Abstract
The A1 allele of the DRD2/ANKK1 Taq1A polymorphism (rs1800497) is associated with reduced striatal D2/3 receptor binding in healthy individuals (Con) as well as depression and addiction. However, the effect of rs1800497 on D2/3 receptor binding in depressed patients as well as the SNP’s effect on D2/3 binding during reward-associated dopamine release is unknown. Twelve unmedicated patients with major depressive disorder (MDD) and 24 Con completed PET scans with [11C]raclopride, once without receiving monetary rewards (baseline) and once while winning money. In Con, the A1 allele was associated with reduced baseline binding potential (BPND) in the middle caudate and ventral striatum. However, in MDD patients the A1 allele was associated with increased baseline BPND in these regions. There were no significant associations between rs1800497 and change in BPND during reward-associated dopamine release. Conceivably, the A1 allele predisposes to depression and addiction via its effect on the post-synaptic D2 receptor.
Keywords: Depression, dopamine 2 receptor, positron emission tomography, reward, Taq1A
Introduction
The majority of dopamine (DA) D2 receptors are densely distributed on post-synaptic, non-dopaminergic neurons in the striatum where D2 signalling modulates a variety of functional domains, including reward processing and appetitive behaviour. Additionally, D2 autoreceptors located in the somas, dendrites and terminals of DA neurons in the ventral tegmentum negatively regulate DA signalling by modulating firing rate (Ford et al., 2010), and DA release and synthesis (Wolf and Roth, 1990), respectively. A widely studied single nucleotide polymorphism (SNP), the so-called DRD2/ANKK1 Taq1A polymorphism (rs1800497, Glu713Lys) is located ~10 kb downstream from the DRD2 gene in the ankyrin repeat and kinase domain containing 1 (ANKK1) gene.
The A1 allele of rs1800497 has consistently been implicated in addiction disorders (Noble, 2000; Smith et al., 2008; Chen et al., 2011) and has also been reported to be a risk factor for depression, possibly via its impact on the quality of early parental interactions. Mills-Koonce et al. (2007) reported that children with the A1 allele showed more negative emotions during interactions with their parents, an effect that was attenuated by maternal sensitivity. Consistent with these data, the A1 allele was associated with ‘social problems’ as measured by the Child Behaviour Checklist questionnaire in children with reading disorders (Marino et al., 2004). In another study the A1 allele was associated with emerging symptoms of anxiety and depression as well as parent× child interactions characterized by negative emotions (Hayden et al., 2010). Similarly, children aged 10–12 yr with the A1 allele were more sensitive to negative feedback during a probabilistic learning task (Althaus et al., 2009). The putative effect of the A1 allele on childhood behaviour and depression may arise from basic physiological differences in the ability to regulate emotions that are present very early in development. Infants aged 3 and 6 months with the A1 allele, who were separated from their parents in an experimental setting, showed reduced ability to regulate vagal tone compared to infants without an A1 allele (Propper et al., 2008). In a longitudinal study of 2347 adult males, the A1 allele was associated with an increased risk of developing depressive symptoms at follow-up (odds ratio 2.55; Roetker et al., 2012). Consistent with these data, veterans with post-traumatic stress disorder who carried the A1 allele had more symptoms of anxiety, depression and social dysfunction than A2/A2 homozygotes (Lawford et al., 2006).
The Taq1A polymorphism or a variant in linkage disequilibrium with Taq1A appears to affect D2 receptor binding, perhaps explaining the reported associations between Taq1A and psychiatric and addiction disorders. Relative to the A2 allele, the A1 allele has been associated with reduced striatal glucose metabolism (Noble et al., 1997) and reduced binding of the D2/3 receptor antagonist, [11C]raclopride, in studies of healthy subjects (Thompson et al., 1997; Pohjalainen et al., 1998; Jonsson et al., 1999). In addition, the DRD2 C957T SNP, which may be in linkage disequilibrium (LD) with rs1800497 (Hirvonen et al., 2009), reportedly affects striatal D2/3 receptor binding in healthy volunteers (Hirvonen et al., 2004).
Although genetic studies have implicated the TaqA1 allele in susceptibility to depression and the TaqA1 allele has been associated with reduced D2 receptor binding in healthy controls, it is unknown how the TaqA1 allele would affect D2 receptor binding in patients with mood disorders. As we reported in our recent review of the positron emission tomography (PET) D2 literature, the data are contradictory and arguably the weight of data is suggestive of increased D2 receptor binding in mood disorders (Savitz and Drevets, 2013). For example, compared with healthy controls, unmedicated patients with major depressive disorder (MDD) and motor retardation displayed increased [11C]raclopride binding potential (BPND) in the caudate and striatum (Meyer et al., 2006) and Kestler et al. (2000) reported a positive correlation between [11C]raclopride binding in the striatum and the ‘depression’ subscale score of the NEO Personality Inventory. Similarly, an increase in striatal D2 receptor binding in depression was reported in single photon emission tomography studies using the radioligand, 123I-iodobenzamide (D’Haenen and Bossuyt, 1994; Shah et al., 1997). Potentially consistent with these data, rats exposed to chronic social stress display elevated D2 receptor binding in the striatum (Lucas et al., 2004); although see (Zhu et al., 2011) who report decreased stress-associated D2 mRNA expression in the striatum. Nevertheless, it is conceivable that the elevations of D2 receptor availability and increased [11C]raclopride binding observed in depressed humans and in rodent depression analogues may arise secondarily to reductions in baseline DA release, consistent with the decreased basal firing activity of DA neurons in rats studied in depression models (Chang and Grace, 2012).
In order to investigate the apparent contradiction between the genetic association studies that implicate the A1 allele in depression, and some of the in vivo human and animal studies that are suggestive of increased D2 receptor binding in MDD or rodent analogues thereof, we measured the effect of the Taq1A SNP on D2 receptor BPND in both healthy volunteers and unmedicated patients with MDD.
Furthermore, in contrast to previous studies that measured the effect of the Taq1A SNP on D2 receptor binding at rest only (i.e. during tonic DA release), we also tested the effect of the Taq1A SNP on D2 receptor BPND during receipt of unpredicted reward (i.e. during phasic DA release). We were able to do this by scanning subjects under two conditions during PET[11C]raclopride imaging. In the first condition the subjects played a slot machine task without receiving monetary rewards (baseline condition) and in the second, subjects received unpredictable monetary rewards while performing the identical slot machine task (reward condition). This approach yielded two measures of striatal DA transmission: (1) D2/3 receptor BPND in the striatum at baseline; (2) the effect of endogenous DA released from DA neurons, measured as the percentage decrease in [11C]raclopride BPND between baseline and reward PET images (ΔBPND).
Method
Detailed descriptions of the subject sample, gambling task and the PET imaging methodology have been previously published (Martin-Soelch et al., 2011). Briefly, medically and psychiatrically-healthy volunteers [n=24, 11 males, aged 35±8 yr, (MADRS) score=0.56±1.5, 42% white, 25% black, 13% Hispanic, 17% Asian, 3% other] and medically healthy patients who met DSM-IV-TR criteria for MDD (current depressive episode; n=12, 4 males, aged 38±11yr, MADRS score= 24.5±7.3, 50% white, 25% black, 8% Hispanic, 8% Asian, 8% other) and who had not used tobacco for at least 1 yr, had no history of substance dependence, substance abuse within 1 yr or pathological gambling behaviour underwent PET-[11C]raclopride imaging using the bolus plus constant infusion method. Both structured (Structured Clinical Interview for the DSM-IV-TR) and unstructured (with a psychiatrist) psychiatric interviews were obtained on all participants. Subjects were excluded if they had taken psychotropic medications or other drugs likely to affect monoamine neurotransmitter function, cerebral physiology or vascular function within 3 wk of scanning (8 wk for fluoxetine). Subjects provided written informed consent after receiving a full explanation of the study procedures and risks, as approved by the National Institute of Mental Health Institutional Review Board.
Scanning was conducted on a GE-Advance scanner in 3D-mode (3D resolution=6 mm full-width at half-maximum). During the scan subjects completed a sensorimotor task (control condition) followed by a gambling task (reward condition). The task consisted of 180 trials, each of average duration 8 s. In the monetary-reward condition subjects received financial rewards unpredictably, in a pseudo-randomized order with an average of one reward per four trials. In the sensorimotor control condition subjects performed the same task without receiving rewards.
Using a region-of-interest (ROI) approach, mean tissue radioactivity concentrations in the anteroventral striatum, middle caudate and cerebellum from the baseline and reward images were calculated using MEDx software. Mean radioactivity in the reference region (cerebellum; C′) was used to control for the effects of free and non-specifically bound [11C]raclopride. The percentage change in [11C]raclopride binding was computed as the difference in BPND (C/C′−1 for each ROI) between baseline and reward images:
The rs1800497 SNP was genotyped using a TaqMan® assay (Life Technologies, USA) and was found to be in Hardy–Weinberg equilibrium in the combined sample (p=1.0), the healthy control sample (p=0.94) and the MDD group (p=0.98). The Illumina GoldenGate platform (Hodgkinson et al., 2008) was used to control for population stratification through the inclusion of 186 ancestry informative markers. To test whether ethnicity predicted D2 [11C]raclopride binding, we used genetic markers of African and European ancestry as predictors of [11C]raclopride binding in separate regression models. There was no effect of either African or European ancestry on baseline BPND of the ventral striatum and the middle caudate as well as ΔBPND of the ventral striatum and the middle caudate (all p values >0.1).
The effect of rs1800497 (A1/A1 vs. A1/A2 vs. A2/A2) on baseline [11C]raclopride BPND in the ventral striatum and middle caudate was assessed using a linear regression model controlling for the effects of age, sex and handedness. Because we previously showed that the Ser9Gly SNP in the D3 receptor gene affects [11C]raclopride BPND during reward-related DA release (but not baseline [11C]raclopride BPND; Savitz et al., 2013) we also controlled for Ser9Gly genotype (Ser/Ser vs. Ser/Gly vs. Gly/Gly) when testing the effect of rs1800497 on the ΔBPND.
Results
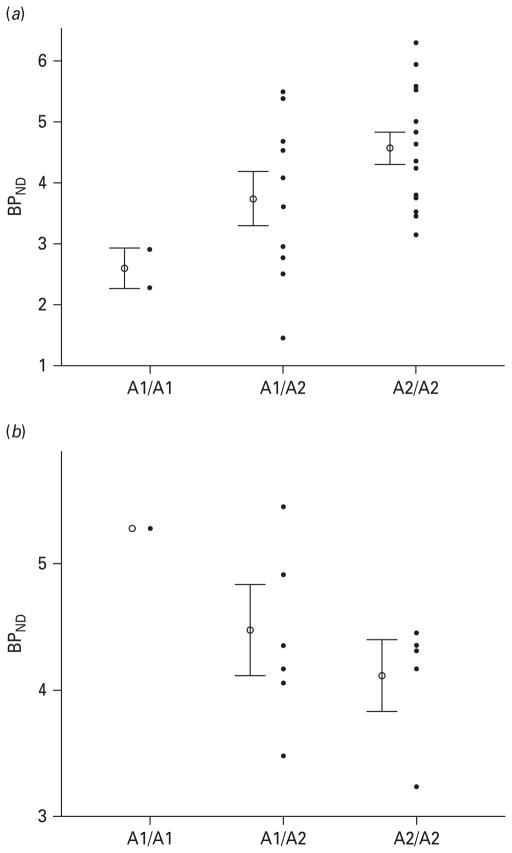
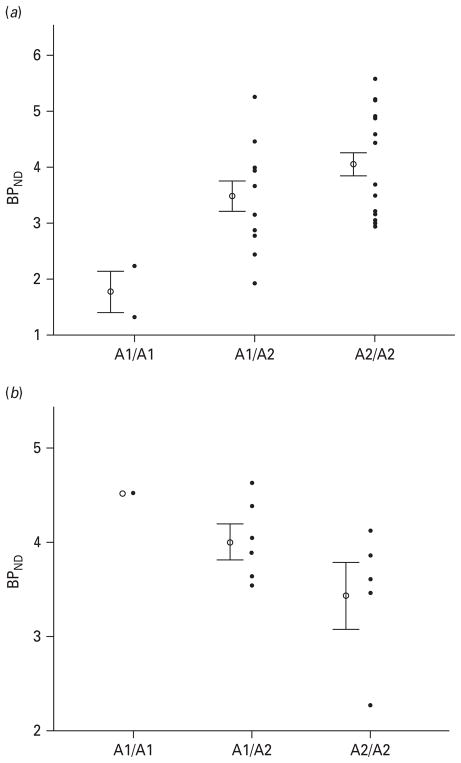
In healthy controls, the A1 allele of the Taq1A SNP was significantly associated with reduced baseline BPND in both the middle caudate (r2 explained=0.20, β-weight=0.47, t=2.9, p=0.009) and ventral striatum (r2 explained=0.20, β-weight=0.46, t=2.6, p=0.016) (Figs. 1 and 2). Conversely, in MDD patients the A1 allele was associated with increased baseline BPND in the middle caudate (r2 explained=0.40, β-weight= 0.65, t=2.6, p=0.033) with a trend towards significance in the ventral striatum (r2 explained=0.28, β-weight= 0.54, t=2.0, p=0.086; Figs. 1 and 2). Severity of depression and self-rated anhedonia were not associated with baseline BPND in the middle caudate or ventral striatum (all p values>0.1). There were no significant associations between Taq1A genotype and ΔBPND during reward-associated DA release in the middle caudate or ventral striatum in either the healthy control or the MDD groups (all p values >0.35).
Fig. 1.
(a) Scatterplot of baseline dopamine D2/3 receptor binding potential (BPND) in the ventral striatum of healthy controls stratified according to genotype. The error bars represent the standard error of the mean. The BPND mean± S.D. for each genotype is as follows: A1/A1=2.6±0.44; A1/A2 =3.8±1.32; A2/A2=4.5±0.98. (b) Scatterplot of baseline D2/3 receptor BPND in the ventral striatum of major depressive disorder patients stratified according to genotype. The error bars represent the standard error of the mean. The BPND mean± S.D. for each genotype is: A1/A1=5.3±0.0; A1/A2=4.4±0.69; A2/A2=4.1±0.50.
Fig. 2.
(a) Scatterplot of baseline dopamine D2/3 receptor binding potential (BPND) in the middle caudate of healthy controls stratified according to genotype. The error bars represent the standard error of the mean. The BPND mean± S.D. for each genotype is as follows: A1/A1=1.8±0.64; A1/A2 =3.5±1.0; A2/A2=4.0±0.96. (b) Scatterplot of baseline D2/3 receptor BPND in the middle caudate of major depressive disorder patients stratified according to genotype. The error bars represent the standard error of the mean. The BPND mean± S.D. for each genotype is: A1/A1=4.5; A1/A2=4.0±0.42; A2/A2=3.5±0.71.
Discussion
Our finding that the TaqA1 allele is associated with reduced D2/3 BPND at baseline in healthy controls replicates the results of two previous PET studies conducted on healthy volunteers reporting reduced availability of the D2/3 receptor in the striatum in A1 allele carriers (Pohjalainen et al., 1998; Jonsson et al., 1999). Our result is also potentially consistent with a more recent study demonstrating an effect of the DRD2 C957T SNP on striatal D2/3 BPND (Hirvonen et al., 2004). The mechanism underlying the contrasting effects of rs1800497 on baseline D2/3 receptor binding in MDD patients vs. healthy subjects remains unclear, but conceivably may reflect interactions between this SNP (or another SNP in LD with rs1800497) and other genetic or environmental factors associated with MDD. There is a precedent for this kind of gene×diagnosis interaction in the literature.
We previously reported that the T allele of the rs324650 SNP in the muscarinic 2 receptor gene was associated with increased M2 receptor binding in healthy controls but decreased M2 receptor binding in patients with bipolar disorder (Cannon et al., 2011). Extant evidence suggests that the short variant of the repeat length polymorphism in the serotonin transporter gene increases the risk for depression when coupled with psychosocial adversity. However, both human and non-human primates who carry the short allele display cognitive and social advantages over long allele homozygotes when raised in a benign environment, possibly because they are more sensitive to positive as well negative stimuli (reviewed in Homberg and Lesch, 2011). Given the Taq1A SNP× psychosocial environment interactions discussed earlier, we raise the possibility that a similar effect may hold for the Taq1A SNP and that this effect may be mediated by differences in D2 receptor density.
The finding that the Taq1A SNP had a significant effect on baseline D2/3 receptor BPND at baseline but not during reward-related DA release was surprising. Because post-synaptic D2 receptors outnumber D2 autoreceptors, the effect of the A1 allele on [11C]raclopride binding at rest would predominantly reflect post-synaptic D2 receptor density (Joyce and Marshall, 1987; Volkow et al., 1996). One possible explanation for the findings is that the Taq1A SNP is associated with decreased coupling of D2 receptors with post-synaptic effectors, such that the effect of a given level of D2 stimulation is diminished. Such a functional effect conceivably could account for the differential effects of the Taq1A SNP on DA D2/3 receptor binding between depressives and controls.
In controls, the decreased coupling of D2 receptors with post-synaptic effectors presumably would be compensated by increased baseline tonic DA concentrations, which could account for the decrease in baseline [11C]raclopride binding through a competition model (Laruelle, 2000). In depression, in contrast, it might be hypothesized that no compensation occurs, so the decreased tonic DA transmission (i.e. decreased number of DA neurons firing) combines with decreased D2 signalling efficacy to result in an even greater reduction in DA transmission, as reflected by increased baseline [11C]raclopride binding (as reviewed earlier) and the emergence of DA deficiency-driven anhedonia.
This hypothesis appears compatible with the extant data from PET studies of the uptake of [18F]fluorodopa ([18F]FDOPA), a radiolabelled analogue of the DA precursor L-DOPA. The uptake of [18F]FDOPA appears to correlate with the number of DA neurons engaged in firing activity (Howes et al., 2007). Laakso et al. (2005) reported that A1 allele carriers had significantly higher [18F]FDOPA uptake in the putamen than A2/A2 homozygotes, a finding that conceivably may reflect the compensatory increase in DA neuron activity that occurs to offset the reduction in D2 receptor signalling efficacy hypothesized. Although depressed subjects have not been studied with regard to the relationship between the Taq1A polymorphism and [18F]FDOPA uptake, it is noteworthy that preliminary studies of DA precursor uptake in depression have shown reductions in [11C]DOPA or [18F]FDOPA uptake in MDD samples (Agren and Reibring, 1994; Martinot et al., 2001), compatible with a reduction in the number of DA neurons firing. Future studies are needed which assess the differential effect of the Taq1A polymorphism on [18F]FDOPA uptake in MDD.
A number of limitations merit comment. The modest sample size may have led to type I or II error, particularly in the smaller MDD group. Large sample sizes are relatively uncommon in PET studies, yet PET has the advantage of allowing a particular molecular target to be assayed directly. It is therefore more likely that true signals can be detected with relatively small sample sizes, potentially explaining our replication of the effect of the Taq1A SNP on D2/3 binding in healthy controls.
Our study design does not allow for a rigorous delineation of the potentially divergent effects of the Taq1A SNP on pre-synaptic vs. post-synaptic D2 receptor binding. The D2 autoreceptor accounts for a proportion of the [11C]raclopride signal at baseline and likewise, the post-synaptic D2 receptor may effect DA release during reward via negative feedback mechanisms.
[11C]raclopride binds to both the D2 and the D3 receptor and thus we cannot exclude the possibility that DRD3 variants interact with the Taq1A SNP to influence D2 receptor function. Nevertheless, in vivo studies using [11C]-(+)-PHNO, a preferential D3 receptor antagonist, indicate that the D3 receptor accounts for <10% of the signal in the striatum and caudate at rest (Rabiner et al., 2009; Tziortzi et al., 2011) and in addition, we controlled for our previous finding (in an overlapping sample) that the Ser9Gly SNP affects D2/3 BPND during the reward task (Savitz et al., 2013) by co-varying for Ser9Gly genotype in the regression analysis.
In summary, we have replicated prior studies showing that in healthy controls the A1 allele of the TaqA1 SNP is associated with a reduction in [11C]raclopride binding at rest. In contrast, however, we found that the A1 allele was associated with increased [11C]raclopride BPND at baseline in depressed subjects with MDD. Our results could conceivably reconcile genetic studies which suggest that the A1 allele is a risk factor for depression and addiction disorders, and some PET studies which report an increase in D2/3 receptor binding in MDD and addiction disorders. Nevertheless, we did not observe a significant effect of the TaqA1 SNP on D2/3 receptor BPND during reward-related DA release. These differential effects on basal D2/3 receptor BPND vs. phasic DA release conceivably may reflect a greater effect of the TaqA1 SNP on post-synaptic compared to pre-synaptic D2 receptor function, or a reduction in the coupling of D2 receptors with post-synaptic effectors, such that the effect of a given level of D2 stimulation is diminished. Studies involving different experimental designs ultimately are needed to rigorously test these hypotheses.
Acknowledgments
This study was funded by the NIH/Division of Intramural Research Programs for the NIMH and the NIAAA. Drs Savitz and Drevets also received support from The William K. Warren Foundation. The Foundation played no role in the writing of the manuscript or in the decision to publish. We thank Jerry Jacobs and staff at the NIH Clinical Center PET Department for their support. We also thank Michele Drevets and Joan Collins, as well as the staff at 5SW for their clinical support. Finally, we acknowledge all those individuals who volunteered to take part in our studies.
Footnotes
Statement of Interest
W.C.D. is an employee of Johnson & Johnson, Inc. and has consulted for Myriad/Rules Based Medicine and Eisai, Inc. A.A.G. has consulted for Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche and Asubio.
References
- Agren H, Reibring L. PET studies of presynaptic monoamine metabolism in depressed patients and healthy volunteers. Pharmacopsychiatry. 1994;27:2–6. doi: 10.1055/s-2007-1014265. [DOI] [PubMed] [Google Scholar]
- Althaus M, Groen Y, Wijers AA, Mulder LJ, Minderaa RB, Kema IP, Dijck JD, Hartman CA, Hoekstra PJ. Differential effects of 5-HTTLPR and DRD2/ANKK1 polymorphisms on electrocortical measures of error and feedback processing in children. Clin Neurophysiol. 2009;120:93–107. doi: 10.1016/j.clinph.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Klaver JK, Gandhi SK, Solorio G, Peck SA, Erickson K, Akula N, Savitz J, Eckelman WC, Furey ML, Sahakian BJ, McMahon FJ, Drevets WC. Genetic variation in cholinergic muscarinic-2 receptor gene modulates M2 receptor binding in vivo and accounts for reduced binding in bipolar disorder. Mol Psychiatry. 2011;16:407–418. doi: 10.1038/mp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Grace AA. Chronic mild stress induces anxiety-like behavior and down-regulation of dopamine system activity in rats. Los Angeles: Society for Neuroscience New Orleans; 2012. [Google Scholar]
- Chen D, Liu F, Shang Q, Song X, Miao X, Wang Z. Association between polymorphisms of DRD2 and DRD4 and opioid dependence: evidence from the current studies. Am J Med Genet. 2011;156B:661–670. doi: 10.1002/ajmg.b.31208. [DOI] [PubMed] [Google Scholar]
- D’Haenen HA, Bossuyt A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiatry. 1994;35:128–132. doi: 10.1016/0006-3223(94)91202-5. [DOI] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PE, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Laptook RS, Dyson MW, Bufferd SJ, Durbin CE, Sheikh HI, Singh SM. The dopamine D2 receptor gene and depressive and anxious symptoms in childhood: associations and evidence for gene-environment correlation and gene-environment interaction. Psychiatr Genetc. 2010;20:304–310. doi: 10.1097/YPG.0b013e32833adccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen M, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry. 2004;9:1060–1061. doi: 10.1038/sj.mp.4001561. [DOI] [PubMed] [Google Scholar]
- Hirvonen MM, Lumme V, Hirvonen J, Pesonen U, Nagren K, Vahlberg T, Scheinin H, Hietala J. C957T polymorphism of the human dopamine D2 receptor gene predicts extrastriatal dopamine receptor availability in vivo. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:630–636. doi: 10.1016/j.pnpbp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Marshall JF. Quantitative autoradiography of dopamine D2 sites in rat caudateputamen: localization to intrinsic neurons and not to neocortical afferents. Neuroscience. 1987;20:773–795. doi: 10.1016/0306-4522(87)90240-5. [DOI] [PubMed] [Google Scholar]
- Kestler LP, Malhotra AK, Finch C, Adler C, Breier A. The relation between dopamine D2 receptor density and personality: preliminary evidence from the NEO personality inventory-revised. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:48–52. [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, Syvalahti E, Hietala J. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenet Genomics. 2005;15:387–391. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with comorbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Marino C, Vanzin L, Giorda R, Frigerio A, Lorusso ML, Nobile M, Molteni M, Battaglia M. An assessment of transmission disequilibrium between quantitative measures of childhood problem behaviors and DRD2/Taql and DRD4/48 bp-repeat polymorphisms. Behav Genet. 2004;34:495–502. doi: 10.1023/B:BEGE.0000038487.80597.7e. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, Carson RE, Drevets WC. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33:1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinot M, Bragulat V, Artiges E, Dolle F, Hinnen F, Jouvent R, Martinot J. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158:314–316. doi: 10.1176/appi.ajp.158.2.314. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, Wilson AA, Houle S. Elevated putamen D(2) receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry. 2006;163:1594–1602. doi: 10.1176/ajp.2006.163.9.1594. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper CB, Gariepy JL, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: the family system as the unit of analyses. Dev Psychopathol. 2007;19:1073–1087. doi: 10.1017/S0954579407000545. [DOI] [PubMed] [Google Scholar]
- Noble EP. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics. 2000;1:309–333. doi: 10.1517/14622416.1.3.309. [DOI] [PubMed] [Google Scholar]
- Noble EP, Gottschalk LA, Fallon JH, Ritchie TL, Wu JC. D2 dopamine receptor polymorphism and brain regional glucose metabolism. Am J Med Genet. 1997;74:162–166. [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, Carbone MA, Cox M. Gene-environment contributions to the development of infant vagal reactivity: the interaction of dopamine and maternal sensitivity. Child Dev. 2008;79:1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, Gunn RN, Laruelle MA. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Roetker NS, Yonker JA, Lee C, Chang V, Basson JJ, Roan CL, Hauser TS, Hauser RM, Atwood CS. Multigene interactions and the prediction of depression in the Wisconsin Longitudinal Study. BMJ Open. 2012;2:e000944. doi: 10.1136/bmjopen-2012-000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Hodgkinson CA, Martin-Soelch C, Shen PH, Szczepanik J, Nugent A, Herscovitch P, Grace AA, Goldman D, Drevets WC. The functional DRD3 Ser9Gly polymorphism (rs6280) is pleiotropic, affecting reward as well as movement. PLoS ONE. 2013;8:e54108. doi: 10.1371/journal.pone.0054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Neuroreceptor imaging in depression. Neurobiol Dis. 2013;52:49–65. doi: 10.1016/j.nbd.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med. 1997;27:1247–1256. doi: 10.1017/s0033291797005382. [DOI] [PubMed] [Google Scholar]
- Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: a HuGE gene-disease association review. Am J Epidemiol. 2008;167:125–138. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN, Court JA. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Logan J, Wang GJ, Ding YS, Dewey S. PET evaluation of the dopamine system of the human brain. J Nucl Med. 1996;37:1242–1256. [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Peng S, Zhang S, Zhang X. Stress-induced depressive behaviors are correlated with Par-4 and DRD2 expression in rat striatum. Behav Brain Res. 2011;223:329–335. doi: 10.1016/j.bbr.2011.04.052. [DOI] [PubMed] [Google Scholar]