pennaceous feathers better fits the inferred scenario of ontogenetic maturation of the plumage in Similicaudipteryx yixianensis.
4. Conclusions
By the Late Jurassic, dinosaurs including birds, were diverse and specialized in their integument. This diversity increased in the Early Cretaceous, with the dominant avian clade, the enantiornithines, possessing a huge diversity of feather patterns and morphologies. Although remiges of modern aspect evolved early, basal birds possess contour feathers different from those of modern birds (and like those of some closely related non-avian dinosaurs), and a unique rectrix feather type unknown in living taxa. This supports hypotheses regarding the acquisition of feather types within Aves that suggest the covert feathers in modern birds are a derived form of the pennaceous feather [67]. The morphology of the unique rectrix of some basal birds suggests that it is a modified pennaceous feather, and could have been developed by modification of the timing (e.g., shortening or lengthening) of specific morphogenetic stages that are today expressed in the development of modern feathers. The known diversity of feather types during the Early Cretaceous, although including morphologies since gone extinct, is still dwarfed by the diversity in morphotypes observed among extant birds.
Acknowledgments
We thank Z-H. Zhou (IVPP), F-C. Zhang (IVPP), C. Gao (DNHM) and G. Sun (LPM) for specimen access, S. Abramowicz (Dinosaur Institute, Natural History of Los Angeles County) for assisting with the figures and photographs, and G. Dyke and the reviewers for the comments on earlier versions of the manuscript. We acknowledge the following funding sources for making this research possible:C. Chuong is supported by NIH grant AR 47364; J. O’Connor is supported by the Chinese National Natural Science Foundation Grant KA 210417 and the Fellowship for Young International Scientists of the Chinese Academy of Sciences grant KC 210201.
Institutional Abbreviations
- DNHM
Dalian Natural History Museum, Dalian, China
- GMV
National Geological Museum of China, Beijing, China
- GSGM
Gansu Geological Museum, Lanzhou, China
- IVPP
Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China
- LPM
Liaoning Paleontological Museum, Shenyang, Liaoning, China
- STM
Shandong Tianyu Museum of Natural History, Linyi, China
Footnotes
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Contributor Information
Luis M. Chiappe, Email: chiappe@nhm.org.
Cheng-ming Chuong, Email: cmchuong@usc.edu.
David J. Bottjer, Email: dbottjer@usc.edu.
Hailu You, Email: youhailu@ivpp.ac.cn.
References
- 1.Ji Q, Ji SA. On discovery of the earliest bird fossil in China and the origin of birds. Chin Geol. 1996;233:30–33. [Google Scholar]
- 2.Ji Q, Currie PJ, Norell MA, Ji SA. Two feathered dinosaurs from northeastern China. Nature. 1998;393:753–761. [Google Scholar]
- 3.Xu X, Zhao Q, Norell MA, Sullivan C, Hone DW, Erickson GM, Wang XL, Han FL, Guo Y. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin Sci Bull. 2009;54:430–435. [Google Scholar]
- 4.Xu X, Zheng XT, You HL. Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature. 2010;464:1339–1341. doi: 10.1038/nature08965. [DOI] [PubMed] [Google Scholar]
- 5.Zheng XT, You HL, Xu X, Dong ZM. An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature. 2009;458:333–337. doi: 10.1038/nature07856. [DOI] [PubMed] [Google Scholar]
- 6.Zhang FC, Zhou ZH, Xu X, Wang XL, Sullivan C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature. 2008;455:1105–1108. doi: 10.1038/nature07447. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Wang KB, Zhang K, Ma QY, Xing LD, Sullivan C, Hu DY, Cheng SQ, Wang S. A gigantic feathered dinosaur from the Lower Cretaceous of China. Nature. 2012;484:92–95. doi: 10.1038/nature10906. [DOI] [PubMed] [Google Scholar]
- 8.Chuong CM, Widelitz RB. Feather morphogenesis: A model of the formation of epithelial appendage. In: Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience; Austin, TX, USA: 1998. pp. 57–74. [Google Scholar]
- 9.Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool B Mol Dev Evol. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- 11.Chang CH, Yu MK, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. Sculpting skin appendages out of epidermal layers via temporally and spatially regulated apoptotic events. J Invest Dermatol. 2004;122:1348–1355. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prum RO. Evolution of the morphological innovations of feathers. J Exp Zool B Mol Dev Evol. 2005;304:570–579. doi: 10.1002/jez.b.21073. [DOI] [PubMed] [Google Scholar]
- 13.Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Curr Opin Cell Biol. 2006;18:730–741. doi: 10.1016/j.ceb.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alibardi L. Cell structure of barb ridges in down feathers and juvenile wing feathers of the developing chick embryo: Barb ridge modification in relation to feather evolution. Ann Anat. 2006;188:303–318. doi: 10.1016/j.aanat.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci USA. 2006;103:951–955. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maderson PF, Hillenius WJ, Hiller U, Dove CC. Towards a comprehensive model of feather regeneration. J Morphol. 2009;270:1166–1208. doi: 10.1002/jmor.10747. [DOI] [PubMed] [Google Scholar]
- 17.Mayr G, Peters DS, Plowdowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturwissenschaften. 2002;89:361–365. doi: 10.1007/s00114-002-0339-6. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Norell MA, Kuang X, Wang X, Zhao Q, Jia C. Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature. 2004;431:680–684. doi: 10.1038/nature02855. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Guo Y. The origin and early evolution of feathers: Insights from recent paleontological and neontological data. Vertebr Palasiat. 2009;47:311–329. [Google Scholar]
- 20.Hu DY, Hou LH, Zhang LJ, You HL. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature. 2009;461:640–643. doi: 10.1038/nature08322. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Zhang F. A new maniraptoran dinosaur from China with long feathers on the metatarsus. Naturwissenschaften. 2005;92:173–177. doi: 10.1007/s00114-004-0604-y. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Zhou Z, Wang X, Kuang X, Du X. Four-winged dinosaurs from China. Nature. 2003;421:335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, You HL, Kai D, Han FL. An Archaeopteryx-like theropod from China and the origin of Avialae. Nature. 2011;475:465–475. doi: 10.1038/nature10288. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Wang XL, Wu XC. A dromaeosaurid dinosaur with a filamentous integument from the Yixian Formation of China. Nature. 1999;401:262–266. [Google Scholar]
- 25.Ji Q, Norell MA, Gao KQ, Ji SA, Ren D. The distribution of integumentary structures in a feathered dinosaur. Nature. 2001;410:1084–1088. doi: 10.1038/35074079. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Zhou Z, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–204. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- 27.Foth C. On the identification of feather structures in stem-line representatives of birds: Evidence from fossils and actuopalaeontology. Palaeontol Z. 2012;86:91–102. [Google Scholar]
- 28.Butler RJ, Upchurch P, Norman DB. The phylogeny of the ornithischian dinosaurs. J Syst Palaeontol. 2008;6:1–40. [Google Scholar]
- 29.Brusatte SL, Nesbitt SJ, Irmis RB, Butler RJ, Benton MJ, Norell MA. The origin and early radiation of dinosaurs. Earth Sci Rev. 2010;101:68–100. [Google Scholar]
- 30.Makovicky PJ, Zanno LE. Theropod diversity and the refinement of avian characteristics. In: Dyke GD, Kaiser G, editors. Living Dinosaurs: The Evolutionary History of Modern Birds. John Wiley & Sons; Oxford, UK: 2011. pp. 9–29. [Google Scholar]
- 31.Rauhut OWM, Foth C, Tischlinger H, Norell MA. Exceptionally preserved juvenile megalosauroid theropod dinosaur with filamentous integument from the Late Jurassic of Germany. Proc Natl Acad Sci USA. 2012;109:11746–11751. doi: 10.1073/pnas.1203238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiappe LM, Ji S, Ji Q, Norell MA. Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of northeastern China. Bull Am Mus Nat Hist. 1999;242:1–89. [Google Scholar]
- 33.Elzanowski A. Biology of basal birds and the origin of avian flight. In: Zhou Z, Zhang F, editors. Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution; Beijing, China. 1–4 June 2000; Beijing, China: Science Press; 2002. pp. 211–226. [Google Scholar]
- 34.Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L. Adaptation to the sky: Defining the feather with integument fossils from Mesozoic China and experimental evidence from molecular laboratories. J Exp Zool B Mol Dev Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F, Zhou Z, Dyke GJ. Feathers and “feather-like” integumentary structures in Liaoning birds and dinosaurs. Geol J. 2006;41:395–404. [Google Scholar]
- 36.Wellnhofer P. Archaeopteryx Der Urvogel von Solnhofen. Friedrich Pfeil; München, Germany: 2008. [Google Scholar]
- 37.Nudds RL, Dyke GD. Narrow primary feather rachises in Confuciusornis and Archaeopteryx suggest poor flight ability. Science. 2010;328:887–889. doi: 10.1126/science.1188895. [DOI] [PubMed] [Google Scholar]
- 38.Zheng XT, Xu X, Zhou ZH, Miao D, Zhang FC. Comment on “Narrow primary feather rachises in Confuciusornis and Archaeopteryx suggest poor flight ability. Science. 2010;330:320. doi: 10.1126/science.1193223. [DOI] [PubMed] [Google Scholar]
- 39.Hou L, Martin LD, Zhou Z, Feduccia A. Early adaptive radiation of birds: Evidence from fossils from northeastern China. Science. 1996;274:1164–1167. doi: 10.1126/science.274.5290.1164. [DOI] [PubMed] [Google Scholar]
- 40.Zhang F, Zhou Z. A primitive enantiornithine bird and the origin of feathers. Science. 2000;290:1955–1960. doi: 10.1126/science.290.5498.1955. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Duan Y, Hu D, Wang L, Cheng S, Hou L. New eoentantiornithid bird from the Early Cretaceous Jiufotang Formation of western Liaoning, China. Acta Geol Sin Engl Ed. 2006;80:38–41. [Google Scholar]
- 42.Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q Rev Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- 43.He T, Wang XL, Zhou ZH. A new genus and species of caudipterid dinosaur from the Lower Cretaceous Jiufotang Formation of western Liaoning, China. Vertebr Palasiat. 2008;46:178–189. [Google Scholar]
- 44.Prum RO. Moulting tail feathers in a juvenile oviraptorisaur. Nature. 2010;468:E1. doi: 10.1038/nature09480. [DOI] [PubMed] [Google Scholar]
- 45.Chiappe LM, Marugán-Lobón J, Ji S, Zhou Z. Life history of a basal bird: Morphometrics of the Early Cretaceous Confuciusornis. Biol Lett. 2008;4:719–723. doi: 10.1098/rsbl.2008.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Q, Chiappe LM, Ji S. A new late Mesozoic confuciusornithid bird from China. J Vertebr Paleontol. 1999;19:1–7. [Google Scholar]
- 47.Zhang F, Zhou Z, Benton MJ. A primitive confuciusornithid bird from China and its implications for early avian flight. Sci China Ser D Earth Sci. 2008;51:625–639. [Google Scholar]
- 48.Stettenheim PR. The integumentary morphology of modern birds—An overview. Am Zool. 2000;40:461–477. [Google Scholar]
- 49.Vinther J, Briggs DEG, Prum RO, Saranathan V. The colour of fossil feather. Biol Lett. 2008:522–525. doi: 10.1098/rsbl.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang FC, Kearns SL, Orr PJ, Benton MJ, Zhou ZH, Johnson D, Xu X, Wang XL. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature. 2010;463:1075–1078. doi: 10.1038/nature08740. [DOI] [PubMed] [Google Scholar]
- 51.Marugán-Lobón J, Chiappe LM, Ji SA, Zhou ZH, Gao CH, Hu DY, Meng QJ. Quantitative patterns of morphological variation in the appendicular skeleton of the Early Cretaceous bird Confuciusornis. J Syst Palaeontol. 2011;9:91–101. [Google Scholar]
- 52.Zheng X, Zhang Z, Hou L. A new enantiornitine bird with four long rectrices from the Early Cretaceous of northern Hebei, China. Acta Geol Sin Engl Ed. 2007;81:703–708. [Google Scholar]
- 53.Chen PJ, Dong Z, Zhen S. An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature. 1998;391:147–152. [Google Scholar]
- 54.Xu X, Tang ZL, Wang XL. A therizinosauroid dinosaur with integumentary structures from China. Nature. 1999;399:350–354. [Google Scholar]
- 55.Xu X, Zhou Z, Wang X. The smallest known non-avian theropod dinosaur. Nature. 2000;408:705–708. doi: 10.1038/35047056. [DOI] [PubMed] [Google Scholar]
- 56.Norell M, Ji Q, Gao K, Yuan C, Zhao Y, Wang L. “Modern” feathers on a non-avian dinosaur. Nature. 2002;416:36–37. doi: 10.1038/416036a. [DOI] [PubMed] [Google Scholar]
- 57.Snow DW. Family Momotidae (motmots) In: Hoyo J, Elliott A, Sargatal J, editors. Handook of the Birds of the World. Vol. 6 Lynx Edicions; Barcelona, Spain: 2001. [Google Scholar]
- 58.Gill FB. Ornithology. 3. W.H. Freeman and Company; New York, NY, USA: 2007. [Google Scholar]
- 59.Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proc Natl Acad Sci USA. 2005;102:11734–11739. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feduccia A. The Origin and Evolution of Birds. Yale University Press; New Haven, CT,USA: 1996. [Google Scholar]
- 61.Peters WS, Peters DS. Life history, sexual dimorphism and “ornamental” feathers in the Mesozoic bird Confuciusornis sanctus. Biol Lett. 2009;5:817–820. doi: 10.1098/rsbl.2009.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peters WS, Peters DS. Sexual size dimorphism is the most consistent explanation for the body size spectrum of Confuciusornis sanctus. Biol Lett. 2010;6:531–532. [Google Scholar]
- 63.Padian K, Horner JR. The evolution of “bizarre structures” in dinosaurs: Biomechanics, sexual selection or species recognition? J Zool. 2011;238:3–17. [Google Scholar]
- 64.Li DS, Sullivan C, Zhou ZH, Zhang FC. Basal birds from China: A brief review. Chin Birds. 2010;1:83–96. [Google Scholar]
- 65.O’Connor JK, Sun CK, Xu X, Wang XL, Zhou ZH. A new species of Jeholornis with complete caudal integument. Hist Biol. 2012;24:29–41. [Google Scholar]
- 66.Xu X, Zheng XT, You HL. Reply: Moulting tail feathers in a juvenile oviraptorosaur. Nature. 2010;468:E2. doi: 10.1038/nature09480. [DOI] [PubMed] [Google Scholar]
- 67.Brush AH. Evolving a protofeather and feather diversity. Am Zool. 2000;40:631–639. [Google Scholar]