Abstract
Some consider the measurements of arterial elasticity and flow-mediated dilation to be an indirect “biomarker” of endothelial dysfunction. As such, we describe the various uses of these techniques in the evaluation of the natural history of vascular disease. These measures are potential markers of disease, as abnormalities reflect changes in the integrity of vascular structure but occur prior to the manifestation of symptomatic cardiovascular events. In this review, the natural history of arterial elasticity is discussed, and the effects of aging and inflammation are reviewed. The role that arterial elasticity and flow-mediated dilation have in predicting future cardiovascular disease, and the effects of pharmacologic agents on these measures, is also reviewed.
Keywords: Arterial elasticity, Arterial stiffness, Flow-mediated dilation
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity in most industrialized countries, and arterial pathology is the major contributor to CVD. However, the initiation and progression of the pathologic changes in the arterial wall is only partially understood. A distinct characteristic of the alterations in arterial wall integrity is arterial stiffness or its inverse, arterial elasticity. The ability to characterize elastic behavior of arteries has become increasingly important, but the mathematical constructs and terminology are not understood by most clinicians, limiting the applicability of arterial elasticity measures in clinical practice. Terminology such as Peterson’s elastic modulus, Young’s modulus, Moens-Korteweg equation, and the differences between stiffness, elasticity, compliance, distensibility, stress, and strain are not part of the clinician’s usual jargon. A review by Lee and Kamm [1] is helpful in this regard. For the purposes of this article, we use the term “arterial elasticity” to represent the changes in structure and function of the large and small arteries, although the use of the term “arterial stiffness” is used in some descriptions.
There are a number of validated techniques to measure arterial elasticity, and a recent review updated their clinical usefulness [2]. The purpose of this current review is to highlight what is known about techniques that assess the degree of stiffness/elasticity of the arterial system (pulse contour analysis [PCA], pulse wave velocity [PWV], and flow-mediated dilation [FMD]) that are indirect novel biomarkers of CVD. We attempt to explain the mathematical and theoretical aspects of these techniques, but whether or not one understands the theory behind the technique, its clinical application has been proven in many clinical arenas. All of the techniques for measuring arterial elasticity require mathematical modeling. For example, one of the common techniques for assessing arterial elasticity is PWV; a technique based upon the fact that less elastic vessels transmit pulse waves more rapidly, a concept relatively easy to understand. However, the conversion of the speed of transmission of the pulse wave into arterial elasticity terms requires solving the following mathematical formula:
where K is the elastic modulus of luminal volume change per unit length and p is the density of blood.
Following is a discussion of some of the clinical areas where measures of the functional and structural integrity of the arterial vasculature have been evaluated.
Natural History of Arterial Elasticity
Because it is well recognized that the vascular changes resulting in CVD events begin in childhood, there has been increased interest in identifying markers of increased risk in early life. Arnett et al. [3] assessed the relation between blood pressure (BP) and arterial elasticity (utilizing the PCA technique) in a healthy sample of young adults. As part of a longitudinal study of school children (aged 10–14 years at entry in 1977–1978), arterial elasticity was measured in 179 children at entry and again at age 23 years. Systolic BP (SBP) was inversely related to small and large artery elasticity after adjustment for gender, height, weight, insulin, and high-density lipoprotein (HDL) and low-density lipoprotein LDL (cholesterol). Bhuiyan et al. [4] reported on the Bogalusa Heart Study experience. In 800 African-American (AA) and white subjects (ages 18–44 years), AA individuals had lower large and small artery elasticity than white individuals and women [4]. In multiple regression analysis, mean arterial pressure, body mass index (BMI), insulin levels, and age were inversely, and body surface area was positively, correlated with large artery elasticity and accounted for 39.2% of the variance. Mean arterial pressure, female gender, age, and triglyceride levels inversely and cardiac output positively correlated with small artery elasticity and explained 56.4% of the variance. There has also been a recent analysis of the year 15 data of the Coronary Artery Risk Development in Young Adults (CARDIA) study assessing biomarker correlates of arterial elasticity [5]. With adjustment for demographic, behavioral, and physiologic characteristics, mean small artery elasticity was 0.084 mL/mm Hg in those with urine albumin/creatinine ratio ≤4 versus 0.075 mL/mm Hg for those with microalbuminuria (ratio >25; P=0.0003). Mean large artery elasticity was 2.59 mL/mm Hg in the lowest P-selectin quintile versus 2.40 mL/mm Hg in the highest quintile (P=0.0003); soluble intercellular adhesion molecule 1 (sICAM-1) was unrelated to either. Plasma triglycerides were inversely related to large artery elasticity (P=0.02). Cigarette smokers had lower mean small artery elasticity than nonsmokers (P=0.006). Arterial elasticity was not significantly related to alcohol consumption or physical inactivity and was positively related to BMI.
Arterial elasticity has become an increasingly important “risk factor” to predict CVD events [6]. In fact, it could be argued that reduced arterial elasticity is not a risk factor at all but an actual marker of early vascular disease, and that changes in smaller vessel elasticity is a “biomarker” of endothelial dysfunction [7]. There is ample evidence to support the hypothesis that endothelial dysfunction with resultant decrease in arterial elasticity precedes the development of clinical vascular disease including hypertension [6]. The Atherosclerosis Risk in Communities (ARIC) study (using a variety of measures of arterial elasticity) did demonstrate that incident hypertension was predicted in those in the highest tertile of arterial stiffness at baseline [7]. However, ARIC is a population study of men and women aged 45 to 64 years. Whether the ARIC findings would apply to a younger cohort is less well studied. In addition, most published studies have used PWV or augmentation index, whereas ARIC used ultrasound-derived indices, as indirect measures of arterial stiffness. Generalized narrowing in smaller arteries has long been recognized as the major pathophysiologic in essential hypertension. The identification of those at risk to develop hypertension may have increased importance given the results of the TROPHY study that demonstrated that treatment of prehypertension decreased the development of hypertension [8]. Early identification of those at risk can also help to prevent clinical manifestations of CVD. Individuals at risk for coronary heart disease (CHD) events are currently identified by screening for risk factors such as cholesterol, glucose, smoking, obesity, and BP. These aforementioned variables are risk factors or correlates of CHD, but do not indicate that subclinical disease is present. Evidence of early vascular disease, evidenced by reduced arterial elasticity in asymptomatic individuals, should provide additional information about future risk (Fig. 1) [9].
Fig. 1.
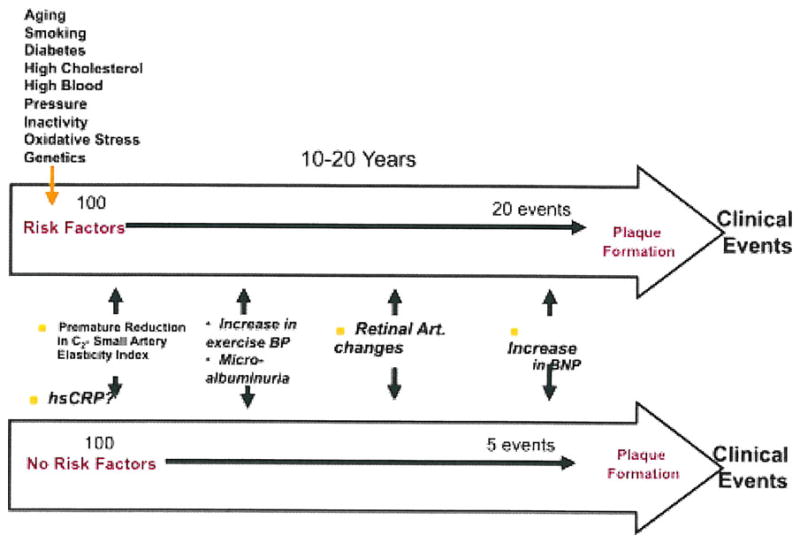
The top arrow is a hypothetical example of a 20% (high Framingham Risk Score) 10–20 year risk of suffering a cardiovascular event, whereas the bottom arrow indicates someone with a low Framingham Risk Score. The risk markers indicate an approximation of where, along the evolution of the natural history of coronary artery disease, the risk markers may become abnormal. Art artery; BNP B-type natriuretic peptide; BP blood pressure; hsCRP high-sensitivity C-reactive protein
The abnormalities of the arterial vasculature that precede CHD events are likely to occur in temporal sequence. The proposed sequence includes an earlier phase in which reduced elasticity, especially in the small arteries, leads to functional changes in those arteries, and a developmental phase in which the arterial system further denigrates arterial structure. The early phase begins in youth, especially in smaller arteries, initially with a defect or injury of arterial endothelial protective function, and progresses with structural remodeling. The late phase is characterized by cellular and lipid accumulation in conduit (larger) arteries followed by calcification and plaque formation, leading ultimately to plaque rupture as the precipitating factor for clot formation and acute morbid and mortal events [10].
Aging and Arterial Elasticity
In recent years, emphasis has been placed on the role of arterial elasticity in the development of CVD. To an increasing extent, various measures of arterial elasticity have begun to be used clinically. However, much remains to be learned about how the different measures change with age, and about the ability of the different measures of elasticity to predict future CVD. Najjar et al. [11••] reviewed extensive evidence that links many biochemical, enzymatic, and cellular alterations, as well as their modulating signals, to accelerated vascular aging and the pathogenesis and progression of arterial diseases such as hypertension and atherosclerosis. The underlying basis for these age-dependent changes is not completely known, but pathologically, fracture and fragmentation of elastin fibers has been observed. Cellular ionic changes (involving calcium and magnesium) have also been reported. It has been shown that aging is associated with an increasing systolic and decreasing diastolic BP, whereas arterial elasticity demonstrates a somewhat linear decrease after the age of 20 years or so, although there is some acceleration of stiffening after the age of 50 years. On average, women have 5% to 10% greater elasticity than men of the same age.
Studies that have shown decreasing arterial elasticity with aging also suggest that the decrease is less for small artery elasticity, and that these changes were independent from BP [12]. The reduced arterial elasticity with aging has been associated with endothelial dysfunction [13, 14]. Weinberger et al. [15] studied 272 participants (50% men, 26% AA, age range of 33–80 years) in a long-term follow up study [15]. They observed an age-related decrease in large and small vessel arterial elasticity with no apparent effect of race or estrogen use. A problem in regard to the issue of decreasing arterial elasticity with aging is that a number of diseases also increase with advancing age, diseases that may alter vascular integrity (eg, atherosclerosis, hypertension). Takeuchi et al. [16] evaluated the association of age (and hypertension) and small vessel elasticity, and the influence of coronary artery disease (CAD) risk factors on that association. They also found an age-related decline in elasticity [16].
Association of Inflammation with Arterial Elasticity
Currently, the association of high-sensitivity C reactive protein (hsCRP) and other inflammatory markers with CVD is under intense investigation. The association of hsCRP and reduced arterial elasticity is also being evaluated in a number of studies as reviewed by Duprez et al. [17], who found in an asymptomatic population undergoing primary prevention cardiovascular screening that hsCRP was associated with reduced large (but not smaller) artery elasticity. Saijo et al. [18•] studied the relationship of another inflammatory protein, gamma-glutamyltransferase (GGT), and found it to be independently associated with increased hsCRP (in both males and females); in males, GGT was related to decreased arterial elasticity. Mahmud and Feely [19], in a hypertensive population, evaluated other markers of inflammation including tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), and hsCRP, finding them all independently associated with arterial elasticity. In a treated, non-diabetic hypertensive population, Kim et al. [20] found hsCRP to be associated with decreased arterial elasticity independent of age, systolic BP, gender, heart rate, glucose, lipid profiles, and medications in treated hypertension.
To further document the role of inflammation and its association with arterial elasticity, Booth et al. [21] studied 31 patients (15 with active disease and 16 in remission) with systemic vasculitis (anti-neutrophil cytoplasmic antibody-associated systemic vasculitis [AASV]) and compared them to 32 age-matched controls. The hsCRP was associated with active AASV and decreased arterial elasticity compared to AASV in remission and controls. Vlachopoulos et al. [22] evaluated 100 healthy individuals each receiving either Salmonella typhi vaccination or sham vaccination in a randomized, double-blind design. Active vaccination was associated with increases in inflammatory markers (CRP, IL-6, and matrix metalloprotienase-9) and decreased arterial elasticity [22]. With aspirin pre-treatment, the arterial elasticity decrease was ameliorated. These data suggest a cause-and-effect relationship of arterial elasticity with acute systemic inflammation. Finally, Tuttolomondo et al. [23••] evaluated in patients with acute ischemic stroke the relationship between immune-inflammatory markers and arterial stiffness indexes in 107 subjects with acute ischemic stroke and 107 controls without stroke. They observed a significant positive relationship, corrected for age and gender between PWV and CRP, TNF-α, IL-1β, von Willebrand factor (vWF), and IL-6. Augmentation index was significantly related to vWF, IL-6, and TNF-α levels. Among lacunar strokes, PWV was significantly related to CRP, IL-1β, IL-6, TNF-α, and vWF. In large artery atherosclerosis subjects, PWV was significantly related to CRP, IL-1β, IL-6, and TNF-α but not with vWF. Among cardioembolic infarcts, PWV was significantly and positively related to CRP, IL-1β, TNF-α, and vWF. They concluded that both aortic stiffness and wave reflection are related to the degree of systemic inflammation in stroke subjects, suggesting that circulating inflammation mediators can influence the stiffness of vessels distant to those involved in the disease process [23••].
Arterial Elasticity and CVD
Numerous cross-sectional studies have shown that the various approximations to arterial elasticity are associated with increased CVD or all-cause mortality risk scores, as well as with presence of CHD, as previously discussed. There have been studies of pressure augmentation due to the reflection wave during systole in CHD patients undergoing cardiac catheterization and procedures such as percutaneous coronary intervention (PCI), balloon angioplasty, and stent placement finding that augmentation index, indicating greater wave reflection and therefore arterial stiffness, predicted adverse cardiovascular events in patients with established CHD.
Longitudinal studies have assessed PWV, large and small artery elasticity, augmentation index, and central pulse pressure on their ability to predict incident CVD. Mitchell et al. [24••] assessed various measures of arterial stiffness and wave reflection and prognosis. Only higher stiffness assessed by PWV was associated with increased risk for first cardiovascular events, and it was additive to standard risk factors. Additionally, PWV has been found to predict subsequent events, including all-cause and cardiovascular mortality, fatal and non-fatal coronary events, and fatal strokes in both the general population and the elderly; however, PWV was not related to incident heart failure in the elderly [11••]. PWV was also predictive of subsequent events in subgroups with various conditions, including patients with acute CHD, patients undergoing PCI, patients with uncomplicated essential hypertension, impaired glucose intolerance, and end-stage renal disease [25]. The Conduit Artery Function Evaluation (CAFE) study indicated that an anti-hypertensive drug regimen can have a different effect on cardiovascular outcome due to its effect on central aortic pressure, despite a nearly equal effect on brachial arterial BP [26]. In a study with self-reported events, it was found that in an asymptomatic population small artery elasticity was associated with future CVD outcomes independent of age, whereas large artery elasticity was not related [27••]. Recent work from the Multi-Ethnic Study of Atherosclerosis (MESA), found that, even after risk factor adjustment, small artery elasticity was predictive of incident CHD, whereas both large artery elasticity (LAE) and small artery elasticity (SAE) were predictive of incident heart failure [28].
Atherosclerosis begins in childhood, and studies such as the Bogalusa Heart Study have demonstrated fatty streaks (the hallmark of future atherosclerotic plaques) in infants [29]. By the time one is in their 20s, over 50% have actual atheromas. But the origins and natural history of vascular disease is still evolving. The current paradigm suggests that changes in endothelial cell function presage measureable vascular abnormalities (Fig. 1). Endothelial cell dysfunction does induce functional changes in smaller vessel vascular elasticity, changes that may well become structural [30]. The role of inflammation at this early point in the natural history of CVD is still under investigation, but abnormal levels of hsCRP may be detected at this early time. As smaller vessel elasticity decreases, microalbuminuria may appear and changes in exercise-induced BP responses may be detected. For example, in a number of studies exaggerated SBP response to exercise was related to the risk of hypertension, total mortality, cardiovascular death, stroke, endothelial dysfunction, and myocardial infarction. Individuals with an exaggerated exercise BP response are not only more likely to develop future hypertension, but it may portend a greater risk for CVD events and death [31••]. Weiss et al. [31••] studied 6578 asymptomatic participants followed for 20 years in the Lipid Research Clinics Prevalence Study. They found that Bruce stage 2 BP >180/90 mmHg identified non-hypertensive individuals at a higher risk of CVD death [31••].
Pulse pressure is also an indirect measure of vascular elasticity. The natural progression with aging is a rise in SBP. After middle age, as age-related decreases in arterial elasticity occur, the SBP continues to rise and the diastolic BP falls, resulting in an increased pulse pressure (PP). Because increased PP is a later manifestation of reduced arterial elasticity, it is not surprising that a number of studies have demonstrated an association of increased PP with CVD, even independent of SBP or DBP. Toward the end of the natural history of arterial elasticity are the clinical manifestations that are the result of changes in vascular integrity (eg, myocardial infarction, sudden cardiac death, hypertension, kidney disease, and stroke).
Arterial Elasticity and Vascular Disease
A number of different techniques for measuring arterial elasticity have shown that a decrease is associated with diseases known to adversely affect the vasculature. In cross-sectional analyses of patients with diabetes mellitus, hypertension, congestive heart failure, and CAD, measures of arterial elasticity have shown decreased values compared to normal subjects [6]. In 24 elderly patients, Duprez et al. [32] found low small and large vessel elasticity to be associated with MRI-diagnosed cerebral white matter lesions. Duprez et al. [32] also found an association of reduced small artery elasticity and carotid intimal-medial thickening as assessed by carotid ultrasound, and in a separate study also found an association of reduced arterial elasticity with peripheral vascular occlusive disease [33]. Small and large artery elasticity is reduced in diabetic patients, and Beltran et al. [34] compared a normotensive population to patients with essential hypertension and a group with isolated systolic hypertension. Small vessel elasticity was reduced in both hypertensive groups but a profound decrease in large vessel elasticity was observed in the isolated systolic hypertension group [34].
Beside the above-mentioned cross-sectional studies that associate abnormal elasticity with vascular disease, there are a number of longitudinal studies that predict future outcome from baseline measures, or measure changes in arterial elasticity with interventions. One longitudinal study using percutaneous coronary angioplasty (PCA) was conducted by Grey et al [35]. Baseline arterial stiffness measures were obtained and 419 high-risk patients returned questionnaires 1 to 7 years after the baseline measurement. Of the 419 patients, 168 had suffered a cardiovascular event. Large artery elasticity was related to age, but only reduced small artery elasticity was predictive of future events [35].
Pharmacologic Agents and Arterial Elasticity
Glasser et al. [36] reviewed the data of the effect of a variety of pharmacologic agents on arterial elasticity up to 1997. Since that time, a variety of agents have been additionally studied, including angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEI), calcium channel blockers (CCBs), beta blockers (BBs), and alpha blockers [30]. One recent study evaluating the pharmacologic effect of drug therapy on arterial wall elasticity was the Atorvastatin and Amlodipine in Patients With Elevated Lipids and Hypertension–Arterial Wall Compliance (AVALON-AWC) trial, a substudy of the AVALON trial [37•]. Of 847 patients randomized in the main trial, 667 (404 men and 263 women) people at 103 centers participated in the arterial wall compliance substudy. The patients were between the ages of 23 and 76 years and all had concomitant hypertension and dyslipidemia. After 8 weeks of treatment, BP and lipids were reduced, and small and large vessel elasticity increased. Small vessel elasticity improved most with the combination of atorvastatin and amlodipine compared to placebo or either treatment alone. Other studies have compared the effects of enalapril to amlodipine in normotensive elderly subjects (>60 years) who had reduced small vessel elasticity at baseline. A differential effect of the agents on large versus small vessel elasticity was observed, with enalapril increasing large vessel elasticity (but not amlodipine) whereas amlodipine increased small vessel elasticity (but not enalapril) [38]. Resnick et al. [39] evaluated four classes of agents and found equal improvement in large vessel elasticity from ACE, ARB, and CCBs but no effect to worsening with BBs, whereas a more disparate effect occurred with small vessel elasticity. The greatest effect on small vessel elasticity was induced by CCBs and ARBs, less with ACEs and again there was no effect to a negative effect from BBs [39].
Flow-Mediated Dilation
Since its first description in 1992 as a non-invasive ultrasound method to measure endothelial-dependent dilation, FMD has been established and widely used as a research tool. Measurement of ultrasound-based FMD in the brachial artery is a non-invasive and relatively repeatable and reproducible method. Endothelial dysfunction is an early event in CVD and is positively correlated with atherogenesis, hypertension, heart failure, cardiovascular events, and CVD outcomes. Endothelium exposed to increased shear stress dilates as a physiologic response. This phenomenon is known as FMD and is non-invasively assessed with ultrasound.
FMD is currently the gold standard in clinical research for the non-invasive assessment of conduit artery endothelial function. In this method, brachial artery diameter is measured before and after an increase in shear stress that is induced by reactive hyperemia (FMD). When a sphygmomanometer cuff is placed on the forearm distal to the brachial artery and is inflated to 200 mm Hg and subsequently released 5 min later, FMD occurs predominantly as a result of local endothelial release of nitric oxide. As in the coronary circulation, this brachial artery response can be contrasted to the endothelium-independent dilator response to sublingual nitroglycerine. FMD is assessed by Doppler ultrasound with a high-resolution, multifrequency linear ultrasound Doppler probe, thus allowing for a direct assessment of endothelial-dependent vascular function.
Despite its widespread application in a variety of studies, the absolute values obtained using FMD vary considerably and mean FMD differs widely between studies. The technical aspects of the measurement, the location, and the duration of the occlusion may explain some of these differences, whereas type of equipment, location of the measurement, and occlusion pressure do not. The technique of FMD also has other challenges due to variations in technique and individual subject characteristics (preparation, vessel size). With the intent to standardize the method, the International Brachial Artery Reactivity Task Force published guidelines in 2002 [40].
The majority of studies investigating flow-mediated dilation focus on the ability of the endothelium to dilate. However, the endothelium has multiple functions and the measurement of endothelial-dependent dilation might be only one functional aspect of vasomotion. The endothelium regulates different physiologic and pathophysiologic mechanisms by secreting numerous thromboregulatory factors growth factors, and vasoactive substances, including prostacyclins, endothelins, endothelin cell growth factors, plasminogen inhibitors, interleukins, and nitric oxide(NO). NO seems to be the major mediator of vasodilation. NO has been intensely studied, and the reduced ability of the endothelium to release NO has been accepted as a surrogate marker of endothelial dysfunction.
There are a variety of cross-sectional studies published that have investigated the impact of major risk factors like aging, male gender, heredity (including race), nicotine, hypercholesterolemia, hypertension, physical inactivity, obesity, and diabetes mellitus on endothelial function and dysfunction as measured by FMD. For example, Celermajer et al. [41] found that increased FMD is associated with progressive endothelial dysfunction in normal humans, and it seems to occur earlier in men than in women [41]. In another controlled study, Celermajer et al. [42] investigated the association of passive and active smoke exposure and endothelium-dependent dilation in asymptomatic young adults [42]. Endothelium-dependent dilation was impaired in healthy young adult passive and active smokers. Endothelial dysfunction has been also demonstrated in patients with uncomplicated hypertension. Gokce et al. [43] investigated the effects of race and hypertension on flow-mediated dilation and found in a controlled study impaired endothelial function in the hypertensive subjects.
Numerous clinical trials were conducted to investigate the effect of commonly used antihypertensive medications like angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and beta-blockers. To investigate the role of ARBs on endothelial function, Koh et al. [44] compared losartan, irbesartan, and candesartan in a placebo-controlled study with 122 subjects [44]. Compared with placebo, angiotensin II type-1 receptor blockers significantly improved the percent flow-mediated dilation. A study conducted by Rajagopalan et al. [45] randomized 35 healthy elderly individuals in a double-blind, placebo-controlled trial. Subjects treated with valsartan in this study showed no alteration in FMD. In a recently published study by Yilmaz et al. [46] in 108 type 2 diabetic hypertensive patients with proteinuria randomized to amlodipine, valsartan or a combination of both, an improvement of FMD and reduced proteinuria was observed in a 12-week intervention. Yilmaz et al. [47] also analyzed data of 49 persons with diabetic chronic kidney disease (stage 1) and 32 healthy subjects in a prospective controlled study. FMD was studied at baseline and after 12 weeks of ramipril therapy [47]. Ramipril treatment significantly improved FMD and normalized urinary protein excretion. Pasini et al. [48] compared the effect of nebivolol and atenolol on endothelial function measured by FMD in 40 essential hypertensive patients matched with 40 healthy subjects. The nebivolol, but not the atenolol, group showed significant improvement in FMD. An improvement in FMD was also observed in a study by Merchant et al. [49] in 43 obese, hypertensive AA patients treated with nebivolol for 8 weeks.
To shed more light on these often conflicting results, Ghiadoni et al. [50] compared effects of antihypertensive drugs in a prospective, randomized study with 168 subjects. Subjects were randomly assigned to nifedipine, amlodipine, atenolol, nebivolol, telmisartan, and perindopril. Interestingly, only perindopril increased flow-mediated dilation. Souza-Barbosa et al. [51] evaluated 63 hypertensive patients in a controlled study. Subjects were divided into four groups (hydrochlorothiazide, irbesartan, quinapril, or irbesartan and quinapril) and followed for 12 weeks. Antihypertensive treatment improved the endothelium-dependent function after renin-angiotensin-aldosterone system blockade. In a further finding, the combined effect of angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade was not superior to the action of either of these treatments separately [51].
In resistant hypertension (BP >140/90 mm Hg, ≥3 antihypertensive medications), hyperaldosteronism is found in approximately 20% of patients. Nishizaka et al. [52] found an impaired endothelium-dependent dilation in resistant hypertensive subjects with hyperaldosteronism. After 3 months of follow-up, treatment with spironolactone resulted in increased FMD. Diabetes is another major risk factor for CVD, and in a controlled study conducted by Singh et al. [53] in 31 diabetic teenagers and 35 age-matched healthy children, endothelium-dependent vasodilation was significantly lower in diabetic children compared with healthy children, and impaired endothelial function was positively correlated with cardiovascular outcome. Interestingly, children with insulin-dependent DM developed endothelial dysfunction within the first decade after the onset of diabetes. These changes were manifest before an increase in carotid intima-media thickness could be identified [53]. CAD is the leading cause of morbidity and mortality in most industrialized countries, and cardiovascular risk factors were shown to impair endothelial function. In order to evaluate the outcome in patients with mild CAD, Suwaidi et al. [54] examined 157 patients with non-obstructive (<40%) CAD in a prospectively designed study, and demonstrated a greater incidence of cardiovascular events during 2.3-year follow-up in patients with impaired endothelium-dependent vasodilation in the coronary resistance and conduit arteries. These data suggest a possible role of endothelial dysfunction in the progression of coronary atherosclerosis [54].
Conclusions
Given the multi-factorial nature of CVD and the increasing burden of CVD, three general preventive strategies apply: 1) population-wide public health measures (eg, national smoking cessation programs), 2) identifying and targeting higher-risk subgroups for preventive interventions, and 3) delivering acute and chronic higher-cost treatments and secondary prevention to those with clinically manifest disease. Issues such as whether we will be able to alter risk factors and behaviors during this phase in order to limit the increase in clinical CVD will be a major challenge. The following facts speak for themselves: 61.8 million Americans have CVD; 50 million have high BP; 12.6 million have CHD; 1 in 5 men and women have some form of CVD; 1 in 3 men will develop major CVD before age 60 years, and the odds for women are 1 in 10; CVD claims more lives than the next seven leading causes of death combined; about one sixth of people killed by CVD are under age 65 years; and the lifetime risk of CHD after age 40 years is 49% for men and 32% for women.
Footnotes
Disclosure Stephen P. Glasser reports no potential conflict of interest relevant to this article. Tanja Dudenbostel reports no potential conflict of interest relevant to this article.
Contributor Information
Stephen P. Glasser, Email: sglasser@uab.edu, Division of Preventive Medicine and Department of Epidemiology, University of Alabama at Birmingham, 1717 11th Avenue South MT638, Birmingham, AL 35205, USA
Tanja Dudenbostel, Email: tduden@uab.edu, Vascular Biology and Hypertension Program, University of Alabama at Birmingham, 933 19th Street South CH19-115, Birmingham, AL 35205, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lee R, Kamm R. Vascular mechanics for the cardiologist. J Am Coll Cardiol. 1994;23(6):1289–95. doi: 10.1016/0735-1097(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 2.Zimlichman R, Boaz M, Duprez D, et al. The Seven European Sites Study of Arterial Elasticity - Using the Blood Pressure Wave Form Analysis - Reliability, Repeatability and Establishment of Normal Values for Healthy European Population with Comparison to Healthy U.S. Population. American Journal of Hypertension. 2003;16(5 Part 2):P315. doi: 10.1016/j.amjhyper.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Arnett DK, Glasser SP, McVeigh G, et al. Blood pressure and arterial compliance in young adults: the Minnesota Children’s Blood Pressure Study. Am J Hypertens. 2001;14(3):200–5. doi: 10.1016/s0895-7061(00)01262-0. [DOI] [PubMed] [Google Scholar]
- 4.Bhuiyan AR, Li S, Li H, et al. Distribution and correlates of arterial compliance measures in asymptomatic young adults: the Bogalusa Heart Study. Am J Hypertens. 2005;18(5 Pt 1):684–91. doi: 10.1016/j.amjhyper.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Valappil NI, Jacobs DR, Gross MD, et al. Correlates of Arterial Compliance in Young Adults: The CARDIA Study. The Journal of Clinical Hypertension Supplement. 2007;95:A10. [Google Scholar]
- 6.Glasser SP, Arnett DK, McVeigh GE, et al. Vascular compliance and cardiovascular disease: a risk factor or a marker? Am J Hypertens. 1997;10(10 Pt 1):1175–89. doi: 10.1016/s0895-7061(97)00311-7. [DOI] [PubMed] [Google Scholar]
- 7.Liao D, Arnett DK, Herman A, et al. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34(2):201–6. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 8.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354(16):1685–97. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 9.Glasser SP. On Arterial Physiology, Pathophysiology of Vascular Compliance, and Cardiovascular Disease. Heart Disease. 2000;2:375–379. [PubMed] [Google Scholar]
- 10.Cohn JN. Vascular wall function as a risk marker for cardiovascular disease. J Hypertens Suppl. 1999;17(5):S41–4. [PubMed] [Google Scholar]
- 11••.Najjar SS, Scuteri A, Shetty V, et al. Pulse Wave Velocity is an Independent Predictor of the Longitudinal Increase in Systolic Glood Pressure and of Incident Hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. This article presents evidence that links many biochemical, enzymatic, and cellular alterations, as well as their modulating signals, to accelerated vascular aging and the pathogenesis and progression of arterial diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McVeigh GE, Bratteli CW, Morgan DJ, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33(6):1392–8. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 13.Gilani M, Alinder C, Kaiser D, et al. Differences in Large and Small Artery Response to Acute Inhibition of Nitric Oxide Synthase in Human Subjects. Am J Hypertens. 2000;13(4 part 2):Abstract No. B003. [Google Scholar]
- 14.McVeigh GE, Allen PB, Morgan DR, et al. Nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci (Lond) 2001;100(4):387–93. [PubMed] [Google Scholar]
- 15.Weinberger MH, Fineberg NS, Weinberg M, et al. The Relationships Between Age, Gender and Blood Pressure and Vascular Compliance and Resistance in Normal and Hypertensive Humans. American Journal of Hypertension. 2001;14(4 Part 2 Orals No O16) [Google Scholar]
- 16.Takeuchi K, Zhang B, Ideishi M, et al. Influence of age and hypertension on the association between small artery compliance and coronary artery disease. Am J Hypertens. 2004;17(12 Pt 1):1188–91. doi: 10.1016/j.amjhyper.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Duprez DA, Somasundaram PE, Sigurdsson G, et al. Relationship between C-reactive protein and arterial stiffness in an asymptomatic population. J Human Hypet. 2005;19:515–519. doi: 10.1038/sj.jhh.1001860. [DOI] [PubMed] [Google Scholar]
- 18•.Saijo Y, Utsugi M, Yoshioka E, et al. The relationship of gamma-glutamyltransferase to C-reactive protein and arterial stiffness. Nutr Metab Cardiovasc Dis. 2008;18(3):211–9. doi: 10.1016/j.numecd.2006.10.002. This article discusses the role of inflammatory markers and vascular disease. [DOI] [PubMed] [Google Scholar]
- 19.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118–22. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Kang TS, Kim JB, et al. Significant association of C-reactive protein with arterial stiffness in treated non-diabetic hypertensive patients. Atherosclerosis. 2007;192(2):401–6. doi: 10.1016/j.atherosclerosis.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Booth AD, Wallace S, McEniery CM, et al. Inflammation and Arterial Stiffness in systemic Vasculitis A model of Vascular Inflammation. Arthritis & Rheumatism. 2004;50:581–588. doi: 10.1002/art.20002. [DOI] [PubMed] [Google Scholar]
- 22.Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 23••.Tuttolomondo A, Di Raimondo D, Pecoraro R, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis. 2010;213(1):311–318. doi: 10.1016/j.atherosclerosis.2010.08.065. This is one of the first studies evaluating the relationship between arterial elasticity and immune inflammatory markers in acute cerebrovascular disease. [DOI] [PubMed] [Google Scholar]
- 24••.Mitchell GF, Hwang S, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. This analysis of 2232 participants from the Framingham Heart Study focuses on aortic PVW as a potential novel biomarker of cardiovascular risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 26.Williams B, O’Rourke M. The Conduit Artery Functional Endpoint (CAFE) study in ASCOT. J Hum Hypertens. 2001;15(Suppl 1):S69–73. [PubMed] [Google Scholar]
- 27••.Duprez DA, Jacobs DR, Jr, Lutseyl P, et al. Small Artery but not Large Artery Elasticity Coronary Heart Disease Events Beyond Coronary Calcium Score and Carotid Intima Media Thickness in an Asymptomatic Population. ACC International Meeting. 2009 The potential value of techniques that differentiate smaller from larger vessel elasticity is suggested in this study. [Google Scholar]
- 28.Duprez DA, Jacobs DR, Jr, Luseyl P, et al. Large and Small Artery elasticity, but not Coronary Calcium Score and Carotid Intima Media Thickness, Predict Incident Congestive Heart Failure Events in an Asymptomatic Population: Results of the Multi-Ethnic Study of Atherosclerosis. Acc International Meeting. 2009 [Google Scholar]
- 29.Bhuiyan AR, Srinivasan SR, Chen W, et al. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis. 2006;189(1):1–7. doi: 10.1016/j.atherosclerosis.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Cohn JN, Duprez DA, Grandits GA. Arterial elasticity as part of a comprehensive assessment of cardiovascular risk and drug treatment. Hypertension. 2005;46(1):217–20. doi: 10.1161/01.HYP.0000165686.50890.c3. [DOI] [PubMed] [Google Scholar]
- 31••.Weiss SA, Blumenthal RS, Sharrett AR, et al. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121(19):2109–16. doi: 10.1161/CIRCULATIONAHA.109.895292. Vascular elasticity is a determinate of the BP response to exercise, and its ability to predict future CVD events in asymptomatic subjects is suggested by this study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duprez D, DeBuyzere M, Van Den Noortgate N, et al. Relationship Between Periventricular or Deep White Matter Lesions and Arterial Elasticity Indices in Very Old People. Age and Ageing. 2001;30:325–330. doi: 10.1093/ageing/30.4.325. [DOI] [PubMed] [Google Scholar]
- 33.Duprez D, DeBuyzere ML, DeBruyne L, et al. Small and Large Artery Elasticity Indices in Peripheral Arterial Occlusive Disease (PAOD) Vascular Medicine. 2001;6:211–214. doi: 10.1177/1358836x0100600402. [DOI] [PubMed] [Google Scholar]
- 34.Beltran A, McVeigh G, Morgan D, et al. Arterial compliance abnormalities in isolated systolic hypertension. Am J Hypertens. 2001;14(10):1007–11. doi: 10.1016/s0895-7061(01)02160-4. [DOI] [PubMed] [Google Scholar]
- 35.Grey E, Bratteli C, Glasser SP, et al. Reduced small artery but not large artery elasticity is an independent risk marker for cardiovascular events. Am J Hypertens. 2003;16(4):265–9. doi: 10.1016/s0895-7061(02)03271-5. [DOI] [PubMed] [Google Scholar]
- 36.Glasser SP, Arnett DK, McVeigh GE, et al. The importance of arterial compliance in cardiovascular drug therapy. J Clin Pharmacol. 1998;38(3):202–12. doi: 10.1002/j.1552-4604.1998.tb04417.x. [DOI] [PubMed] [Google Scholar]
- 37•.Cohn JN, Goldman JM. Establishing a new option for target-organ protection: rationale for ARB plus ACE inhibitor combination therapy. Am J Hypertens. 2008;21(3):248–56. doi: 10.1038/ajh.2007.56. The fact that different drugs impact arterial elasticity differentially is emphasized in this study. [DOI] [PubMed] [Google Scholar]
- 38.Bratteli CW, Alinder CM, Cohn JN. Contrasting Arterial Compliance Effects of Enalapril and amlodipine in Normotensive Elderly Subjects. American Journal of Hypertension. 1999;12(4 part 2):Abstract No B015. [Google Scholar]
- 39.Resnick LM, Lester MH, Chesney CF. Differential Effects of Antihypertensive Drug Therapy on Arterial Compliance. American Journal of Hypertension; 15th Scientific Meeting of the American Society of Hypertension; 2000. p. 20A. [DOI] [PubMed] [Google Scholar]
- 40.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 41.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 42.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334(3):150–4. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 43.Gokce N, Holbrook M, Duffy SJ, et al. Effects of race and hypertension on flow-mediated and nitroglycerin-mediated dilation of the brachial artery. Hypertension. 2001;38(6):1349–54. doi: 10.1161/hy1201.096575. [DOI] [PubMed] [Google Scholar]
- 44.Koh KK, Han SH, Chung WJ, et al. Comparison of effects of losartan, irbesartan, and candesartan on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am J Cardiol. 2004;93(11):1432–5. A10. doi: 10.1016/j.amjcard.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 45.Rajagopalan SR, Kariisa M, Dellegrottaglie S, et al. Angiotensin Receptor Blockade Imporves Vascular Compliance in Healthy Normotensive elderly Individuals: Results from a Randomized Double-Blind Placebo-Controlled Trial. The Journal of Clinical Hypertension. 2006;8(11):783–790. doi: 10.1111/j.1524-6175.2006.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yilmaz MI, Carrero JJ, Martin-Ventura JL, et al. Combined therapy with renin-angiotensin system and calcium channel blockers in type 2 diabetic hypertensive patients with proteinuria: effects on soluble TWEAK, PTX3, and flow-mediated dilation. Clin J Am Soc Nephrol. 2010;5(7):1174–81. doi: 10.2215/CJN.01110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yilmaz MI, Axelsson J, Sonmez A, et al. Effect of renin angiotensin system blockade on pentraxin 3 levels in type-2 diabetic patients with proteinuria. Clin J Am Soc Nephrol. 2009;4(3):535–41. doi: 10.2215/CJN.04330808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasini AF, Garbin U, Stranieri, et al. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21(11):1251–7. doi: 10.1038/ajh.2008.260. [DOI] [PubMed] [Google Scholar]
- 49.Merchant N, Searles CD, Pandian A, et al. Nebivolol in high-risk, obese African Americans with stage 1 hypertension: effects on blood pressure, vascular compliance, and endothelial function. J Clin Hypertens (Greenwich) 2009;11(12):720–5. doi: 10.1111/j.1751-7176.2009.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41(6):1281–6. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 51.Souza-Barbosa L, Ferreira-Melo SE, Ubaid-Girioli S, et al. Endothelial Vascular Function in Hypertensive Patients After Renin—Angiotensin System Blockade. The Journal of Clinical Hypertension. 2006;8:803–811. [PubMed] [Google Scholar]
- 52.Nishizaka MK, Zaman MA, Green SA, et al. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109(23):2857–61. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 53.Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 2003;41(4):661–5. doi: 10.1016/s0735-1097(02)02894-2. [DOI] [PubMed] [Google Scholar]
- 54.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]


