Abstract
Background
The development of depressive symptomatology is a recognised complication of treatment with the cytokine, interferon-α, and has been seen as supporting inflammatory theories of the pathophysiology of major depression. Major depression has been associated with changes in glutamatergic activity and recent formulations of interferon-induced depression have implicated neurotoxic influences which could also lead to changes in glutamate function. The present study used magnetic resonance spectroscopy (MRS) to measure both glutamate and its major metabolite, glutamine in patients with hepatitis C who received treatment with pegylated-interferon-α and ribavirin.
Methods
MRS measurements of glutamate and glutamine were taken from a 25×20×20mm voxel including pregenual anterior cingulate cortex in 12 patients before and after 4-6 weeks treatment with interferon.
Results
Interferon treatment led to an increase in cortical levels of glutamine (p= 0.02) and a significant elevation in the ratio of glutamine to glutamate (p<.01). Further, changes in glutamine level correlated significantly with ratings of depression and anxiety at the time of the second scan.
Conclusions
We conclude that treatment with interferon-α is associated with MRS-visible changes in glutamatergic metabolism. However, the changes seen differ from those reported in major depression which suggests that the pathophysiology of interferon-induced depression may be distinct from that of major depression more generally.
Introduction
Administration of interferon-α (IFN-α) for the treatment of hepatitis C is associated with a significant incidence of depressive symptomatology, with up to 40% of patients developing major depression over three months of IFN-α therapy (Hauser et al. 2002, Raison et al. 2005, Leutscher et al. 2010). This observation has been taken as evidence of the possible role of inflammatory mechanisms in the pathophysiology of major depression more generally (Raison et al. 2006), and is consistent with reports of elevated plasma levels of pro-inflammatory mediators such as tumor necrosis factor (TNF) and interleukin-6 (IL-6) in depressed patients (Dowlati et al. 2010).
The mechanism by which IFN-α might produce depression has been explored in several previous studies. One potential mechanism involves induction of indoleamine 2,3 dioxygenase (IDO) with consequent alterations in the metabolism of the serotonin precursor, tryptophan. While central tryptophan depletion was originally suggested as a cause of IFN-α-induced depression (Bonaccorso et al. 2000, Capuron et al. 2002), more recent formulations have implicated increased production of tryptophan metabolites such as quinolinic and kynurenic acids which have the potential to alter central glutamatergic neurotransmission (Wichers and Maes 2004, Miller et al. 2009, Raison et al. 2010). It has also been suggested that the response to inflammatory mediators such as IFN-α might include impairment of the ability of astrocytes to take up synaptic glutamate, thus predisposing to glutamate toxicity (Tavares et al. 2002, McNally et al. 2008). This is of interest in view of the postulated role of glutamate abnormalities (Sanacora et al. 2008) and glial cell deficits (Rajkowska et al. 2007) in major depression.
Magnetic Resonance Spectroscopy (MRS) provides a safe non-invasive method for measurement of cortical glutamate. A growing number of studies demonstrate MRS abnormalities in glutamine and glutamate in brain regions such as anterior cingulate cortex in mood disorders (Yüksel et al. 2010). Typically, reliable differentiation of glutamate from glutamine with MRS is highly challenging so the combined total glutamate with glutamine, often termed Glx, is reported. Recent technical advances allow for more confident estimation of glutamate and glutamine individually (Mekle et al. 2009). This is an important development, since while Glx reflects a total glutamatergic pool, the ratio of glutamine to glutamate is believed to more closely reflect glutamatergic function (Théberge et al. 2002, Yüksel et al. 2010).
The aim of the present study was to use proton MRS to assess the effect of IFN-α on cerebral glutamate and glutamine levels in patients receiving IFN-α treatment for the management of hepatitis C. We also investigated whether changes in these brain neurochemicals correlated with emergent depressive symptomatology.
Methods
Participants
We recruited 17 participants, aged between 18 and 65, who had chronic hepatitis C viral infection without evidence of cirrhosis, and were scheduled to receive treatment with pegylated-interferon-α and ribavirin (IFN) as part of their routine clinical care. Participants were recruited from NHS hepatology clinics at two centres (Oxford and Bristol, UK). They were scanned at baseline, prior to starting treatment, and again after 4-6 weeks of treatment with IFN. Those with current DSM-IV axis I disorders by Structured Clinical Interview (First et al. 2002), taking a non-stable dose of psychotropic medication, or with any contraindication to MR scanning were excluded. Clinical or biochemical evidence of cirrhosis was an exclusion criterion and all participants had had liver biopsy or fibroscan. Past psychiatric history such as history of opiate dependence was not an exclusion criterion. Concomitant medication was continued at the same dose throughout the study. The majority of participants (16 of 17) were taking no psychotropic medication, the remaining participant was taking a low dose antidepressant, but does not contribute to the glutamine analyses presented below. Patients were included taking vitamin supplements, erythromycin, thyroxine, the oral contraceptive pill and regular inhalers. The study was approved by the Oxfordshire Research Ethics Committee A and all participants gave written informed consent. Participants were reimbursed for their time and any other expenses.
Imaging was performed on a 3 Tesla Siemens TIM Trio scanner with a body coil transmitter and a 12-channel head receiver array. MRS measurements were obtained from a 25×20×20mm voxel of medial prefrontal cortex including pregenual anterior cingulate cortex (Figure 1B) using SPECIAL (Mekle et al. 2009) with TR/TE: 3000/8.5ms. Water suppressed (192 averages) and water unsuppressed data (8 averages) were obtained. The voxel was positioned manually by reference to an axial T1-weighted gradient-echo image. Prior to spectral analysis, motion corrupted averages were discarded, and the effects of scanner drift were removed by performing a frequency and phase alignment of the averages. Spectra were analysed with LCModel version 6.2 (Provencher 1993) using a set of simulated metabolite basis spectra. Eddy current correction was employed. Metabolite estimates with Cramér-Rao Lower Bounds (CRLB) >20% were excluded. One baseline glutamine estimate was excluded for this reason. Concentrations were expressed relative to creatine (Cr), and ratio of glutamate to glutamine also calculated. In a secondary analysis, concentrations relative to tissue water were also calculated. T1-weighted structural images of whole brain were acquired with 1mm3 voxel resolution. FSL FAST was employed to segment the structural brain images into grey matter, white matter, and CSF, to allow estimation of voxel composition.
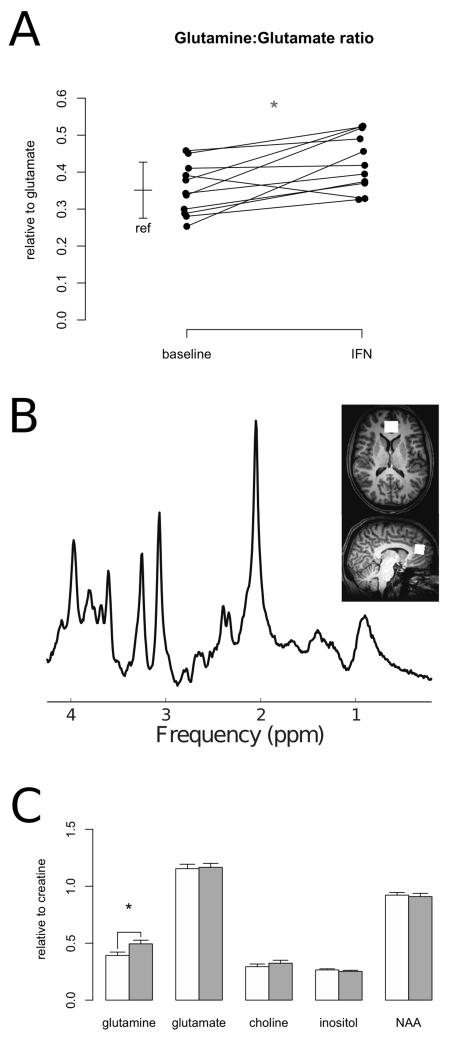
Figure 1.
A: Glutamine:glutamate ratio at baseline and during treatment with interferon-a (IFN). Bar: reference values (mean and standard deviation) for healthy controls with this method.
B: Sample SPECIAL spectrum and voxel position in anterior cingulate cortex.
C: MRS measures at baseline (white) and during interferon treatment (grey). NAA:N-acetyl-aspartate. *: p<.05.
Ratings
Self-report measures of mental state were obtained at baseline and at the time of second scan using the 16 item self-report Quick Inventory of Depressive Symptomatology (QIDS, Rush et al. 2003), Spielberger State Anxiety (STAI, Spielberger et al. 1970) and the Fatigue Severity Scale (FSS, Krupp et al. 1989). FSS ratings were not available for three participants (Table 1).
Table 1.
Participant characteristics. n=12 except where indicated. Means with standard deviations.
| Baseline (n=12) | During interferon treatment (n=12) |
|
|---|---|---|
| Age (yr) | 38.5 ± 10.5 | - |
| Gender | 8 male, 4 female | - |
| QIDS-SR16 | 3.67 ± 3.53 | 7.67 ± 4.66 * |
| State anxiety | 32.2 ± 7.42 | 35.2 ± 9.48 |
| Fatigue Severity Scale | 2.69 ± 1.23 (n=9) | 3.86 ± 1.33 (n=10) |
: p<.05 difference between baseline and treatment
Statistics
Group comparisons were performed using the general linear model in PASW Statistics v18 (SPSS Inc). MRS measures were tested for correlation with age and voxel composition. No significant effects were seen so these were not included as covariates in the model. Correlations were tested using Pearson’s r. All statistical tests were two-tailed with 0.05 significance level.
Results
Imaging data were obtained at baseline and during IFN treatment from 12 patients with chronic hepatitis C. Of the seventeen patients recruited, one did not tolerate baseline scanning, two withdrew before completing the protocol, and for two the MRS acquisition failed for technical reasons. Six of the twelve patients demonstrated a rapid virologic response with undetectable serum viral load at week four of treatment. Treatment was associated with a statistically significant increase in depression symptoms (p=0.01; Table 1) but not in anxiety or fatigue ratings (p>0.15 both cases).
IFN treatment was associated with a significant increase in anterior cingulate cortex glutamine levels (Gln/Cr baseline 0.40 ± .10, IFN 0.50 ± .12, p=0.02; Figure 1C). No effect on glutamate was observed (p>0.7; Figure 1C). A significant increase in glutamine: glutamate ratio was also apparent (baseline 0.35 ± .07, IFN 0.43 ± .08, p<0.01; Figure 1A). Concentrations estimates for both metabolites were obtained with low estimated errors (CRLB glutamate 4.8% ± .7, glutamine 11.3% ± 3.0). The majority (eight) of these participants were taking no concomitant medication, the others were taking vitamin supplements, erythromycin, oral contraceptive and an antihistamine, respectively.
No significant effect of IFN was observed on other MRS measures relative to creatine (choline, NAA, inositol; p>0.2 all cases). No significant correlation was observed between change in viral load and any MRS measure. A secondary analysis of concentrations relative to tissue water showed the same pattern of effect, with a significant effect of IFN on glutamine (p=0.03) but not on other measures (p>0.3 in all cases).
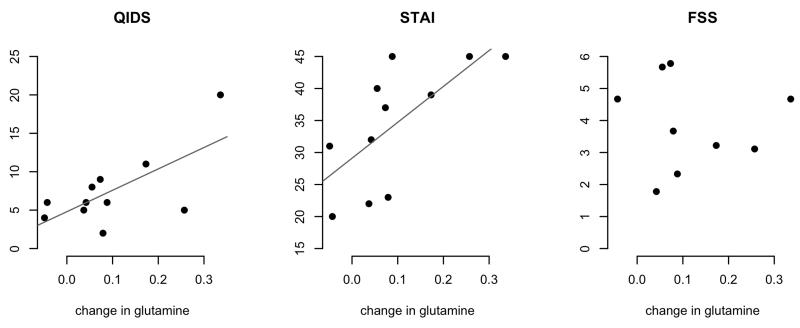
The observed change in cortical glutamine concentration correlated with symptom ratings at the time of the second scan (Figure 2) for depression (QIDS: r .68, p=0.02), and anxiety (STAI: r .69, p=0.02), but not fatigue (FSS: p>0.8). Anxiety scores also correlated with change in glutamine:glutamate ratio (r .68, p=0.02).
Figure 2.
Change in glutamine against mental state during treatment. QIDS: Quick Inventory of Depressive Symptomatology, STAI: Spielberger state anxiety, FSS: Fatigue Severity Scale. Significant correlations with QIDS (r .68, p=.02) and STAI (r .69, p=.02).
Discussion
Our findings suggest that treatment with IFN-α over four to six weeks leads to an increase in glutamine levels in anterior cingulate cortex. However, levels of glutamate were unchanged. Interestingly, this increase in glutamine showed some correlation with measures of depression and anxiety. This effect on cortical glutamine has not to our knowledge previously been described.
The cycling of glutamine and glutamate is a key process in the control of glutamate neurotransmission. Glutamate released from synaptic terminals is taken up into astrocytes where it is converted to glutamine by the enzyme, glutamine synthetase. Glutamine is subsequently released by astrocytes and accumulates in glutamatergic neurones where it is converted back to glutamate by glutaminase. Our data suggest that IFN-α treatment is associated with an increase in glutamine relative to glutamate. This might be consistent with increased turnover of glutamate, because with elevated glutamate levels one would expect more glutamate to be made available for astrocytes for conversion to glutamine (Yüksel et al. 2010). Because glutamate itself can be neurotoxic there are several mechanisms which prevent glutamate levels becoming excessive, particularly uptake into astrocytes (Danbolt 2001). Functional interpretation of changes in spectroscopic measures is not always straightforward, however there is growing evidence that increased glutamine relative to glutamate is consistent with glutamatergic overactivity (Ongur et al, 2008) and thus altered resting state activity (Alcaro et al, 2009). Given the well established role of medial prefrontal cortex including anterior cingulate in emotional processing and mood disorders (Strakowski et al, 2012; Kupfer et al, 2012) such changes are well placed to influence risk of developing depression.
As noted above, accurately differentiating glutamine from glutamate with MRS is often challenging. Methods such as those used here with very short echo times assist in precise glutamate quantitation (Wijtenburg and Knight-Scott 2011). In this study, as in previous reports with this particular technique (Mekle et al. 2009), both glutamine and glutamate were measured with low errors, lending confidence to the finding that glutamine increases relative to glutamate during IFN-α treatment.
There are a number of processes by which IFN-α might increase glutamate release. IFN-α has been shown to elevate CSF levels of quinolinic acid which can increase glutamate release from synaptosomes (Raison et al. 2010). Inflammatory mediators can also lead to decreased uptake of glutamate into astrocytes which could result in increased synaptic glutamate levels (McNally et al. 2008). However, diminished glutamate uptake into astrocytes would not be expected to be lead to increased glutamine as observed here, because of reduced access of glutamate to the astrocytic enzyme, glutamine synthetase.
It is possible to elevate central glutamine concentrations in people with hepatic cirrhosis by inducing hyperammonaemia (Mardini et al. 2011). This effect is associated with compensatory reductions in myo-inositol concentrations (Mardini et al. 2011). A similar pattern can be observed when cirrhosis is accompanied by hepatic encephalopathy (Haussinger et al. 1994, Weissenborn et al. 2007). In this study we excluded patients with evidence of cirrhosis a priori to avoid this potential confound. The absence of any decrease in myo-inositol levels makes it less likely that the effect on glutamine seen here can be explained by alterations in circulating ammonia but it could be of interest in future studies to measure this directly. While participants in this study were recruited from a general clinic population, replication in other samples would increase confidence further that the effect seen is generalisable.
In a previous IFN study, increased CSF levels of quinolinic acid were reported to correlate with scores on the Montgomery-Åsberg Depression Rating Scale during IFN-α treatment (Raison et al. 2010). We also saw a correlation between increases in cortical glutamine and the depression and anxiety ratings. It clearly would be of interest to measure both glutamine and quinolinic acid in patients receiving IFN-α to see whether the concentrations of these two neurochemicals correlate with each other and with depression scores.
It is known that chronic hepatitis C viral infection can affect mental state in the absence of IFN-α treatment, and indeed that it can be associated with MRS abnormalities such as elevated levels of choline (Forton et al. 2001). A recent study found that elevated basal ganglia choline and inositol reduced in people with sustained response to antiviral treatment (Byrnes et al. 2012). This study lacks the statistical power to investigate such subgroup effects robustly. Previous studies have not used MRS techniques optimised to specifically identify alterations in glutamine levels such as those described here. The pre-treatment glutamine:glutamate ratios found here are similar to those in healthy volunteers at our centre with this combination of MRS acquisition and analysis (Figure 1A) suggesting that IFN-α treatment is causing a change rather than normalising a baseline abnormality, but clearly further investigation of baseline effects of viral infection on such measures would be of interest. Potential participants with current axis I disorders were excluded from taking part in this study. If participants with existing depression at baseline had been included, this might have affected the magnitude of neurochemical effects seen and could be investigated in future studies.
There have been several studies using MRS to examine cortical glutamate levels in patients experiencing major depressive episodes. Generally in patients with current unipolar depression, MRS levels of total glutamate and glutamine are reported to be lower than controls while elevated levels are associated with bipolar depression (Yüksel and Öngür 2010). It also appears that unipolar depression tends to be associated with a reduction of the ratio of glutamine relative to glutamate (Yüksel and Öngür 2010). If this is the case, the neurochemical changes produced by IFN-α as measured by MRS appear distinct from those seen in unipolar depression. This raises the possibility that depression associated with inflammation may have a profile of glutamatergic changes more similar to that seen in bipolar depression.
A similarity in emergent neurochemical profile in this model and that seen in bipolar depression may be of particular interest given recent reports of emergent manic or mixed affective episodes during IFN treatment (Constant et al. 2005) and association of the presence of trait markers of vulnerability to bipolar disorder with higher rates of psychiatric complications of IFN treatment (Lim et al. 2010). The changes seen in glutamate activity in major depression and bipolar disorder seem relatively independent of concomitant psychotropic medication. In the present study the effect observed cannot be secondary to psychotropic medication, which would have the greatest potential to influence MRS results, however some participants were taking medication for physical reasons. We tried to deal with this as far as we could by only recruiting patients whose drug treatment was stable. We suspect that it might be impracticable to recruit a group of hepatitis C patients receiving IFN who are otherwise medication-free.
Here we have observed that treatment with IFN-α over four to six weeks leads to an increase in glutamine levels in anterior cingulate cortex. This effect correlates with depressive symptoms but the neurochemical effect seen differs from that observed in unipolar depression. This may have implications for the management and prevention of IFN-induced depression. Our study requires replication in a larger group of IFN-treated participants where it would also be desirable to have a direct comparison group of patients with major depression.
Acknowledgments
We thank the nurses Denise O’Donnell, Jane Phillips and Elizabeth Sims and the patients for their participation in this study. This study was supported by the Medical Research Council, the Academy of Medical Sciences and Wellcome Trust, and the John Fell Oxford University Press (OUP) Research Fund. Dr Taylor was a NIHR Clinical Lecturer through the Oxford University Clinical Academic Graduate School. Additional support was received from the Oxford NIHR Biomedical Research Centre.
Footnotes
Declaration of Interest
Professor Cowen has been a member of advisory boards of Eli Lilly, Servier and Lundbeck and has been a paid lecturer for Eli Lilly, Servier, Lundbeck and GlaxoSmithKline. Dr Taylor has been a paid lecturer for Bristol-Myers Squibb and his spouse is an employee of GlaxoSmithKline. Drs Godlewska, Near, Christmas, Potokar, Collier, Klenerman and Barnes reported no biomedical financial interests or potential conflicts of interest.
References
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neuroscience and Biobehavioral Reviews. 2010;34:592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Meltzer H, Maes M. Psychological and behavioural effects of interferons. Current Opinion in Psychiatry. 2000;13:673–677. [Google Scholar]
- Byrnes V, Miller A, Lowry D, Hill E, Weinstein C, Alsop D, Lenkinski R, Afdhal NH. Effects of anti-viral therapy and HCV clearance on cerebral metabolism and cognition. Journal of Hepatology. 2012;56:549–556. doi: 10.1016/j.jhep.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Constant A, Castera L, Dantzer R, Couzigou P, de Ledinghen V, Demotes-Mainard J, Henry C. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. Journal of Clinical Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–39. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Molecular Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- Häussinger D, Laubenberger J, vom Dahl S, Ernst T, Bayer S, Langer M, Gerok W, Hennig J. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology. 1994;107:1475–1480. doi: 10.1016/0016-5085(94)90552-5. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Leutscher PDC, Lagging M, Buhl MR, Pedersen C, Norkrans G, Langeland N, Morch K, Farkkila M, Hjerrild S, Hellstrand K, Bech P. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C. Hepatology. 2010;52:430–435. doi: 10.1002/hep.23699. [DOI] [PubMed] [Google Scholar]
- Lim C, Olson J, Zaman A, Phelps J, Ingram KD. Prevalence and impact of manic traits in depressed patients initiating interferon therapy for chronic hepatitis C infection. Journal of Clinical Gastroenterology. 2010;44:e141–146. doi: 10.1097/MCG.0b013e3181dc24f8. [DOI] [PubMed] [Google Scholar]
- Mardini H, Smith FE, Record CO, Blamire AM. Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. Journal of Hepatology. 2011;54:1154–1160. doi: 10.1016/j.jhep.2010.09.030. [DOI] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectrums. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magnetic Resonance Medicine. 2009;61:1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biological Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory: Test manual. Consulting Psychologist Press; Palo Alto, CA: 1970. [Google Scholar]
- Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. Journal of Clinical Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Molecular Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurological Disorders Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, DelBello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disorders. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares RG, Tasca CI, Santos CES, Alves LB, Porciuncula LO, Emanuelli T, Souza DO. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochemistry International. 2002;40:621–627. doi: 10.1016/s0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- Théberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, Densmore M, Al-Semaan Y, Williamson PC. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. American Journal of Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biological. Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. Journal of Psychiatry and Neuroscience. 2004;29:11–17. [PMC free article] [PubMed] [Google Scholar]
- Wijtenburg SA, Knight-Scott J. Very short echo time improves the precision of glutamate detection at 3T in (1) H magnetic resonance spectroscopy. Journal of Magnetic Resonance Imaging. 2011;34:645–52. doi: 10.1002/jmri.22638. [DOI] [PubMed] [Google Scholar]