Abstract
BACKGROUND
Oral fluid (OF) is a valuable biological alternative for clinical and forensic drug testing. Evaluating OF to plasma (OF/P) cannabinoid ratios provides important pharmacokinetic data on the disposition of drug and factors influencing partition between matrices.
METHODS
Eleven chronic cannabis smokers resided on a closed research unit for 51 days. There were four 5-day sessions of 0, 30, 60, and 120 mg oral Δ9-tetrahydrocannabinol (THC)/per day followed by a 5-puff smoked cannabis challenge on Day 5. Each session was separated by 9 days ad-libitum cannabis smoking. OF and plasma specimens were analyzed for THC and metabolites.
RESULTS
During ad-libitum smoking, OF/P THC ratios were high (median 6.1, range 0.2– 348.5) within 1 h after last smoking, decreasing to 0.1–20.7 (median 2.1) by 13.0–17.1 h. OF/P THC ratios also decreased during 5-days oral THC dosing, and after the smoked cannabis challenge, median OF/P THC ratios decreased from 1.4–5.5 (0.04–245.6) at 0.25 h to 0.12–0.17 (0.04–5.1) at 10.5 h post smoking. In other studies, longer exposure to more potent cannabis smoke and oromucosal cannabis spray was associated with increased OF/P THC peak ratios. Median OF/P 11-nor-9-carboxy-THC (THCCOOH) ratios were 0.3–2.5 (range 0.1–14.7) ng/µg, much more consistent in various dosing conditions over time.
CONCLUSIONS
OF/P THC, but not THCCOOH, ratios were significantly influenced by oral cavity contamination after smoking or oromucosal spray of cannabinoid products, followed by time-dependent decreases. Establishing relationships between OF and plasma cannabinoid concentrations is essential for making inferences of impairment or other clinical outcomes from OF concentrations.
Keywords: cannabis, marijuana, delta9-tetrahydrocannabinol, oral fluid, plasma, ratio
Introduction
Around the globe, cannabis is the most widely consumed illicit drug with an estimated 119–224 million users in 2010 [1]. Cannabis consumption is increasing in the US where the prevalence of current (past month) cannabis use increased from 5.8 to 7.0% between 2007 and 2011 [2]. In Europe and Australia, 3.6 and 5.6% of the general population reported using cannabis in the past month from 2008–10 [3] and 2010 [4], respectively.
Cannabis intoxication may induce a wide range of physiological and behavioral changes that can disrupt normal daily performance, including altered time perception, mood alteration, impaired learning, memory, and motor coordination, and lack of concentration [5]. As a result of these effects, cannabis is associated with loss of productivity in the workplace [6–7] and increased risk of work-related and road traffic accidents [7–10]. In clinical settings, problems related to cannabis use accounted for 18% of 2010 US substance abuse treatment admissions, the highest after alcohol and opiates [11], and also a major contributor to treatment demand worldwide [1,12]. Thus, there are clear circumstances under which monitoring cannabis consumption is a means for improving public health and safety.
Although urine and blood are commonly utilized for cannabinoid testing, the acceptance of oral fluid (OF) as an alternative matrix increased in the past 2 decades [13]. OF is an attractive drug testing matrix because the procedures for obtaining observed specimens are easier, safer, and less invasive compared with urine and blood. Another advantage is that OF has a strong linear correlation over time with blood, which is temporally associated with the pharmacological effects of cannabinoids [14–15]. Establishing relationships between OF and plasma cannabinoid concentrations is essential for using OF test results to make inferences of impairment or other clinical outcomes associated with cannabis intake.
Δ9-tetrahydrocannabinol (THC) is the primary psychoactive constituent of cannabis and also one of the main analytes detected in both OF and blood. Multiple factors affect transfer of drugs from blood to OF, including OF pH, flow rate, composition, a drug’s physicochemical properties (e.g., protein binding, pKa, lipophilicity, molecular weight), and sample collection method [16–17]. THC’s high lipophilicity results in extensive adsorption to oral mucosal membranes following cannabis smoking, with minimal partitioning into blood [18–19]. Several studies evaluated THC OF/plasma (OF/P) or OF/blood ratios. Samyn and van Haeren reported a 0.2–3.1 THC expectorated OF/P ratio range in 6 individuals suspected of intoxication [20]. In contrast, Kauert et al. found much higher mean (SD) OF/serum THC ratios of 46 (27) and 36 (20) within 6 h after smoking cannabis cigarettes containing 250 and 500 µg/kg body weight, respectively [21]; OF was collected with the Intercept® device and participants were recreational cannabis smokers (≥5 times in the previous 12 months). Wille et al. also reported high Intercept OF/blood THC ratios (n = 277) with a median (range) of 15.4 (0.01–568.9) in motorists suspected of driving under the influence of drugs (DUID), and drivers who were randomly stopped [18].
There are fewer OF/P cannabinoid data after oral THC dosing. To date, there is only one study, in which 37 doses of 20 mg oral THC were administered with increasing frequency over 8 days to 10 daily cannabis smokers; median (range) Quantisal™ OF/P THC ratios were 0.5 (0.03–12.0) on admission, decreased over time, and could not be determined by Day 2, due to few positive OF samples during oral THC dosing [22]. Oral THC capsules did not contaminate the oral mucosa, and OF THC concentrations, primarily from previously self-administered smoked cannabis, decreased over time [22]. Peak THC plasma concentrations occurred on Day 5 of dosing. In addition to THC, 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) metabolite concentrations may be useful for interpreting cannabinoid results and accompanying effects. In the oral THC study described [22], 11-OH-THC and THCCOOH plasma concentrations increased over time, whereas THC concentrations did not. Plasma metabolite accumulation could be due to more rapid THC oxidation than metabolite excretion [23]. Oral THC dosing increased both OF and plasma THCCOOH concentrations, leading to median OF/P THCCOOH ratios of 0.5–1 ng/µg over 8 days; the ng/µg units reflect 1000-fold concentration differences between OF and plasma THCCOOH [22]. Because THCCOOH results from THC metabolism and is not present in cannabis smoke [24], OF/P THCCOOH ratios after smoking could be similar to those after oral THC doses, but no study evaluated OF/P THCCOOH ratios after controlled smoking.
Variation in OF/P cannabinoid ratios across studies could have been influenced by differences in sample storage conditions that affect analyte stability, collection method, dose, route of administration, analytical sensitivity, and time interval between drug intake and sample collection. Furthermore, large inter-subject variability was repeatedly documented [22,18,21], arguing against mathematical conversion of OF cannabinoid results to plasma or blood cannabinoid concentrations.
In the present report, we evaluated the relationship between OF and plasma cannabinoid concentrations with respect to dose, route of administration and time after dosing. We characterized time courses of OF/P THC and THCCOOH ratios during short-term oral THC administration and following smoking of single and multiple cannabis cigarettes. These findings also were compared to prior research involving controlled administration of smoked cannabis, oral THC, and Sativex®.
Materials and Methods
Participants
Cannabis smokers, age 18 years or older, were recruited via newspaper advertisements and flyers distributed in the Baltimore area for a 51-day, within-subject study [25]. Inclusion criteria were self-reported cannabis smoking ≥25 days per month during the past 3 months, negative urine immunoassay test for drugs other than cannabinoids, negative breath alcohol test, and negative urine pregnancy test on admission, reported ≥2 cannabis withdrawal symptoms of at least moderate severity in prior periods of abstinence, and ≥8th grade level of education and demonstrated literacy. Participants were excluded if they received psychoactive medication; met clinical criteria for Axis I psychiatric disorders (DSM-IV-TR) other than cannabis or nicotine dependence; were seeking treatment for cannabis-related problems or using cannabis for medical purposes; or donated blood within 6 weeks of admission. Participants also were required to have no history of seizure, severe head trauma, dementia, or other condition associated with significant cognitive impairment, heart attack or major cardiac event in the prior 6 months, abnormal electrocardiogram or allergy to sesame oil (dronabinol capsule ingredient). The Johns Hopkins Medicine Institutional Review Board approved the study and participants provided written informed consent.
Study design
Participants resided on the closed Johns Hopkins Bayview Behavioral Pharmacology Research Unit to evaluate dronabinol (0, 30, 60, and 120 mg/day) effects on cannabis withdrawal, side effects, cognitive performance, and subjective and physiological responses to smoked cannabis challenge [25]. Briefly, the study design included a 4-day baseline to acclimate to the research unit and receive training on study procedures; ad libitum cannabis smoking was allowed from 12:00 to 23:00 h each day. Four 5-day oral THC sessions (Days 5–9, 19–23, 33–37, and 47–51) followed, during which oral synthetic THC was administered at 9:00, 14:00, and 19:00 h each day. Participants received in a counterbalanced order, 1 of 4 doses of oral THC: placebo (0 mg tid), 30 mg/day (10 mg tid), 60 mg/day (20 mg tid), or 120 mg/day (40 mg tid). Cannabis smoking was prohibited except on the 5th day (Days 9, 23, 37, and 51) when participants were challenged with 5 controlled puffs of smoked cannabis at approximately 11:30 h. The paced puff procedure consisted of 5 sec inhalation, 10 sec breath holding, and 40 sec inter-puff interval. Each oral THC session was separated by 9-days of ad libitum cannabis smoking between 12:00 and 23:00 h (Days 10–18, 24–32, and 38–46). Cannabis cigarettes for baseline, three ad libitum cannabis smoking sessions, and the smoked cannabis challenges were obtained from the National Institute on Drug Abuse; mean (SD) cannabis cigarette weight was 0.9 (0.07) g and contained 5.9 (0.3)% THC, 0.36 (0.04)% cannabinol (CBN), and 0.01 (0.00)% cannabidiol (CBD), yielding approximately 53.1, 3.2, and 0.1 mg per cigarette, respectively.
Current OF/P cannabinoid ratio data were compared to those determined in our previous research; in one study, we measured OF [26] and plasma [27] cannabinoid concentrations in 10 chronic cannabis smokers after smoking a single cannabis cigarette (6.8% THC) ad-libitum over 10 min. In another study, we evaluated OF [28] and plasma [29] cannabinoid disposition in 11 occasional cannabis smokers who received in random order 5 or 15 mg synthetic oral THC, 2 (5.4 mg THC + 5.0 mg CBN) or 6 (16.2 mg THC + 15.0 mg CBD) actuations of Sativex, or placebo oral THC and placebo Sativex.
Chemicals and reagents
THC, 11-OH-THC, THCCOOH, CBD, and CBN for calibrators and quality control samples and corresponding internal standards (THC-d3, 11-OH-THC-d3, THCCOOH-d3, CBD-d3) were purchased from Cerilliant (Round Rock, TX). N,O-Bis(trimethylsilyl) trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane was obtained from Thermo Fisher Scientific (Rockford, IL) for OF and from Regis Technologies (Morton Grove, IL) for plasma analysis. Trifluoroacetic anhydride (TFAA) and hexafluoroisopropanol (HFIP) were acquired from Campbell Science (Rockton, IL). CEREX® Polycrom™ THC (3 cc/35 mg) solid-phase extraction (SPE) columns were from SPEware (Baldwin Park, CA) for OF analysis and Clean Screen THC SPE columns (ZSTHC020) were from United Chemical Technologies (Bristol, PA) for plasma analysis.
Instrumentation
An Agilent 6890/7890 gas chromatograph was utilized, configured with Agilent 7683/7693 automated liquid sampler, microfluidic Deans switch, flame ionization detector, and interfaced to an Agilent 5973/5975 mass selective detector (Agilent Technologies, Wilmington DE) and quipped with a cryogenic focusing trap (Joint Analytical System, Marlton, NJ). The GC was equipped with a DB-1MS (Agilent Technologies) primary column (15 m × 0.25 mm i.d., 0.25 µm film thickness) and a ZB-50 (Phenomenex, Torrance, CA) secondary column (30 m x 0.32 mm i.d., 0.25 µm film thickness). The MSD was operated in electron ionization-selected ion monitoring (SIM) mode for THC, 11-OH-THC, and THCCOOH in plasma and THC, 11-OH-THC, CBD, and CBN in OF. The MSD for OF THCCOOH was operated in negative chemical ionization-SIM mode; pure ammonia (99.999%) was the reagent gas with a flow control setting of 35 (1.8 × 10−4 Torr).
Plasma and OF collection and analysis
Blood specimens were collected on ice in 6 mL sodium heparin Vacutainer® tubes, centrifuged within 2 h, and plasma separated and stored frozen at −20°C until analysis. Plasma THC, 11-OH-THC, and THCCOOH concentrations were analyzed by a previously published method [30]; minor changes were made to improve analysis productivity and the modified method’s performance was comparable to that of the original method. Briefly, 2 mL ice-cold acetonitrile was added drop wise to 1 mL plasma to precipitate proteins. Four mL sodium acetate buffer (2N, pH = 4.0) were added. SPE columns were conditioned with 1 mL elution solvent (hexane:ethyl acetate, 80:20), 3 mL methanol, 3 mL deionized water, and 2 mL 0.1 N hydrochloric acid. Buffered supernatants were added to conditioned columns. Columns were washed with 3 mL deionized water and 2 mL 0.1 N hydrochloric acid:acetonitrile (70:30) and dried by vacuum for 10 min. After priming columns with 0.2 mL hexane, analytes were eluted with 5 mL elution solvent into 10 mL centrifuge tubes containing 0.5 mL ethanol.. Eluates were dried under nitrogen at 40°C and derivatized with 25 µL BSTFA at 70°C for 30 min. Three µL was injected splitless onto 2-dimensional GCMS. Limits of quantification (LOQ) were 0.5 µg/L for THC and THCCOOH and 1.0 µg/L for 11-OH-THC. Electronic Supplemental Material Table S1 describes the plasma method modifications and resultant performance parameters.
OF (1±0.1 mL) was collected with the Quantisal™ device (Immunalysis, Pomona, CA), which contains 3 mL buffer. OF specimens were refrigerated for 24 h and then stored at −20°C until analysis. THC, CBD, CBN, 11-hydroxy-THC and THCCOOH in OF were quantified according to our previously published 2-dimensional GCMS method [31]; minor changes were made to improve analysis productivity and the modified method’s performance was comparable to that of the original method. In short, 1 mL cold acetonitrile was added to 1 mL Quantisal OF-buffer mixture. Supernatants were decanted onto SPE columns conditioned with 1 mL methanol and columns were washed with 3 mL deionized water/acetonitrile/ammonium hydroxide (85:15:1), dried under positive pressure, and primed with 0.4 mL hexane.. THC, CBD, CBN, and 11-OH-THC were eluted with 3 mL hexane/acetone/ethyl acetate (60:30:20), followed by THCCOOH elution into separate tubes with 3 mL hexane/ethyl acetate/glacial acetic acid (75:25:2.5). Eluates were dried under nitrogen at 35°C and derivatized with 20 µL HFIP and 40 µL TFAA for THCCOOH and 20 µL BSTFA for the other analytes at 65°C for 35 min. THCCOOH derivatives were evaporated and reconstituted in 20 µL toluene before GC-NCI-MS analysis. LOQs were 0.5 µg/L for THC and CBD, 1 µg/L for CBN and 11-OH-THC, and 15 ng/L for THCCOOH. Electronic Supplemental Material Table S2 describes the OF method modifications and resultant performance parameters.
Data analysis
Statistical analysis utilized Microsoft Excel 2007 and IBM SPSS version 20. OF/P THCCOOH ratios are presented in ng/µg units because OF THCCOOH concentrations were 1000-fold lower than plasma concentrations. Non-normal data distribution was determined by the Kolmogorov-Smirnov normality test and Normal Q-Q plot. Comparisons of OF/P ratios among different oral THC dosing sessions were evaluated with the Related-Samples Wilcoxon Signed Rank test. Correlations between the ratios and time and oral THC doses were determined with the nonparametric Spearman’s rho (ρ). Results with 2-tailed P <0.05 were considered significant.
Results
Eleven daily cannabis smokers (ages 22–52; 1 female) completed the 51-day study, and self-reported prior mean cannabis smoking of 3.7 (SD 1.4) times per day. The total amount of blood that could be collected in the study was restricted to 591 mL; 77 plasma and 169 OF specimens per participant were collected over the study. For each participant, OF/P pairs were scheduled for collection under 3 different dosing conditions (Table 1): 20 pairs were collected during ad-libitum cannabis smoking, 24 pairs were collected during oral THC maintenance, and 32 pairs were collected during combined oral THC maintenance with smoked cannabis challenge. Specimens positive in both plasma and OF were included in the ratio analysis. Eleven plasma and 11 OF specimen results were not obtained due to missed collection (N = 6), participant refusal for collection (N = 7), or loss during extraction (N = 9).
Table 1.
Study design for oral fluid (OF) and plasma (P) specimen collection.
| Study day | # OF/P pairs | Collection time, h | Treatment |
|---|---|---|---|
| 1 : 4 |
10 | 9, 11, 11:45, 12:30, 14, 15:30, 17, 19, 20:30, 22 | Ad-lib cannabis smoking |
| 5 : 9 |
4 2 + 8* |
9, 14, 19, 22 9, 11, 11:45*, 12:30, 14, 15:30, 17, 19, 20:30, 22 |
Oral THC tid |
| 10 : 18 |
10 | 9, 11, 11:45, 12:30, 14, 15:30, 17, 19, 20:30, 22 | Ad-lib cannabis smoking |
| 19 : 23 |
4 2 + 8* |
9, 14, 19, 22 9, 11, 11:45*, 12:30, 14, 15:30, 17, 19, 20:30, 22 |
Oral THC tid |
| 24 : 32 |
Ad-lib cannabis smoking |
||
| 33 : 37 |
4 2 + 8* |
9, 14, 19, 22 9, 11, 11:45*, 12:30, 14, 15:30, 17, 19, 20:30, 22 |
Oral THC tid |
| 38 : 46 |
Ad-lib cannabis smoking |
||
| 47 : 51 |
4 2 + 8* |
9, 14, 19, 22 9, 11, 11:45*, 12:30, 14, 15:30, 17, 19, 20:30, 22 |
Oral THC tid |
The first 4 days served as an acclimation and training period during which participants smoked cannabis ad-libitum from 12:00 to 23:00 h each day. Subsequently, participants (n = 11) received in counterbalanced order, 1 of 4 oral THC doses: placebo (0 mg tid), 30 (10 mg tid), 60 (20 mg tid), or 120 (40 mg tid) mg/day at 9:00, 14:00 and 22:00 h. Each of the 5-day oral THC sessions was separated by 9-days of ad libitum cannabis smoking from 12:00 to 23:00 h. On the last days of the oral THC sessions, 5 controlled puffs of smoked cannabis were administered approximately at 11:30 h
collected after smoked cannabis challenge.
Ad-libitum cannabis smoking
On the last day of each of the first 2 ad-libitum cannabis smoking sessions (Days 4 and 18), 207, 204, and 11 OF/P pairs were analyzed for THC, THCCOOH, and 11-OH-THC concentrations, respectively. Time since last smoking ranged from 0.1 to 17.1 h; data were divided into six Δtime intervals with approximately similar numbers of samples (N) (Figure 1). Participants could have several data points within an interval. OF/P THC ratios were generally higher <1 h post smoking (median 6.1, range 0.2–348.5, Figure 1A). Median ratios were relatively stable in the 5 time intervals from 1.0 to 17.1 h after smoking: 2.1–2.7 (range 0.04– 47.7). OF/P THC ratios were greater than 1 in 81% of specimens, likely because THC OF concentrations were elevated due to oral mucosal cannabis smoke contamination. Large inter-subject variability was observed (Figure 2), which precluded time-course estimation. OF/P THCCOOH ratios were lower and more stable (range for all specimens 0.2–7.9 ng/µg). Median OF/P THCCOOH ratios in all 6 Δtime intervals were similar, 0.8–1.0 ng/µg (Figure 1C); 57% of specimens had ratios ≤1 ng/µg. Δtimes were significantly correlated with OF/P THC ratios (ρ = − 0.265; P <0.001) but not for THCCOOH ratios (ρ = −0.120; P = 0.086).
Fig. 1.
Median oral fluid to plasma THC (A, B) and THCCOOH (C, D) ratios in 11 chronic cannabis smokers following multiple cannabis smoking episodes and single smoked cannabis preceded by 5-day 0, 30, 60, and 120 mg oral THC daily administrations
The ratios were plotted with respect to time since last smoking. During ad-libitum smoking sessions (A, C), participants could smoke cannabis from 12:00 – 23:00 h each day. A single participant may contribute multiple data points to a single interval based on their smoking frequency. During the smoked cannabis challenge, a participant inhaled 5 puffs of 5.9% THC according to a controlled, paced procedure. Data tables indicate the number of samples (A, C) or participants (B, D) included in each time point. Error bars represent interquartile ranges.
Fig. 2.
Individual oral fluid to plasma THC and THCCOOH ratios during multiple, ad libitum cannabis smoking
Total 207 (THC; A) and 204 (THCCOOH; B) pairs of oral fluid and plasma samples were collected from 11 chronic cannabis smokers. The ratios were plotted with respect to time since last smoking.
11-OH-THC detection in OF was rare. Only 5.9% of specimens (N = 13) were 11-OH-THC positive, with none positive more than 0.5 h after smoking. OF/P 11-OH-THC ratios were 0.1–0.7 (median 0.2), as 11-OH-THC plasma concentrations were always higher than OF concentrations.
Oral THC maintenance
Over 5-days maintenance at each oral THC dose, each participant provided 4 OF/P pairs on the 1st day (9:00, 14:00, 19:00, and 22:00) and 2 pairs on the 5th day (9:00 and 11:00) before the smoked cannabis challenge. The collection times corresponded to approximately 11, 16, 21, 24, 107, and 109 h since last smoking. A total of 39, 43, 40, and 42 pairs were available for OF/P THC ratio calculations and 58, 60, 60, and 60 pairs for OF/P THCCOOH ratio calculations in 0, 30, 60, and 120 mg oral THC sessions, respectively. Differences in sample size between OF/P THC and THCCOOH ratios were due to differences in the number of samples positive for each analyte in both matrices. OF/P 11-OH-THC ratios were not determined because no OF specimen was positive for 11-OH-THC after oral THC dosing.
OF/P THC ratio ranges during the 1st day of oral THC administration were comparable to those during ad-libitum smoking (Figure 3 A–D). Median ratios generally decreased over time on the 1st day from 2.6–7.8 (9:00 h) to 0.9–2.0 (14:00 h), 0.5–0.9 (19:00 h), and 0.4–1.8 (22:00 h). On the 5th day of oral THC maintenance, prior to the smoked cannabis challenge, OF/P THC ratios ranged from 0.02–0.2 (N = 5); oral THC doses contribute to plasma but not OF THC concentrations, leading to a low OF THC detection rate on the 5th day (6.0% in OF vs. 98.9% in plasma). OF/P THC ratios on the 1st day 9:00 h were significantly higher than later times on the 1st day in all sessions (P’s ≤0.028). During maintenance on the 60 mg/day dose, the ratios at 22:00 h were significantly lower than at all earlier time points that day. During maintenance on the 120 mg/day dose, the ratios decreased over time, with significant differences observed between 9:00 and 14:00 and 14:00 and 19:00. OF/P THC ratios on the 5th day could not be compared due to too few data points. OF/P THC ratios had a significant inverse correlation with time since last smoking (ρ = −0.452–0.666; P <0.002). There was no significant difference in OF/P THC ratios based on the active doses of oral THC maintenance (P’s ≥0.872). However, the OF/P THC ratios in the 120 mg dosing session were significantly lower than those during placebo administration (P = 0.036), likely because the high oral THC dose significantly increased plasma concentrations, while plasma THC concentrations decreased during placebo administration.
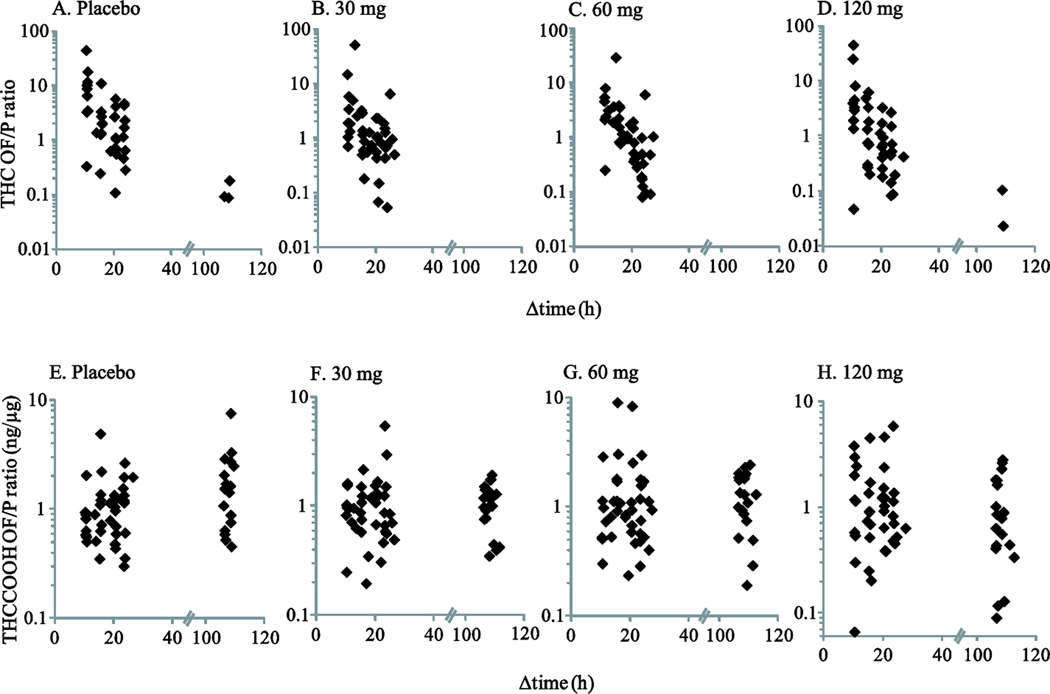
Fig 3.
Individual oral fluid to plasma THC (A–D) and THCCOOH (E–H) ratios in 11 chronic cannabis smokers during 5-day 0, 30, 60, and 120 mg oral THC daily administrations
Specimens were collected on the 1st day (9:00, 14:00, 19:00, and 22:00) and on the 5th day (9:00 and 11:00) before smoked cannabis challenge, which corresponded to approximately 11, 16, 21, 24, 107, and 109 h post smoking. The ratios were plotted with respect to time since last smoking.
In contrast to THC ratios, OF/P THCCOOH ratios remained relatively stable throughout the oral THC maintenance period with medians of 0.6–1.6 ng/µg (Figure 3E–H). During placebo maintenance, lower OF/P THCCOOH ratios were observed for specimens obtained on the 1st day at 9:00 h compared with those obtained at later times (except 19:00 h) on the 1st and at 9:00 and 11:00 h on the 5th day. The ratios reflect equilibrium between OF and plasma with a 1000-fold concentration difference, and that oral THC dosing contributed to both OF and plasma THCCOOH concentrations. OF/P THCCOOH was significantly and positively correlated with time since last smoking during placebo maintenance (ρ = 0.309, P = 0.018), but not during active oral THC maintenance (P’s ≥0.225). No significant differences in OF/P THCCOOH ratios were observed between active oral THC dose conditions (P’s ≥ 0.237), but the OF/P THCCOOH ratios obtained during 120 mg/day maintenance were significantly lower than those during placebo maintenance (P = 0.039), possibly because equilibrium between OF and plasma had not yet been reached with the high dose.
Oral THC maintenance with smoked cannabis challenge
After the smoked cannabis challenge at 11:30 h on the 5th day of each oral THC session, a total of 64, 70, 66, and 67 OF/P ratios were available for THC and 71, 87, 83, and 82 pairs for THCCOOH in 0, 30, 60, and 120 mg oral THC sessions, respectively, for 0.25–10.5 h post smoking. OF/P THC ratios exhibited significant inverse correlations with time since smoking (ρ = −0.423–0.720, P ≤0.001) in all 4 dose conditions; median (range) ratios decreased from 1.4–5.5 (0.04–245.6) at 0.25 h to 0.12–0.17 (0.04–5.1) at 10.5 h post smoking (Figure 1B). OF/P THC ratios were significantly different during the 30 mg oral THC maintenance condition compared with the 60 mg (P = 0.022) and 120 mg (P = 0.001) conditions; no other placebo and active dosing comparisons showed significant differences. Median ratios at 0.25 h were 1.4, 2.3, 4.1, and 5.5 in the 0, 30, 60, and 120 mg oral THC sessions, respectively. Unlike THC ratios, OF/P THCCOOH ratios were not significantly correlated with time (ρ = −0.053–0.187, P ≥0.118). OF/P THCCOOH ratios in the 30 mg session were significantly different from those in the 0 mg (P = 0.004) and 60 mg (P = 0.014) sessions; no other dosing comparisons showed significant differences. Median (range) ratios across time and dose stabilized to 0.7–2.0 (0.1–14.7) (Figure 1D). Large inter-subject variability was observed for both analyte ratios, but was more pronounced for THC (Figure 4).
Fig 4.
Individual oral fluid to plasma THC (A–D) and THCCOOH (E–H) ratios in 11 chronic cannabis smokers after smoked cannabis challenge on the last day of the 5-day 0, 30, 60, and 120 mg oral THC daily administrations
Participants inhaled 5 puffs of 5.9% THC according to a controlled, paced procedure. The ratios were plotted with respect to time since last smoking.
Cross-study comparisons
OF/P THC and THCCOOH ratios after smoking a single cannabis cigarette with slightly higher THC potency (6.8%) were calculated from our previously published OF [26] and plasma [14] pharmacokinetic data; participants smoked a cannabis cigarette ad-libitum over 10 min. Median OF/P THC ratios in that study peaked later (1 h post smoking; Figure 5A) compared with the peak at 0.2–0.6 h post-smoking in the present study (Figure 1B). Furthermore, the median peak ratios were higher (17.1 vs. 1.4–5.5) with longer exposure to more potent cannabis smoke. More extensive THC contamination of the oral cavity conceivably widened concentration and time-course differences between OF and plasma THC that subsequently led to higher OF/P THC ratios and a later maximum ratio time (Tmax). In contrast, OF/P THCCOOH ratios showed no time-dependent changes with medians similar to those after smoked cannabis challenge (0.9–2.5 vs. 0.7–2.0 ng/µg) over the 22 and 10.5 h collection periods, respectively.
Fig 5.
Median oral fluid to plasma THC (A, B) and THCCOOH (C, D) ratios after single smoked cannabis, oral THC, and Sativex administrations
These data are from our previously published oral fluid [26,32] and plasma [27,29] studies in which participants were administered single doses of smoked cannabis (6.8% THC), oral THC (5 or 15 mg), or Sativex [2 (low) or 6 (high) actuations]. The ratios were plotted with respect to time since last smoking. Data tables indicate the number of participants included in each time point. Error bars represent interquartile ranges.
OF/P THC and THCCOOH ratios also were evaluated after single 5 or 15 mg oral THC and 2 (5.4 mg THC + 5.0 mg CBD) or 6 (16.2 mg THC + 15.0 mg CBD) actuations of Sativex® administrations from our other published OF [32] and plasma [29] pharmacokinetic data. Sativex, a whole cannabis plant extract delivered via spray onto oral mucosa, extensively contaminates the oral cavity. This led to high THC OF concentrations, whereas encapsulated oral THC doses did not contaminate the cavity. OF THC after oral THC reflected previously self-administered smoked cannabis and the limited THC transfer from blood. Conversely, in plasma, there were no statistically significant differences in Cmax, Tmax, or AUC between similar Sativex or oral THC doses (2 actuations vs. 5 mg or 6 actuations vs. 15 mg) [29]. OF/P THC ratios, accordingly, were high after 2 and 6 actuations of Sativex with medians of 481.7 (range 166.6– 6964) and 2018 (479.4–14234), respectively, 0.25 h post dose decreasing to 30.1 (12.2–255.2) and 33.3 (9.1–280.0), respectively, 4.5 h post dose (Figure 5B). A significant dose-difference was observed at 0.25 (P = 0.046) and 1 (P = 0.017) h but not at 4.5–10.5 h. However after single oral THC doses, the OF/P THC ratios never exceeded 4.5 with medians of 0.2–2.2 (Figure 5B). Again, OF/P THCCOOH ratios reflected a stronger relationship between OF and plasma THCCOOH concentrations with medians of 0.3–0.9 (range 0.1–1.7 except 2 samples with 27.7 and 110.4, 0.25–1 h after Sativex) ng/µg and no change over time after single oral THC and Sativex doses (P’s ≥ 0.723) (Figure 5D).
Discussion
As expected during ad-libitum smoking sessions, OF/P THC ratios were highly elevated, up to 348.5, within 1 h after last smoking. The extensive oral cavity contamination from cannabis smoke resulted in high THC OF concentrations; however, 17.8% OF/P THC ratios were <1 within 1 h, yielding a median of 6.1 with large inter-subject variability. OF/P THC ratios decreased over time as oral mucosal THC contamination dissipated; the median (range) over 13.0–17.1 h post smoking was 2.1 (0.1–20.7), comparable to 0.5 (0.03–12.0) found on study admission after 17.5–21 h of cannabis abstinence [22]. While OF/P THC was significantly and inversely correlated with Δtime, OF/P THCCOOH was not, possibly because OF THCCOOH was not influenced by oral mucosal contamination. THCCOOH accordingly showed lower and narrower OF/P ratio ranges, suggesting stronger association between OF and plasma concentrations.
During 5 days of daily 0, 30, 60, or 120 mg oral THC administration, OF/P THC ratios decreased over time, as high OF THC concentrations from smoked cannabis contamination dissipated over time. Oral THC maintenance resulted in increased THC plasma, but not OF concentrations. As a result, OF/P THC ratios were significantly lower after 5 days of maintenance on the 120 mg/day dose compared with maintenance on placebo. OF/P THC ratios did not differ between active oral THC maintenance doses, or between placebo and the 30 and 60 mg THC doses, despite qualitative increase in plasma THC during maintenance on 30 and 60 mg THC. The lack of statistical significance in OF/P THC ratios between placebo and the two lower oral THC doses probably is due to large inter-subject variability in OF and plasma THC concentrations.
Milman et al. reported that despite around-the-clock oral THC dosing over 8 days, OF THC continued to decrease over time while OF THCCOOH concentrations increased similarly to plasma THCCOOH time course [22]. The present study also found that OF THC decreased even during up to 120 mg/day oral THC maintenance, whereas OF THCCOOH decreased in the placebo but not active dosing sessions. These data indicate that THC transfer from plasma is a minor contribution to overall OF THC concentrations. Thus, OF/P THC ratios conceivably reflect differences in THC concentration time profiles in the two matrices more than equilibrium between the two matrices. Although mean THC elimination half-lives were similar in OF and plasma/serum (1.5–1.6 h vs. 1.4–1.9 h) [21,33–34], the primary sources of OF and plasma THC differed during oral THC maintenance. Oral cavity contamination from prior smoked cannabis was the major contributor to OF THC, but not plasma THC, whereas, oral THC dosing was the major contributor to plasma THC but not OF THC.
Alternatively, the THC elimination rate from plasma may be too short to fully achieve equilibrium with OF. Plasma THC elimination half-lives of 117 and 93 min were reported, shorter than THCCOOH half-lives of 5.2 and 6.2 days in frequent and infrequent users, respectively [33]. Furthermore, plasma THC did not accumulate over 8 days of multiple 20 mg oral THC dosing, but THCCOOH did [23]. Additional research is needed to characterize the extent of OF THC transfer from blood. One approach could include administering multiple oral THC doses to individuals with negative OF and plasma cannabinoid concentrations, and analyzing their OF samples for THC with a low ng/L LOQ.
OF/P THCCOOH ratios better represented the equilibrium between OF and plasma because of the lack of contribution from oral cavity contamination and the 1000-fold lower LOQ for OF THCCOOH. OF/P THCCOOH ratios were consistent throughout active oral THC maintenance without significant correlation with time since last smoking. In contrast, there was a significant positive correlation between OF/P THCCOOH ratios and time since last smoking in the placebo session, implying that THCCOOH elimination rate is slower in OF than plasma. OF THCCOOH was suggested as a possible long-term cannabis marker in chronic smokers [35].
After smoked cannabis challenge on the 5th day of oral THC maintenance, OF/P THC and THCCOOH ratios followed similar time courses to those observed during ad-libitum cannabis smoking; OF/P THC ratios exhibited a significant inverse correlation with time since last smoking but OF/P THCCOOH did not. OF/P THC ratios in the 30 mg session were overall higher 0.8–9.8 h post smoking than those in the 60 and 120 mg sessions (Figure 1B), likely due to smaller oral THC contribution to plasma THC concentrations. Surprisingly, OF/P THCCOOH ratios in the 30 mg session were significantly different from those in the 0 and 60 mg sessions, although ratio changes over time were not significant in all dosing sessions (Figure 1D). Collective influence of THC dose, small study size for multiple comparisons, and large inter-subject variability in OF and plasma cannabinoid concentrations may have been responsible for this finding.
11-OH-THC disposition in OF has not been clearly elucidated due to lack of an analytical method sensitive enough to detect 11-OH-THC at ng/L OF concentrations [26,28,36]. With the present method’s 11-OH-THC LOQ of 1 µg/L, only 5.9% of samples were positive within 0.5 h of ad-libitum smoking and none during oral THC administration. As a metabolite, 11-OH-THC is likely present in OF at concentrations similar to those of THCCOOH, although the time course could be different. As peak plasma 11-OH-THC concentrations after smoking were 6–10% of that of THC [37] and approximately equal after oral THC intake owing to first-pass metabolism [29], OF/P 11-OH-THC may also differentiate smoked and oral administrations.
Current study results were compared with ratio data determined from our previous OF [26,32] and plasma [27,29] studies to further evaluate effects of different doses and drug delivery systems. Smoking a slightly more potent (6.8 vs. 5.9%) THC cigarette for a longer period (10 vs. 3.9 min) led to higher median peak OF/P THC ratios and Tmax (Figure 5A). The sudden drop in OF/P THC and THCCOOH ratios 3 h post smoking (Figure 5A, C) was possibly due to having lunch 2.5 h post smoking, which may have reduced OF cannabinoid concentrations [26]. Oromucosal Sativex extensively contaminated the oral cavity that led to high OF/P THC ratios. Interestingly, there was a significant difference in OF/P THC ratios between low- and high-dose Sativex only within 1 h post dose. Kauert et al. found that mean (range) OF THC and serum THC elimination half-lives of 1.5 (1–3) and 1.4 (1–2) h, respectively, were not significantly different between two smoked cannabis dosing groups [21]. While these authors also did not find significant differences in maximum OF and plasma THC concentrations after smoking [21], we did after low- and high-dose Sativex [32,29], which may explain the initial dose-dependent difference in OF/P THC ratios. These previous data confirmed that OF/P THCCOOH ratios were not influenced by time or routes of administration with medians of 0.3–2.5. Two OF/P pairs 0.25 and 1 h after Sativex showed unusually high OF/P THCCOOH ratios of 27.7 and 110.4 ng/µg, respectively; in both participants, OF THCCOOH peaked 0.25 h post dose (19.6 and 184.6 ng/L) while plasma THCCOOH peaked at 4.5 and 7.5 h (2.0 and 36.4 µg/L). Plasma THCCOOH concentrations were only 0.7 µg/L 0.25 and 1 h post dosing, leading to high OF/P THCCOOH ratios at those times.
These findings indicate that while high OF/P THC ratios (>10) may document recent smoked cannabis or Sativex intake within approximately 15 h, lower OF/P THC ratios do not exclude recent intake as ratios <1 can occur even within 1 h post smoking. The window of high OF/P THC ratios potentially encompasses the window of acute cannabis impairment, which may last for 3–6 h [7,15,38]. This could be useful for DUID and post-accident investigations when both matrices are collected; however, the effects of Δtime, route of administration, dose, and inter-subject variability limits the interpretive value of OF/P THC ratios. Direct prediction of plasma THC concentrations from OF concentrations is not appropriate regardless of drug delivery system. On the other hand, OF THCCOOH could estimate plasma THCCOOH concentrations, but it is an inactive metabolite and will not reflect performance impairment. These data also provide valuable information for understanding mechanisms of cannabinoids transfer from blood to OF.
Supplementary Material
Acknowledgements
We acknowledge Sebastien Anizan and Marisol Castaneto for analytical assistance in data collection; Erin Karschner and David Schwope for plasma data in cross-study comparisons; and contributions from the clinical staff at the NIDA Intramural Research Program and Johns Hopkins Behavioral Pharmacology Research Unit.
Research Funding
This research was funded by grant R01 DA025044 from the National Institute on Drug Abuse and by the Intramural Research Program, National Institute on Drug Abuse, NIH. The funding sources had no role in study design, data collection and analysis, or presentation of results. This study was registered on clinicaltrials.gov (NCT00893074).
Abbreviations
- OF
Oral fluid
- THC
Δ9-tetrahydrocannabinol
- OF/P
Oral fluid to plasma
- DUID
Driving under the influence of drugs
- THCCOOH
11-nor-9-carboxy-THC
- CBN
Cannabinol
- CBD
Cannabidiol
- 11-OH-THC
11-hydroxy-THC
- LOQ
Limit of quantification
- Tid
Ter in die (three times a day)
References
- 1.United Naitons Office on Drugs and Crime. World Drug Report 2012. Vienna, Austria: United Nations Publication, Sales No. E.12.XI.1; 2012. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: NSDUH Series H-44, HHS Publication No. (SMA) 12–4713; 2012. [Google Scholar]
- 3.European Monitoring Centre for Drugs and Drug Addiction. Annual Report 2012. Publications Office of the European Union. Luxembourg: The State of the Drugs Problem in Europe; 2012. [Google Scholar]
- 4.Australian Institute of Health and Welfare. 2010 National Drug Strategy Household Survey report. Canberra: Drug statistics series no. 25. Cat. no. PHE 145; 2011. [Google Scholar]
- 5.Huestis MA (2002) Cannabis (marijuana) - effects on human behavior and performance. The Effects of Drugs on Human Performance and behavior. Taipei: Central Police University Press; [Google Scholar]
- 6.Wadsworth EJ, Moss SC, Simpson SA, Smith AP. Cannabis use, cognitive performance and mood in a sample of workers. J Psychopharmacol. 2006;20:14–23. doi: 10.1177/0269881105056644. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald S, Hall W, Roman P, Stockwell T, Coghlan M, Nesvaag S. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408–416. doi: 10.1111/j.1360-0443.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Baker SP, Zhao Q, Brady JE, Lang BH, Rebok GW, DiMaggio C. Drug violations and aviation accidents: findings from the US mandatory drug testing programs. Addiction. 2011;106:1287–1292. doi: 10.1111/j.1360-0443.2011.03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li MC, Brady JE, DiMaggio CJ, Lusardi AR, Tzong KY, Li G. Marijuana use and motor vehicle crashes. Epidemiol Rev. 2012;34:65–72. doi: 10.1093/epirev/mxr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartman RL, Huestis MA. Cannabis Effects on Driving Skills. Clin Chem. 2012;59:478–492. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TED) 2000–2010. Rockville, MD: National Admissions to Substance Abuse Treatment Services. DASIS Series S-61, HHS Publication No. (SMA) 12-4701; 2012. [Google Scholar]
- 12.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 13.Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwope DM, Bosker WM, Ramaekers JG, Gorelick DA, Huestis MA. Psychomotor performance, subjective and physiological effects and whole blood Δ9-tetrahydrocannabinol concentrations in heavy, chronic cannabis smokers following acute smoked cannabis. J Anal Toxicol. 2012;36:405–412. doi: 10.1093/jat/bks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006;85:114–122. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Aps JK, Martens LC. Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci Int. 2005;150:119–131. doi: 10.1016/j.forsciint.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Gallardo E, Barroso M, Queiroz JA. Current technologies and considerations for drug bioanalysis in oral fluid. Bioanalysis. 2009;1:637–667. doi: 10.4155/bio.09.23. [DOI] [PubMed] [Google Scholar]
- 18.Wille SM, Raes E, Lillsunde P, Gunnar T, Laloup M, Samyn N, Christophersen AS, Moeller MR, Hammer KP, Verstraete AG. Relationship between oral fluid and blood concentrations of drugs of abuse in drivers suspected of driving under the influence of drugs. Ther Drug Monit. 2009;31:511–519. doi: 10.1097/FTD.0b013e3181ae46ea. [DOI] [PubMed] [Google Scholar]
- 19.Drummer OH. Review: Pharmacokinetics of illicit drugs in oral fluid. Forensic Sci Int. 2005;150:133–142. doi: 10.1016/j.forsciint.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Samyn N, van Haeren C. On-site testing of saliva and sweat with Drugwipe and determination of concentrations of drugs of abuse in saliva, plasma and urine of suspected users. Int J Legal Med. 2000;113:150–154. doi: 10.1007/s004140050287. [DOI] [PubMed] [Google Scholar]
- 21.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288–293. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 22.Milman G, Schwope DM, Schwilke EW, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA. Oral fluid and plasma cannabinoid ratios after around-the-clock controlled oral delta9-tetrahydrocannabinol administration. Clin Chem. 2011;57:1597–1606. doi: 10.1373/clinchem.2011.169490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem. 2009;55:2180–2189. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day D, Kuntz DJ, Feldman M, Presley L. Detection of THCA in oral fluid by GC-MS-MS. J Anal Toxicol. 2006;30:645–650. doi: 10.1093/jat/30.9.645. [DOI] [PubMed] [Google Scholar]
- 25.Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, Lee D. The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend. 2012;128:64–70. doi: 10.1016/j.drugalcdep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin Chem. 2012;58:748–756. doi: 10.1373/clinchem.2011.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57:1406–1414. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee D, Karschner EL, Milman G, Barnes AJ, Goodwin RS, Huestis MA. Can oral fluid cannabinoid testing monitor medication compliance and/or cannabis smoking during oral THC and oromucosal Sativex administration? Drug Alcohol Depend. 2013;130:68–76. doi: 10.1016/j.drugalcdep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66–75. doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe RH, Karschner EL, Schwilke EW, Barnes AJ, Huestis MA. Simultaneous quantification of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. J Chromatogr A. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chromatogr A. 2010;1217:1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D, Karschner EL, Milman G, Barnes AJ, Goodwin RS, Huestis MA. Can oral fluid cannabinoid testing monitor medication compliance and/or cannabis smoking during oral THC and oromucosal Sativex administration? Drug Alcohol Depend In press. 2012 doi: 10.1016/j.drugalcdep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly P, Jones RT. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. J Anal Toxicol. 1992;16:228–235. doi: 10.1093/jat/16.4.228. [DOI] [PubMed] [Google Scholar]
- 34.Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Pharmacokinetic properties of delta9-tetrahydrocannabinol in oral fluid of occasional and chronic users. J Anal Toxicol. 2010;34:216–221. doi: 10.1093/jat/34.4.216. [DOI] [PubMed] [Google Scholar]
- 35.Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily Cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–1136. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- 36.Milman G, Barnes AJ, Schwope DM, Schwilke EW, Darwin WD, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56:1261–1269. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 38.Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109–119. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.