Abstract
Background
Sickle cell disease is associated with extensive healthcare utilization; estimated lifetime costs exceed $460,000 per patient. Approximately 30% of chronically transfused sickle cell patients become alloimmunized to red cell antigens, but these patients cannot be identified a priori. Prospective antigen-matching can prevent alloimmunization, but is costly and may not benefit most patients.
Study Design and Methods
A Markov-based model was constructed to compare the health and financial implications of four alternative antigen-matching strategies for chronically transfused sickle cell patients. The strategies varied by the selection method of patients receiving matched blood (contingent on prior alloimmunization or prospectively for all patients) and the extent of antigen-matching (limited or extensive). Direct medical costs and alloimmunization events were assessed over 10 and 20-year periods, for a hypothetical cohort of initially transfusion-naïve patients and for a dynamic population.
Results
Within a hypothetical cohort of initially transfusion-naïve patients, implementing prophylactic limited matching for chronically transfused patients instead of history-based limited matching is expected to cost an additional $766 million over 10 years, but result in 2,072 fewer alloimmunization events. Within the same cohort, implementing prospective extensive matching is expected to cost $1.86 billion more than history-based extensive matching, but result in 2,424 fewer alloimmunization events. Averting a single alloimmunization event using prospective matching would cost $369,482–769,284. Among a dynamic population over 10 years, prospective limited matching is expected to cost $358 million more than history-based limited matching.
Conclusions
While prospective matching for all transfused patients would reduce alloimmunization, this benefit requires considerable expenditure.
Keywords: cost-effectiveness, delayed hemolytic transfusion reaction (DHTR), red blood cells, transfusion, alloimmunization, phenotype matching, sickle cell disease, Markov model, decision-tree
Introduction
Sickle cell disease (SCD) is one of the most common genetic disorders in the United States, affecting an estimated 70,000 to 100,000 people.1–3 Despite improvements in management and survival, SCD remains associated with extensive medical resource utilization; the lifetime cost of care for an SCD patient is greater than $460,000.4 Red blood cell (RBC) transfusion plays an important role in the management of SCD. Unfortunately, RBC transfusion among SCD patients may result in alloimmunization, defined by the development of alloantibodies directed against donor RBC antigens.5 This immune response may be partly explained by racial antigenic differences; SCD patients are predominantly of African descent, while blood donors are often white.6 Approximately 30% of transfused SCD patients are likely to become alloimmunized.5,7–10
Although many alloimmunized patients do not experience associated adverse reactions, others may experience delayed serologic transfusion reactions (DSTRs) or delayed hemolytic transfusion reactions (DHTRs), which can lead to worsened anemia and possibly hyperhemolysis.6 RBC alloimmunization has also been associated with hemolytic disease of the fetus and newborn (HDFN) and increased morbidity following organ transplantation.11,12 These complications may present challenges for transfusion management and cause delays in patient care.5 Warm autoantibody formation occasionally occurs in patients with alloantibodies, and may exaggerate difficulties in identifying alloantibodies and finding compatible blood for these patients.5
Phenotypic matching of RBC antigens between donors and recipients has been shown to reduce the risk of alloimmunization in transfused SCD patients.13,14 Some transfusion services routinely conduct prophylactic matching for all transfused SCD patients. However, because it is not clear which patients are likely to become alloimmunized5,15 and because prophylactic matching is costly and time consuming, other transfusion services only provide antigen-matched blood once a patient has already developed an alloantibody. Furthermore, while some transfusion services consider only the most frequently implicated antigens (C, E, K) in matching, others match for an extensive set of antigens. There is no standard policy across transfusion services to prevent alloimmunization and associated adverse effects.16 An expert panel convened by the National Institutes of Health (NIH) recently identified knowledge gaps in the transfusion management of SCD patients, highlighting the need for efficacy and cost-effectiveness evaluations of antigen-matching strategies to reduce alloimmunization among these patients.17
To our knowledge, a cost-effectiveness analysis evaluating potential antigen-matching strategies to prevent alloimmunization and DSTRs/DHTRs has not been conducted, but these results would be valuable in developing appropriate transfusion medicine policy. This study compares the health and financial implications of prospective versus history-based antigen-matching, in addition to evaluating the effects of variation in the set of antigens considered.
Materials and Methods
A Markov-based decision tree model was constructed (TreeAge Pro Suite 2012, Williamstown, MA) to compare RBC antibody formation and transfusion-related costs across four alternative strategies of antigen-matching for SCD patients undergoing chronic RBC transfusions. Markov models have been used extensively to simulate recurring processes,18 and are thus well-suited to describing transfusion therapy. This model simulated a population of male and female SCD patients undergoing chronic transfusion therapy, incorporating an initial prevalent SCD patient cohort supplemented annually by incident cohorts of newly diagnosed SCD patients. Each year, transfused SCD patients experienced risks from alloimmunization and DSTRs/DHTRs. Transfusions, immunization events, and associated costs were tracked for SCD patients over 10 and 20 year periods. Outcomes were assessed separately for a cohort of chronically transfused SCD patients who had been transfusion-naïve initially (at the start of the simulation), as well as for a dynamic sample of SCD patients, which included patients with a prior history of transfusion and possible alloimmunization.
Model Structure
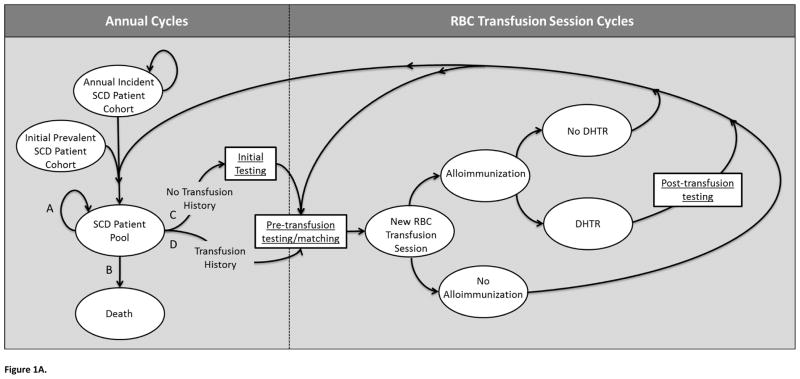
Under all evaluated antigen-matching strategies, simulated SCD patients traversed the same model, illustrated in Figure 1A. Over a series of 1-year cycles, each annual incident cohort joined an initial existing prevalent cohort, forming a comprehensive SCD patient pool. Each year, these patients could follow one of four paths, each defined by particular Markov state transitions: the patient could (A) remain in the patient pool without beginning chronic transfusion therapy, (B) die, (C) initiate transfusion therapy, having no prior history of transfusion, or (D) initiate a transfusion session, having had previous transfusions. Patients who began transfusion therapy were assumed to continue therapy for the remainder of the simulation until death. Patients undergoing chronic transfusion therapy (paths C or D) underwent “Pre-transfusion testing/matching” before beginning a transfusion session, with those patients who had no history of transfusion (path C) first undergoing “Initial Testing.” Each year, patients receiving transfusions progressed through a series of 12 subcycles to model monthly transfusion sessions. Each new RBC transfusion session was associated with with a per-unit alloimmunization risk, and the possibility of experiencing subsequent DSTRs or DHTRs. We assumed no cases of HDFN. All transfused patients, with or without antibody formation, transitioned back to the “SCD patient pool” if no further transfusion sessions were received during the year, or to “Pre-transfusion testing/matching” to prepare for an additional transfusion session during the current annual cycle. Time was tracked and incremented explicitly, to allow for yearly cycles and monthly sub-cycles.
Figure 1.
Figure 1A. Illustration of Markov model.
A portion of the simulation operates through annual cycles, where simulated patients enter the model as part of an “Annual Incident SCD Patient Cohort,” or as part of the “Initial Prevalent SCD Patient Cohort.” Each annual incident cohort joins this initial prevalent cohort to form the “SCD Patient Pool.” Individuals of this pool may follow one of four paths each year (A, B, C, or D). Path A is defined by continuing in the SCD Patient Pool without undergoing transfusion therapy. Path B reflects leaving the simulation via death. Path C and D allow simulated patients to undergo chronic transfusion therapy. Patients with no previous transfusion history follow Path C, which first leads to an “Initial Testing” phase, and then to “Pre-transfusion testing/matching,” while patients with a previous history of transfusion progress directly to “Pre-transfusion testing/matching” (Path D). Patients undergoing chronic transfusion therapy (Paths C or D) enter a portion of the model with each cycle defining a single RBC transfusion session. While patients following paths A or B continue through a series of annual cycles, others transition to “New RBC Transfusion Session,” Each transfusion session could lead to an alloimmunization event, which could result in a DSTR or DHTR, or only a positive antibody screen. In the event that a transfusion session resulted in a DHTR, “Post-transfusion testing” would be conducted. Regardless, patients would subsequently either return to “Pre-transfusion testing/matching” for another transfusion session within the same annual cycle or transition back to the “SCD Patient Pool” if no further transfusion sessions were required during the year.
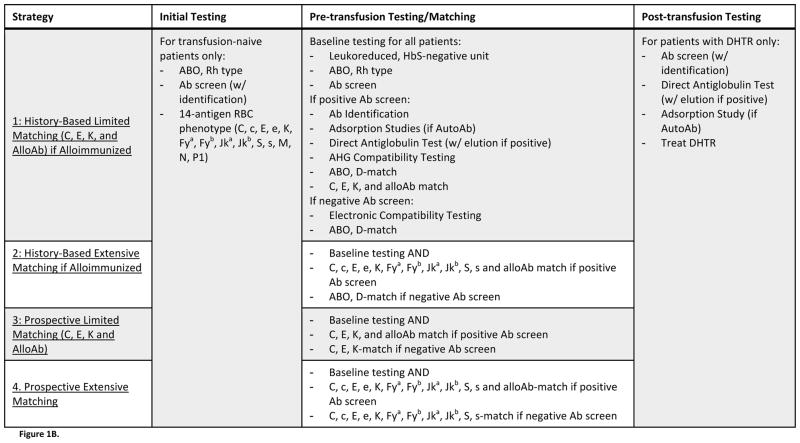
Figure 1B. Description of Evaluated Strategies. Four strategies of antigen-matching, which differed by the protocols used for preventing and managing alloimmunization, were evaluated. All strategies incorporated identical “Initial Testing” and “Post-transfusion Testing” procedures, but strategies differed by the procedure implemented for “Pre-transfusion Testing/Matching.” Strategies 1 and 2 characterized history-based antigen-matching, where only those patients who had previously formed alloantibodies would receive antigen-matched blood. Strategies 3 and 4 characterized prospective antigen-matching, where all patients – regardless of alloimmunization history – would receive antigen-matched blood. Under Strategies 1 and 3, antigen-matching would be limited, with units screened for C, E, K, and any other antigens against which the patient had formed alloantibodies. Under Strategies 2 and 4, however, antigen-matching would be extensive, with units screened for 11 antigens (C, c, E, e, K, Fya, Fyb, Jka, Jkb, S, s). Under any matching protocol, compatible units would be negative for any of the screened antigens that the patient lacked, and for any other antigens against which patients had formed alloantibodies.
Transfusion Procedures
Only individuals undergoing chronic transfusion therapy during their lifetimes were modeled, assuming that 4.67% of pediatric patients undergo chronic transfusion,19 and 10% of SCD patients would be chronically transfused at some point in their lifetimes. 64.3% of pediatric patients receiving chronic transfusion were assumed to undergo simple transfusion,19 receiving 1–3 units per transfusion session (triangular distribution with mode = 1), while the remainder of pediatric patients were assumed to receive exchange transfusion,19 receiving 6–12 units (mode = 8) per session. Half of adult patients receiving chronic transfusion underwent simple transfusion,20 receiving 2–4 units per session (mode = 2), while the other half of adult patients underwent exchange transfusion, 20 receiving 8–14 units (mode = 10) per session. The number of units transfused per exchange transfusion session is an estimate based upon conventional red cell exchange, and not isovolemic hemodilution procedures.
Antigen-Matching Strategies
Four antigen-matching strategies were evaluated (Figure 1B). Under all strategies, “Initial Testing” included ABO and Rh typing, antibody screening with subsequent antibody identification for positive screens, and 14-antigen phenotyping. “Pre-transfusion testing/matching” under all strategies incorporated an ABO and Rh type and antibody screen. Patients with negative antibody screens received electronic compatibility testing, while patients with positive screens underwent antibody identification, a direct antiglobulin test, and AHG compatibility testing. Furthermore, adsorption studies were performed if antibody screens suggested autoantibody formation, and an elution was conducted for positive direct antiglobulin tests. The four strategies differed by the antigen-matching protocol used to select RBC units for patients with or without alloimmunization.
Under all four strategies, when a unit was antigen-matched, an entire set of antigens was considered for compatibility, but the selected unit was matched only for antigens for which the patient was negative or for antigens against which the patient had formed alloantibodies. Patient phenotypes were defined and tracked using published rates of antigens among SCD patients, and it was assumed that the appropriately matched units were available. Two of the evaluated strategies incorporated antigen-matching for patients only after they had experienced alloimmunization (history-based), while the other two involved prospective antigen-matching for all patients, irrespective of alloantibody development. Under all strategies, RBC units were, at a minimum, HbS-negative, leukocyte-reduced, and ABO and D antigen-matched. Under history-based antigen-matching strategies (Strategies 1 and 2), patients with no history of alloimmunization were chronically transfused with this baseline level of matching.
Strategy 1 used limited RBC antigen-matching only for those chronically transfused SCD patients who had previously developed alloantibodies. In addition to the baseline requirements for units, patients with an alloimmunization history would receive RBC units matched for the antigens C, E, and K, as well as for any other antigens against which alloantibodies had been formed. Strategy 2 involved the same history-based antigen-matching as Strategy 1, but used an extensive matching protocol (C, c, E, e, K, Fya, Fyb, Jka, Jkb, S, and s) for patients with an alloimmunization history. Strategy 3 and 4 involved prophylactic antigen-matching for all patients, regardless of alloimmunization history, with Strategy 3 characterizing limited prophylactic matching (C, E, and K), and Strategy 4 characterizing an extensive antigen-match (C, c, E, e, K, Fya, Fyb, Jka, Jkb, S, and s).
Input Parameters
Individual patients were tracked as they traversed the model, experiencing transfusion-related events and accumulating associated expenses, which were discounted to the beginning of the simulation and expressed in 2012US$. Both costs and events were discounted at a rate of 3% per year. The analysis focused on the perspective of a hospital transfusion service, with each component of a transfusion session –“initial testing,” “pretransfusion testing/matching,” “posttransfusion testing,” and the transfusion itself – associated with a cost. Only direct medical expenses were included, and these were estimated by 2012 Medicare reimbursement rates.21,22 The cost associated with a DHTR was approximated by previously reported hospital expenses for SCD patients presenting with painful crises.23 The expense for selecting an antigen-matched unit was assumed to be $80 per negative antigen.24 Input parameters are provided in Table 1.
Table 1.
Model Input Parameters (Base-Case Values and Ranges for Sensitivity Analysis)
| Input Parameter | Base-Case Value (Range) | Source |
|---|---|---|
| Initial Testing | ||
| Cost: ABO Type | $7.71 (5.78, 9.64) | 22 |
| Cost: Rh Type | $7.71 (5.78, 9.64) | 22 |
| Cost: Antibody Screen | $14.95 (11.21, 18.69) | 22 |
| Cost: Antibody Identification (for patients with positive screen only) | $24.77 (18.58, 30.96) | 22 |
| Cost: Initial RBC Antigen Phenotyping (14-antigen)a | $364 (273, 455) | 24 |
| Pre-transfusion Testing/Matching | ||
| Cost: Leukoreduced RBC unit | $198.87 (149.15, 248.59) | 21 |
| Cost: ABO Type | $7.71 (5.78, 9.64) | 22 |
| Cost: Rh Type | $7.71 (5.78, 9.64) | 22 |
| Cost: Antibody Screen | $14.95 (11.21, 18.69) | 22 |
| Cost: Antibody Identification (for patients with positive screen only) | $24.77 (18.58, 30.96) | 22 |
| Cost: Direct Antiglobulin Test (for patients with positive screen only) | $7.71 (5.78, 9.64) | 22 |
| Cost: Elution (for patients with positive DAT only) | $24.77 (18.58, 30.96) | 22 |
| Cost: Adsorption Study (for patients with positive screen indicating AutoAB only)b | $24.77 (18.58, 30.96) | 22 |
| Cost: Electronic Compatibility Testing (for patients with negative screen) | $14.95 (11.21, 18.69) | 22 |
| Cost: AHG Compatibility Testing (for patients with positive screen) | $24.77 (18.58, 30.96) | 22 |
| Cost: Negative Antigens (per antigen negative, per unit) | $80 (60, 100) | 24 |
| Post-transfusion Testing | ||
| Cost: DHTR Hospitalization | $1392.09 (1044.07, 1740.11) | 23 |
| Cost: Antibody Screen | $14.95 (11.21,18.69) | 22 |
| Cost: Antibody Identification (for patients with positive screen only) | $24.77 (18.58,30.96) | 22 |
| Cost: Direct Antiglobulin Test (for patients with positive screen only) | $7.71 (5.78,9.64) | 22 |
| Cost: Elution (for patients with positive DAT only) | $24.77 (18.58,30.96) | 22 |
| Cost: Adsorption Study (for patients with positive screen indicating AutoAB only) | $24.77 (18.58,30.96) | 22 |
| Alloimmunization Rate | ||
| Portion of Patients Experiencing Alloimmunization Risk (portion of “responders”) | 30% (25, 35) | 5,8–10 |
| Matching ABO, D Only (among ”responders”, per 100 units transfused) | 3.27 (1–5) | 14,28 |
| Percent Reduction in Alloimmunization Risk from Limited Matching (ABO, D, C, E, K) | 85% (75–95) | 13,28,29 |
| Percent Reduction in Alloimmunization Risk from Extensive Matching | 99% (90–100) | Assumed 30 |
| Portion of Patients with Positive DAT (among those with positive screen) | 25% (15–35) | 6 |
| DHTR | ||
| Portion of alloimmunization events leading to DHTRs (Pediatric/Adult) | 17.3/3.4 (15–20/1–5) | 7 |
| Patient/Background Characteristics | ||
| Portion of initial cohort of patients with transfusion and AlloAb history | Varies by age | 27 |
| Annual SCD Incident Patient Population (National)c | 1674 (1256, 2093) | 3,8,34 |
| Initial Prevalent SCD Patient Population | 85000 (72000, 98000) | 3 |
| Portion of Pediatric SCD Patients Undergoing Chronic Transfusion Therapy | 4.67% (2, 6) | 19 |
| Portion of SCD Patients Undergoing Chronic Transfusion Therapy (over lifetime) | 10% (5, 15) | Assumed |
| Transfusion Sessions per Year for Chronic Therapy | 12 (6–20) | Assumed |
| Units per Simple Transfusion Session (Pediatric Patients) | 1 (1–3) | Assumed |
| Units per Exchange Transfusion Session (Pediatric Patients) | 8 (6–12) | Assumed |
| Portion of Chronically Transfused Pediatric Patients Undergoing Exchange Transfusion | 64.3% (40–80) | 19 |
| Units per Simple Transfusion Session (Adult Patients) | 2 (2–4) | Assumed |
| Units per Exchange Transfusion Session (Adult Patients) | 10 (8–14) | Assumed |
| Portion of Chronically Transfused Adult Patients Undergoing Simple Transfusion | 50% (40–60) | 20 |
| Age and Sex-specific Mortality Rate | Varies by age, sex | 34 |
Note: All costs are expressed in 2012 US$.
Assumes $26 per antigen as reported.
Cost estimate for adsorption studies was not reported in the original source, but it was assumed that the reimbursement rate for adsorption would be comparable to rates for elution.
Incidence estimated from birth cohort SCD prevalence by race and estimates of birth cohort populations by race.
Rates of alloimmunization among chronically transfused SCD patients in the absence of antigen-matching were drawn from existing literature, and patients undergoing simple transfusion were assumed to face the same per-unit alloimmunization risk as patients undergoing exchange transfusion. It has previously been reported that only about 30% of transfused SCD patients are ever expected to become alloimmunized.25,26 Therefore, at the beginning of the simulation, 30% of transfused SCD patients were randomly identified as “responders.” Only these “responders” had potential to develop alloantibodies and experience DHTRs/DSTRs. Alloimmunization from transfusion prior to the beginning of the simulation was incorporated using age-specific alloimmunization rates.27
The reported efficacy of antigen-matching in reducing alloimmunization and associated delayed transfusion reactions varies widely. We assumed that while 30% of SCD patients would ultimately develop alloantibodies when not provided with antigen-matched RBCs10 at a rate of 3.27 alloantibodies per 100 units transfused,28 a limited antigen-matching strategy would reduce alloimmunization events by 85%,13,28,29 and an extensive matching strategy would reduce alloimmunization events by 99%.30
Analysis
Under the base-case scenario, strategies were analyzed over 10 and 20 year periods to reflect estimated outcomes over a policy-relevant mid-range and a long-range time period. Each simulation was run using 100,000 individual trials. Transfusion costs and alloimmunization events were reported for each strategy, both for a hypothetical cohort of initially transfusion-naïve patients and for a dynamic population incorporating patients with a history of transfusion. Costs were expressed per transfused unit and for a comprehensive patient population, assuming that chronically transfused patients received monthly transfusions, beginning in the year they initiated transfusion therapy. Furthermore, to evaluate the effect of history-based versus prospective antigen-matching, Strategy 1 was compared to Strategy 3, and Strategy 2 was compared to Strategy 4. The cost to avert each alloimmunization event using prospective or extended matching was calculated. One-way sensitivity analyses were used to evaluate the impact of variation in the cost of obtaining antigen-negative RBC units, the efficacy of antigen-matching in reducing alloimmunization risk, and the portion of individuals likely to become alloimmunized. Probabilistic sensitivity analysis, using 10,000 samples of 10,000 trials each evaluated the impact of uncertainty in input parameters. Costs were varied by 25% in either direction using an adjustment factor sampled from a triangular distribution (mode=1; min=0.75, max=1.25). Efficacy estimates and incidence rates were drawn from beta distributions, using the 95% CI reported by the original data source wherever possible.
Results
Under the base-case scenario, history-based antigen-matching (Strategies 1 and 2) was less costly than prospective antigen-matching for all patients (Strategies 3 and 4) (Table 2). Within a hypothetical chronically transfused cohort of initially transfusion-naïve patients in the United States (8,500 patients), implementing prospective limited matching (Strategy 3) over a 10-year period is expected to cost $766 million more than history-based limited matching (Strategy 1), but result in 2,072 fewer alloimmunization events. Within the same cohort, implementing prospective extensive matching (Strategy 4) is expected to cost $1.86 billion more than history-based extensive matching (Strategy 2), but result in 2,424 fewer alloimmunization events. Thus, it would cost $369,482–769,284 to prevent a single alloimmunization event using prospective (instead of history-based) matching. The increase in costs associated with prospective matching was largely attributed to increased expenses for matched units. Strategies 1 and 2 did, however, exhibit slight increases in testing and complication costs.
Table 2.
Base-Case Results: Cohort of Initially Transfusion-Naive Chronically Transfused Sickle Cell Patients
| Strategy
|
||||
|---|---|---|---|---|
| History-Based Matching | Prospective Matching | |||
|
|
|
|||
| Time Period | 1: Limited | 2: Extended | 3: Limited | 4: Extended |
|
|
|
|||
| 10 years | ||||
| Total Cost per Transfused Unit: All Patients ($)a | 252.33 | 301.82 | 367.50 | 581.47 |
| Cost of Testing | 19.53 | 19.50 | 18.45 | 16.99 |
| Cost of Units | 232.47 | 282.19 | 348.79 | 564.47 |
| Cost of Complications | 0.34 | 0.12 | 0.25 | 0.02 |
| Total Cost per Transfused Unit: Alloimmunized Patients Only ($)b | 397.45 | 564.57 | 371.43 | 580.51 |
| Cost of Testing | 25.96 | 25.87 | 21.59 | 14.76 |
| Cost of Units | 370.34 | 538.28 | 348.91 | 565.48 |
| Cost of Complications | 1.16 | 0.41 | 0.93 | 0.27 |
| Entire SCD Cohort (over 10 year period)c | ||||
| Total Cost (millions, $) | 1665.65 | 2014.31 | 2431.21 | 3879.20 |
| Net Change (Extended vs. Limited) | 348.66 | 1447.99 | ||
| Net Change (Prospective vs. History-Based) | 765.56 | 1864.89 | ||
| Alloimmunization Events | 11058 | 3081 | 8986 | 657 |
| Net Change (Extended vs. Limited) | −7977 | −8329 | ||
| Net Change (Prospective vs. History-Based) | −2072 | −2424 | ||
| Cost per Alloimmunization Event Avoided (thousands, $)d | ||||
| Extended vs. Limited | 43.71 | 173.84 | ||
| Prospective vs. History-Based | 369.48 | 769.28 | ||
| 20 years | ||||
| Total Cost per Transfused Unit: All Patients ($)a | 220.37 | 264.16 | 320.70 | 506.63 |
| Cost of Testing | 15.99 | 15.99 | 15.40 | 13.93 |
| Cost of Units | 204.09 | 248.11 | 305.06 | 492.69 |
| Cost of Complications | 0.28 | 0.06 | 0.24 | 0.02 |
| Total Cost per Transfused Unit: Alloimmunized Patients Only ($)b | 351.73 | 500.60 | 325.51 | 504.45 |
| Cost of Testing | 21.70 | 21.67 | 19.60 | 12.77 |
| Cost of Units | 329.06 | 478.72 | 305.06 | 491.55 |
| Cost of Complications | 0.97 | 0.22 | 0.85 | 0.14 |
| Entire SCD Cohort (over 20 year period)c | ||||
| Total Cost (millions, $) | 2928.62 | 3533.02 | 4274.97 | 6796.30 |
| Net Change (Extended vs. Limited) | 604.41 | 2521.33 | ||
| Net Change (Prospective vs. History-Based) | 1346.36 | 3263.28 | ||
| Alloimmunization Events | 17887 | 3475 | 15786 | 1083 |
| Net Change (Extended vs. Limited) | −14412 | −14703 | ||
| Net Change (Prospective vs. History-Based) | −2101 | −2391 | ||
| Cost per Alloimmunization Event Avoided (thousands, $)d | ||||
| Extended vs. Limited | 41.94 | 171.49 | ||
| Prospective vs. History-Based | 640.79 | 1364.56 | ||
Total cost per transfused unit reflects the average expected cost per unit transfused across all chronically transfused patients over a period of time (10 or 20 years). Cohort only includes patients without a history of transfusion/alloimmunization.
Total cost per transfused unit reflects the average cost per unit transfused across all alloimmunized patients in the cohort.
Outcomes for an entire cohort of chronically transfused SCD patients. Assumes a population of 85,000 SCD patients, of which 10% are chronically transfused. Net change outcomes calculated as Extended-Limited or Prospective – History-Based.
Positive outcomes for cost per alloimmunization event avoided indicate additional required expenditure to avert a single alloimmunization event. Negative outcomes reflect a decrease in costs associated with either extended or prospective matching.
Analysis of outcomes for a dynamic population, incorporating patients with a history of transfusion and previous alloimmunization, suggested similar cost savings associated with history-based instead of prospective antigen-matching (Table 3). Slight differences in the average cost per transfusion session were evident between the cohort and dynamic population due to differences in the transfusion and alloimmunization history of the patient populations. Over 10 years, implementing history-based (Strategy 1) instead of prospective limited matching (Strategy 3) among a national population of chronically transfused SCD patients would cost $358 million less, but lead to an expected 1,417 more alloimmunization events. Thus, over this time period, it would cost $252,816 to prevent one alloimmunization event using limited prospective matching instead of limited history-based matching. Comparing history-based and prospective extensive matching indicated that history-based matching would result in nearly $931 million in savings, but lead to 1,717 more alloimmunization events.
Table 3.
Base-Case Results: Dynamic Population of Chronically Transfused Sickle Cell Patients
| Strategy
|
||||
|---|---|---|---|---|
| History-Based Matching | Prospective Matching | |||
|
|
|
|||
| Time Period | 1: Limited | 2: Extended | 3: Limited | 4: Extended |
|
|
|
|||
| 10 years | ||||
| Total Cost per Transfused Unit: All Patients ($)a | 190.73 | 238.98 | 257.59 | 409.85 |
| Cost of Testing | 10.38 | 10.36 | 9.68 | 8.97 |
| Cost of Units | 180.16 | 228.54 | 247.78 | 400.87 |
| Cost of Complications | 0.20 | 0.08 | 0.13 | 0.01 |
| Total Cost per Transfused Unit: Alloimmunized Patients Only($)b | 394.27 | 564.21 | 370.71 | 590.35 |
| Cost of Testing | 18.50 | 18.45 | 16.08 | 16.66 |
| Cost of Units | 375.09 | 545.48 | 354.11 | 573.61 |
| Cost of Complications | 0.69 | 0.28 | 0.52 | 0.08 |
| Entire SCD Population (over 10 year period)c | ||||
| Total Cost (millions, $) | 1084.28 | 1377.14 | 1442.62 | 2307.65 |
| Net Change (Extended vs. Limited) | 292.86 | 865.02 | ||
| Net Change (Prospective vs. History-Based) | 358.34 | 930.51 | ||
| Alloimmunization Events | 6870 | 2085 | 5452 | 368 |
| Net Change (Extended vs. Limited) | −4785 | −5085 | ||
| Net Change (Prospective vs. History-Based) | −1417 | −1717 | ||
| Cost per Alloimmunization Event Avoided (thousands, $)d | ||||
| Extended vs. Limited | 61.20 | 170.11 | ||
| Prospective vs. History-Based | 252.82 | 541.93 | ||
| 20 years | ||||
| Total Cost per Transfused Unit: All Patients ($)a | 172.69 | 215.64 | 235.69 | 374.96 |
| Cost of Testing | 9.52 | 9.51 | 8.97 | 8.28 |
| Cost of Units | 162.98 | 206.06 | 226.59 | 366.66 |
| Cost of Complications | 0.19 | 0.07 | 0.13 | 0.01 |
| Total Cost per Transfused Unit: Alloimmunized Patients Only($)b | 358.99 | 514.96 | 336.38 | 547.93 |
| Cost of Testing | 16.77 | 16.74 | 14.72 | 14.91 |
| Cost of Units | 341.54 | 497.98 | 321.12 | 532.95 |
| Cost of Complications | 0.68 | 0.24 | 0.54 | 0.07 |
| Entire SCD Population (over 20 year period)c | ||||
| Total Cost (millions, $) | 1710.13 | 2167.44 | 2305.81 | 3687.35 |
| Net Change (Extended vs. Limited) | 457.31 | 1381.54 | ||
| Net Change (Prospective vs. History-Based) | 595.68 | 1519.91 | ||
| Alloimmunization Events | 10639 | 2575 | 8964 | 614 |
| Net Change (Extended vs. Limited) | −8064 | −8350 | ||
| Net Change (Prospective vs. History-Based) | −1675 | −1961 | ||
| Cost per Alloimmunization Event Avoided (thousands, $)d | ||||
| Extended vs. Limited | 56.71 | 165.45 | ||
| Prospective vs. History-Based | 355.54 | 774.95 | ||
Total cost per transfused unit reflects the average expected cost per unit transfused across all chronically transfused patients over a period of time (10 or 20 years). Dynamic population includes patients with a history of transfusion and possible alloimmunization.
Total cost per transfused unit reflects the average cost per unit transfused across all alloimmunized patients in the dynamic population. Includes patients who began the analysis period with a history of alloimmunization.
Outcomes for an entire dynamic population of chronically transfused SCD patients. Assumes an initial population of 85,000 SCD patients, of which 10% are chronically transfused, and an annual incident population of 1674, of whom 10% undergo chronic transfusion. Net change outcomes calculated as Extended-Limited or Prospective – History-Based.
Positive outcomes for cost per alloimmunization event avoided indicate additional required expenditure to avert a single alloimmunization event. Negative outcomes reflect a decrease in costs associated with either extended or prospective matching.
Under the base-case scenario, the cost of selecting a unit matched for a limited set of antigens (C, E, K) is up to $240. One-way sensitivity analysis varying the cost of selecting a limited matched unit suggested that history-based limited matching would continue to be cost-saving over prospective limited matching while this expense was greater than $20. Probabilistic sensitivity analysis (Table 4) demonstrated that trends in financial outcomes between strategies withstood variation in input parameters. Outcomes were not sensitive to variation in the portion of SCD patients undergoing chronic transfusion or number of units per transfusion session for adults or pediatric patients. However, an increase in the portion of SCD patients likely to become alloimmunized is associated with a decrease in cost per averted alloimmunization event using prospective or extended antigen-matching. Over a 10 year period, varying the portion of “high-risk” chronically transfused SCD patients from 25 to 35% suggests that the cost per alloimmunization event averted using prospective limited instead of history-based limited matching varies from the base-case value by 2–4%, from $293,777 (35%) to $493,289 (25%). Outcomes were also sensitive to variation in the cost of selecting antigen-matched units and the effectiveness of limited or extended matching in reducing alloimmunization.
Table 4.
Probabilistic Sensitivity Analysis Results: Cohort of Initially Transfusion-Naive Chronically Transfused Sickle Cell Patients
| Time Period | Strategy
|
|||
|---|---|---|---|---|
| History-Based Matching
|
Prospective Matching
|
|||
| 1: Limited | 2: Extended | 3: Limited | 4: Extended | |
|
|
|
|||
| 10 years | ||||
| Total Cost per Transfused Unit: All Patients ($)a | 253.33 [218.83, 289.13] | 304.06 [262.36, 345.24] | 367.99 [320.51, 418.92] | 583.26 [503.49, 666.25] |
| Cost of Testing | 19.49 [16.75, 22.28] | 19.48 [16.78, 22.24] | 18.35 [15.86, 20.98] | 16.83 [14.48, 19.22] |
| Cost of Units | 233.49 [199.11, 269.32] | 284.46 [242.81, 325.50] | 349.38 [302.15, 400.83] | 566.42 [484.37, 650.41] |
| Cost of Complications | 0.35 [0.25, 0.46] | 0.12 [0.08, 0.17] | 0.26 [0.17, 0.37] | 0.02 [0.01, 0.03] |
| Entire SCD Cohort (National)b | ||||
| Total Cost (millions, $) | 1746.98 [1460.89, 2059.62] | 2113.27 [1772.12, 2483.83] | 2551.05 [2161.97, 2988.67] | 4073.02 [3396.32, 4792.12] |
| Net Change (Extended vs. Limited)c | 366.29 [351.67, 380.92] | 1521.97 [420.66, 454.90] | ||
| Net Change (Prospective vs. History-Based)d | 804.08 [788.04, 820.11] | 1959.75 [1934.80, 1984.70] | ||
| Alloimmunization Events | 11587.11 [9029.94, 14291.35] | 3123.06 [2776.06, 3487.79] | 9452.74 [6893.78, 12216.03] | 630.28 [445.80, 840.90] |
| Net Change (Extended vs. Limited)c | −8464.06 [−8553.23, −8374.90] | −8822.46 [−8912.34, −8732.58] | ||
| Net Change (Prospective vs. History-Based)d | −2134.38 [−2260.35, −2008.40] | −2492.77 [−2505.42, −2480.12] | ||
| Cost per Alloimmunization Event Avoided: Extended vs. Limited (thousands, $) | 43.28 [14.47, 72.09] | 172.51 [117.49, 227.53] | ||
| Cost per Alloimmunization Event Avoided: Prospective vs. Limited (thousands, $) | 376.73 [−1.70, 755.16] | 786.17 [612.43, 959.92] | ||
| 20 years | ||||
| Total Cost per Transfused Unit: All Patientsa | 220.88 [189.38, 253.69] | 266.41 [228.82, 303.37] | 319.01 [274.99, 362.85] | 505.86 [431.60, 580.45] |
| Cost of Testing | 15.97 [13.88, 18.12] | 15.96 [13.87, 18.07] | 15.35 [13.32, 17.37] | 13.81 [11.89, 15.74] |
| Cost of Units | 204.62 [173.24, 237.72] | 250.38 [213.10, 287.05] | 303.42 [260.48, 347.92] | 492.03 [418.13, 566.76] |
| Cost of Complications | 0.29 [0.20, 0.40] | 0.07 [0.04, 0.09] | 0.25 [0.17, 0.35] | 0.02 [0.01, 0.03] |
| Entire SCD Cohort (National)b | ||||
| Total Cost (millions, $) | 3062.88 [2556.47, 3603.88] | 3716.09 [3097.83, 4319.63] | 4449.14 [3750.08, 5195.19] | 7104.07 [5936.56, 8397.29] |
| Net Change (Extended vs. Limited)c | 653.21 [351.67, 380.92] | 2654.93 [420.66, 454.90] | ||
| Net Change (Prospective vs. History-Based)d | 1386.27 [788.04, 820.11] | 3387.99 [1934.80, 1984.70] | ||
| Alloimmunization Events | 18842 [14143.70, 24564.27] | 3613 [3203.90, 4112.99] | 16681 [11878.90, 22386.42] | 1106 [801.43, 1435.17] |
| Net Change (Extended vs. Limited)c | −15230 [−8553.23, −8374.90] | −15575 [−8912.34, −8732.58] | ||
| Net Change (Prospective vs. History-Based)d | −2162 [−2260.35, −2008.40] | −2507 [−2505.42, −2480.12] | ||
| Cost per Alloimmunization Event Avoided: Extended vs. Limited (thousands, $) | 42.89 [14.20, 71.58] | 170.46 [114.93, 226.00] | ||
| Cost per Alloimmunization Event Avoided: Prospective vs. Limited (thousands, $) | 641.26 [−480.74, 1763.27] | 1351.56 [1029.50, 1673.62] | ||
Total cost per transfused unit reflects the average expected cost per unit transfused. Average across all patients in a cohort of initially transfusion-naïve SCD patients over a period of chronic transfusion.
Outcomes for an entire cohort of chronically transfused SCD patients. Assumes a population of 85,000 SCD patients, of which 10% are chronically transfused.
Net change calculated as: Extended-Limited.
Net change calculated as: Prospective-History-Based.
Discussion
Antigen-matching of transfused RBC units can reduce the risk of alloimmunization.8,9,13 However, since only 30% of chronically transfused SCD patients are likely to become alloimmunized,5,8–10 prospective matching for all transfused patients may not be the optimal strategy. There is currently no standard antigen-matching policy across transfusion services. Although their final report has not yet been published, an NIH-convened committee has stated that further research on the use of antigen-matching to prevent alloimmunization is critical to improving the management of SCD.17
This analysis compared the health and financial implications of history-based antigen-matching, where only those patients who previously formed alloantibodies received matched units, to prospective matching, where all patients - irrespective of alloantibody formation - received matched units. Strategies were also distinguished by the extent of antigen-matching, with outcomes evaluated for both limited (C, E, and K) and extensive matching. The rationale for not prophylactically matching RBC antigens hinges on the understanding that most patients are unlikely to ever experience alloantibody formation. This analysis demonstrates that in addition to the lack of clinical benefit for the majority of SCD patients, prospective antigen-matching is an extremely expensive strategy as compared to history-based matching.
These results suggest that prospective antigen-matching is expected to be substantially more costly but prevent slightly more alloimmunization events than history-based matching. The additional cost to prevent each alloimmunization event increases over time, as many individuals at risk of alloimmunization first develop alloantibodies soon after initiating chronic transfusion therapy. At a national scale, assuming 10% of the SCD patient population undergoes chronic transfusion, a strategy of prospective limited antigen-matching (Strategy 3) is expected to cost up to $766 million more over 10 years than a strategy of history-based limited antigen-matching (Strategy 1). Incorporating a dynamic population over the 10 year period suggests that at a national level, Strategy 3 would cost $358 million more than Strategy 1. Over a 10-year period, it would cost an estimated $252,817 to prevent a single alloimmunization event using prospective limited (Strategy 3) rather than history-based limited (Strategy 1) antigen-matching. Over 20-years, this cost is expected to increase to $355,544.
Although advocates of prophylactic matching justify this practice as a means to reduce the complications of alloimmunization, it is not clear that the high costs of antigen-matching outweigh its benefits. The majority of patients who become alloimmunized develop an antibody to a single common red cell antigen, without evidence of hemolysis. A previous study which studied DSTRs and DHTRs in transfused patients found 34 cases of alloantibody formation with 28 having no evidence of hemolysis.31 Among the six cases with clinical evidence of hemolysis, 4 were missed by clinicians prospectively. Although occasional cases of DHTR can impact patient morbidity, the majority are asymptomatic and clinically benign. There is no evidence that patients who make red cell antibodies develop multiple alloantibodies as an early response. There is also no evidence that prophylactic antigen-matching will prevent alloimmunization to patients who lack rare high incidence antigens that would not be avoided in current matching protocols. Thus, prophylactic antigen-matching will not prevent the difficult problems with multiple alloantibodies or single antibodies to high incidence antigens that can harm patients or cause transfusion delays due to extensive blood screening and limited inventories.
This analysis relied on a simplified model of chronic transfusion therapy among SCD patients, and outcomes presented here may vary after incorporating other elements. The costs incorporated were drawn from Medicaid reimbursement rates and included direct medical expenses only. These likely underestimate the comprehensive cost of transfusion-related events to hospitals and do not account for additional non-medical or indirect costs borne by patients, caregivers, or communities. Furthermore, it was assumed that antigen-matched units were available whenever required under any of the four strategies. The availability of extensively matched units may require additional donation campaigns or other additional procurement expenses. These additional costs were not included in this analysis, suggesting that outcomes reported here may underestimate the expenditure associated with prospective antigen-matching and that expected costs per averted alloimmunization event are likely underestimates. In addition, the model did not incorporate the potential development of warm autoantibodies, which may be associated with alloimmunization. Finally, we assumed that patients remained on chronic transfusion for the duration of the simulation, when, in fact, some patients may cease chronic transfusion for various reasons after shorter transfusion courses.
Currently, there is no method to identify a priori which patients will form alloantibodies from transfusion. If methods were developed to identify these at-risk patients, the optimal transfusion strategy may involve prophylactic matching for only those patients identified as “at-risk.” Given current limitations, transfusion services must decide whether or not to prospectively match blood for all transfused SCD patients. The price to avert alloimmunization through prospective antigen-matching seems substantial, particularly after considering the often referenced incremental cost effectiveness ratio of $50,000–$100,000 per quality-adjusted life year gained.32 However, because transfusion safety is highly prioritized, transfusion-related interventions that are not cost-saving or cost-effective may still be widely implemented. For example, HIV nucleic acid amplification testing has been shown to have a marginal cost-effectiveness of $2 million per additional quality-adjusted life year gained.33 This analysis suggests that while prospective antigen-matching provides limited clinical benefit over history-based matching to some patients, this benefit comes with significant costs. This evidence must be interpreted in the larger context of transfusion medicine-related interventions to evaluate and establish appropriate policy.
Acknowledgments
A.A.R.T. was supported by the NIH 1K23AI093152-01A1 and Doris Duke Charitable Foundation Clinician Scientist Development Award (#22006.02). WJS was supported by an American Society of Hematology Scholar award and NIH R21HL107828-01A1.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. [Accessed September 10, 2012];Sickle Cell Disease: Data and Statistics. 2011 Sep; http://www.cdc.gov/NCBDDD/sicklecell/data.html.
- 2.Meny GM. Transfusion protocols for patients with sickle cell disease: working toward consensus? Immunohematology. 2012;28:1–2. [PubMed] [Google Scholar]
- 3.Hassell KL. Population estimates of sickle cell disease in the U. S. Am J Prev Med. 2010;38:S512–21. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84:323–7. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- 5.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–37. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–21. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 7.Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 8.Karafin MS, Shirey RS, Ness PM, King KE. Antigen-matched red blood cell transfusions for patients with sickle cell disease at The Johns Hopkins Hospital. Immunohematology. 2012;28:3–6. [PubMed] [Google Scholar]
- 9.King KE, Shirey RS. Transfusion management of patients with sickle cell disease: the continuing dilemma. Transfusion. 2010;50:2–4. doi: 10.1111/j.1537-2995.2009.02527.x. [DOI] [PubMed] [Google Scholar]
- 10.Osby M, Shulman IA. Phenotype matching of donor red blood cell units for nonalloimmunized sickle cell disease patients: a survey of 1182 North American laboratories. Arch Pathol Lab Med. 2005;129:190–3. doi: 10.5858/2005-129-190-PMODRB. [DOI] [PubMed] [Google Scholar]
- 11.Lerut E, Van Damme B, Noizat-Pirenne F, Emonds MP, Rouger P, Vanrenterghem Y, Pirenne J, Ansart-Pirenne H. Duffy and Kidd blood group antigens: minor histocompatibility antigens involved in renal allograft rejection? Transfusion. 2007;47:28–40. doi: 10.1111/j.1537-2995.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 12.Boyd SD, Stenard F, Lee DK, Goodnough LT, Esquivel CO, Fontaine MJ. Alloimmunization to red blood cell antigens affects clinical outcomes in liver transplant patients. Liver Transpl. 2007;13:1654–61. doi: 10.1002/lt.21241. [DOI] [PubMed] [Google Scholar]
- 13.Lasalle-Williams M, Nuss R, Le T, Cole L, Hassell K, Murphy JR, Ambruso DR. Extended red blood cell antigen matching for transfusions in sickle cell disease: a review of a 14-year experience from a single center (CME) Transfusion. 2011;51:1732–9. doi: 10.1111/j.1537-2995.2010.03045.x. [DOI] [PubMed] [Google Scholar]
- 14.Ambruso DR, Githens JH, Alcorn R, Dixon DJ, Brown LJ, Vaughn WM, Hays T. Experience with donors matched for minor blood group antigens in patients with sickle cell anemia who are receiving chronic transfusion therapy. Transfusion. 1987;27:94–8. doi: 10.1046/j.1537-2995.1987.27187121485.x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–53. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 16.Afenyi-Annan A, Willis MS, Konrad TR, Lottenberg R. Blood bank management of sickle cell patients at comprehensive sickle cell centers. Transfusion. 2007;47:2089–97. doi: 10.1111/j.1537-2995.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute. Expert Panel Report on the Management of Sickle Cell Disease is Available for Public Comment Until. Office of the Director; Aug 31, [Accessed March 18, 2013]. http://www.nhlbi.nih.gov/about/directorscorner/messages/2012-messages/august-2012/expert-panel-report-on-the-management-of-sickle-cell-disease-is-available-for-public-comment-until-august-31/index.html. [Google Scholar]
- 18.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 19.Fasano RM, Paul W, Siegal E, Luban NL. Transfusion protocol for patients with sickle hemoglobinopathies at Children’s National Medical Center. Immunohematology. 2012;28:13–6. [PubMed] [Google Scholar]
- 20.Vichinsky EP. The prevention and management of alloimmunization in sickle cell disease: the benefit of extended phenotypic matching of red blood cells. Immunohematology. 2012;28:20–3. [PubMed] [Google Scholar]
- 21.Centers for Medicare & Medicaid Services. Hospital Outpatient Prospective Payment System: Anndendum A Update. 2012 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates-Items/2012-July-Addendum-A.html.
- 22.AABB. 2013 Proposed Medicare Payments for Hospital Outpatient Services [monograph on the internet] 2012 Available from: http://www.aabb.org/programs/reimbursementinitiatives/Pages/13hoppsruleprop.aspx.
- 23.Moore RD, Charache S, Terrin ML, Barton FB, Ballas SK. Cost-effectiveness of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Am J Hematol. 2000;64:26–31. doi: 10.1002/(sici)1096-8652(200005)64:1<26::aid-ajh5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Winkler AM, Josephson CD. Transfusion practices for patients with sickle cell disease at major academic medical centers participating in the Atlanta Sickle Cell Consortium. Immunohematology. 2012;28:24–6. [PubMed] [Google Scholar]
- 25.Ness PM. To match or not to match: the question for chronically transfused patients with sickle cell anemia. Transfusion. 1994;34:558–60. doi: 10.1046/j.1537-2995.1994.34794330007.x. [DOI] [PubMed] [Google Scholar]
- 26.Castro O, Sandler SG, Houston-Yu P, Rana S. Predicting the effect of transfusing only phenotype-matched RBCs to patients with sickle cell disease: theoretical and practical implications. Transfusion. 2002;42:684–90. doi: 10.1046/j.1537-2995.2002.00126.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller ST, Kim HY, Weiner DL, Wager CG, Gallagher D, Styles LA, Dampier CD, Roseff SD. Red blood cell alloimmunization in sickle cell disease: prevalence in 2010. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakhalkar VS, Roberts K, Hawthorne LM, McCaskill DM, Veillon DM, Caldito GC, Cotelingam JD. Allosensitization in patients receiving multiple blood transfusions. Ann N Y Acad Sci. 2005;1054:495–9. doi: 10.1196/annals.1345.072. [DOI] [PubMed] [Google Scholar]
- 29.Vichinsky EP, Luban NL, Wright E, Olivieri N, Driscoll C, Pegelow CH, Adams RJ. Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: a multicenter transfusion trial. Transfusion. 2001;41:1086–92. doi: 10.1046/j.1537-2995.2001.41091086.x. [DOI] [PubMed] [Google Scholar]
- 30.Tahhan HR, Holbrook CT, Braddy LR, Brewer LD, Christie JD. Antigen-matched donor blood in the transfusion management of patients with sickle cell disease. Transfusion. 1994;34:562–9. doi: 10.1046/j.1537-2995.1994.34794330008.x. [DOI] [PubMed] [Google Scholar]
- 31.Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long-term serologic findings, and clinical significance. Transfusion. 1990;30:688–93. doi: 10.1046/j.1537-2995.1990.30891020325.x. [DOI] [PubMed] [Google Scholar]
- 32.Chambers JD, Neumann PJ, Buxton MJ. Does Medicare have an implicit cost-effectiveness threshold? Med Decis Making. 2010;30:E14–27. doi: 10.1177/0272989X10371134. [DOI] [PubMed] [Google Scholar]
- 33.van Hulst M, de Wolf JT, Staginnus U, Ruitenberg EJ, Postma MJ. Pharmaco-economics of blood transfusion safety: review of the available evidence. Vox Sang. 2002;83:146–55. doi: 10.1046/j.1423-0410.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. National Center for Health Statistics. [Accessed October 2, 2012];VitalStats. http://www.cdc.gov/nchs/vitalstats.htm.