Abstract
Objective
To evaluate the risk of cancer in patients with heart failure (HF) compared to community controls and its impact on outcome.
Background
HF is associated with excess morbidity and mortality. Non-cardiac causes of adverse outcomes in HF are increasingly recognized but not fully characterized.
Methods
In a case-control study, we compared history of cancer among community subjects newly diagnosed with HF from 1979–2002 to age-, sex-, and date-matched community controls without HF (961 pairs). Individuals without cancer at index (596 pairs) were followed for cancer in a cohort design, and the survival of HF patients who developed cancer was assessed.
Results
Before index, 22% of HF cases and 23% of controls had a history of cancer (odds ratio 0.94; 95% CI 0.75–1.17). During 9,203 person-years of follow-up [mean (SD), 7.7 (6.4) years], 244 new cancer cases were identified; HF patients had a 68% higher risk of developing cancer [hazard ratio (HR) 1.68; 95% CI 1.13–2.50] adjusted for body mass index, smoking and comorbidities. The HRs were similar for men and women with a trend toward a stronger association among subjects ≤75 years (P=0.22) and during the most recent time period (P=0.075). Among HF cases, incident cancer increased the risk of death (HR 1.56; 95% CI 1.22–1.99) adjusted for age, sex, year and comorbidities.
Conclusions
HF patients are at increased risk of cancer, which appears to have increased over time. Cancer increases mortality in HF underscoring the importance of non-cardiac morbidity and of cancer surveillance in the management of HF patients.
Keywords: heart failure, cancer, risk, epidemiology, follow-up studies
Introduction
Heart failure (HF) is a major cause of morbidity and mortality (1). As survival among patients with HF improves and the incidence remains the same (2), the prevalence of HF is increasing (3). While patients with HF have increased morbidity and mortality compared to disease-free individuals, the causes for these are often non-cardiac. Indeed, non-cardiovascular causes for hospital readmissions after HF diagnosis are more common than cardiovascular (4), and mortality in patients with HF is commonly attributed to non-cardiovascular causes, particularly if the ejection fraction (EF) is preserved (5). While these data are thought-provoking, they typically reflect the evaluation of comorbidity using composite indices (6), such that individual comorbid conditions associated with HF are not well delineated, the excess burden of a given comorbidity in HF compared to HF-free subjects is not known, and the impact on the outcome of a disease already characterized by poor survival is unknown. These gaps in knowledge underscore that non-cardiac causes of morbidity in HF warrant further investigation.
Cancer is a major cause of morbidity in the population. A total of 1,638,910 new cancer cases and 577,190 deaths from cancer are projected to occur in the United States in 2012 (7). Yet the link between HF and cancer is not well characterized. In particular, it is not known whether cancer is associated with a risk of developing HF compared to matched controls and whether HF is associated with an excess risk of cancer post-HF compared to HF-free individuals. Further, the impact of cancer on the outcome of HF is not well delineated. These questions are important as therapies for advanced HF are expanding in use with its associated patient and societal burden (8).
We hypothesized there may be an increased risk of cancer among HF patients. Therefore, the aims of our study were to assess the association between cancer and risk of HF, measure the excess risk of cancer among HF patients and determine its impact on survival in a community population of optimal clinical relevance.
Methods
Study Setting
This study was conducted in Olmsted County, Minnesota, under the auspices of the Rochester Epidemiology Project. As previously described, Olmsted County is isolated from other urban centers, and thus only a few providers deliver nearly all medical care to local residents (9). The medical records from these providers are indexed through the Rochester Epidemiology Project resulting in the linkage of in- and outpatient records from all sources of care used by the population, therefore providing a unique infrastructure to analyze disease determinants and outcomes (9).
Study Design
This study was carried out in two stages. First, a case-control study was performed with newly diagnosed HF patients serving as cases and subjects free of HF, selected from the general population, serving as the controls. Prior cancer history (verified by dates, classified by cancer site) was the primary exposure of interest. Known risk factors for HF or cancer were considered confounding factors. Second, the HF patients and their matched controls were followed to compare their long-term risk of incident cancer using a cohort design. The study was approved by the appropriate Institutional Review Boards.
Selection of Cases and Controls
Case subjects were Olmsted County residents with an incident diagnosis of HF between 1979 and 2002. As previously described (2), HF was defined using the Framingham criteria (10).
Control subjects were selected from the same county population. Controls were individually matched (1:1) to cases on age (±3 years), sex and date. The index date for the control corresponds to the incidence date of the matched HF case. Potential controls with HF prior to the index date were excluded.
In any 3-year period, over 90% of residents are seen at Mayo Clinic, and the majority are attended annually by some local health care provider (9). Thus, the Rochester Epidemiology Project medical records linkage system provides a virtually complete enumeration of the population from which to sample controls. Since information on exposures prior to the index date was obtained from these community medical records, this ensures similar opportunities for ascertainment of risk factors in the two groups and avoids biases inherent in many case-control studies (e.g., differential recall, non-response bias, and survivor bias).
Follow-up
Cases and controls were subsequently followed through the medical records. Follow-up began at the index date and lasted until death or the most recent clinical contact, whichever came first (last follow up, January 2012). In the cohort, 92% of the HF cases and 89% of those without HF (p=0.09) stayed in Olmsted County or within a 30-mile radius, and thus continued to receive their care in Olmsted County. As previously described, death was ascertained using multiple sources (2).
Cancer Data
Each subject's complete medical history was searched for the occurrence of any cancer through the comprehensive diagnostic and surgical indices that are part of the Rochester Epidemiology Project (9). Cancer types were classified by anatomic and system primary involvement (11); non-melanoma skin cancers were excluded from the study. The date of first cancer diagnosis was used as the diagnosis date.
Clinical Characteristics
Nurse abstractors collected clinical data from the medical record at the time when the cases and controls were assembled. Myocardial infarction was ascertained using a clinical diagnosis. The National Diabetes Data Group criteria (12) was used to define diabetes. Clinical definitions were used to assess hypertension and dyslipidemia. Body mass index (BMI, kg/m2) was calculated using the last weight prior to HF diagnosis and earliest adult height. Smoking was dichotomized as current (at time of evaluation or within the previous 6 months) versus not current smoker. Comorbidity was summarized with the Charlson comorbidity index (6). Ejection fraction (EF) was derived from echocardiography reports at time of diagnosis of HF (±90 days) and dichotomized into “reduced” (<50%) or “preserved” (≥50%).
Statistical Analysis
For the case-control study, a matched analysis was conducted. Baseline characteristics and prior cancer were compared between cases and controls using conditional logistic regression with the matching identification number as the stratification variable.
For the cohort study, the risk of developing incident cancer in the HF patients during follow-up was directly compared to their matched controls using a stratified proportional hazards regression model with the strata being the case/control pairs. Multivariable adjustment was made for suspected risk factors for cancer. Stratified analyses by age, gender and index year were also performed. Differences in age, gender and index year were tested by including HF×age, HF×gender and HF×year interaction terms in the model individually. Differences in the associations between HF and cancer subtypes were tested and were not statistically significant. Among HF patients with EF measured, the association between EF and incident cancer was assessed with a proportional hazards regression model. The cumulative incidence of cancer for up to 10 years following index was plotted with death as a competing event (13). Death as a competing risk should be accounted for since the traditional Kaplan-Meier method could substantially overestimate the cumulative cancer incidence in the presence of strong competing risks.
Finally, survival among HF cases and among controls was assessed with the Kaplan-Meier method. The association between cancer and death was evaluated using proportional hazards modeling with cancer treated as a time-dependent variable.
The proportional hazards assumption was tested for unadjusted and adjusted models and found to be valid. A pre-specified P value of 0.05 was used as the cutoff for statistical significance except when testing interactions, when a pre-specified P value of 0.10 was used. Analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC) and R 2.14.0 (The R Foundation for Statistical Computing).
Results
Clinical Characteristics at Index
The study included 961 patients with incident HF (mean [SD] age: 75.5 [12.7] years; 54% women) and 961 matched controls. Subject characteristics by HF status are presented in Table 1. HF patients had a higher frequency of prior myocardial infarction as well as traditional cardiovascular risk factors including hypertension, diabetes and smoking but not hyperlipidemia. In addition, they had higher mean BMI and more comorbidities than controls. Before the index date of HF diagnosis, 209 (22%) HF patients and 219 (23%) controls had a history of cancer recorded. No association existed between cancer diagnosis and development of subsequent HF (odds ratio 0.94; 95% CI: 0.75–1.17).
Table 1.
Patient characteristics among Olmsted County, Minnesota residents with incident heart failure diagnosed in 1979–2002 compared with age-, sex-, and year-matched community controls, stratified by study design.
| Characteristic | Case-Control |
P | Cohort |
P | ||
|---|---|---|---|---|---|---|
| HF | Control | HF | Control | |||
| n | 961 | 961 | 596 | 596 | ||
| Age, years, mean (SD) | 75 (13) | 75 (13) | 0.98 | 73 (14) | 73 (14) | 0.54 |
| Female, (%) | 517 (54) | 517 (54) | 1.00 | 317 (53) | 317 (53) | 1.00 |
| Body mass index, mean (SD) | 26.9(6.4) | 25.4 (6.5) | <0.00 | 27.5 | 25.6 | <0.001 |
| 1 | (6.6) | (7.3) | ||||
| Prior myocardial infarction, n (%) | 208 (22) | 80 (8) | <0.00 | 125 (21) | 38 (6) | <0.001 |
| 1 | ||||||
| Hypertension, n (%) | 643 (67) | 563 (59) | <0.00 | 401 (67) | 322 (54) | <0.001 |
| 1 | ||||||
| Hyperlipidemia, n (%) | 279 (29) | 302 (31) | 0.21 | 174 (29) | 177 (30) | 0.83 |
| Diabetes mellitus, n (%) | 181 (19) | 78 (8) | <0.00 | 116 (20) | 47 (8) | <0.001 |
| 1 | ||||||
| Current smoking, n (%) | 155 (16) | 84 (9) | <0.00 | 109 (19) | 54 (9) | <0.001 |
| 1 | ||||||
| Peripheral vascular disease, n (%) | 199 (21) | 121 (13) | <0.00 | 116 (20) | 74 (12) | 0.001 |
| 1 | ||||||
| Dementia, n (%) | 64 (7) | 99 (10) | 0.003 | 30 (5) | 54 (9) | 0.006 |
| Chronic obstructive pulmonary disease, n (%) | 198 (21) | 114 (12) | <0.00 | 111 (19) | 63 (11) | <0.001 |
| 1 | ||||||
| Renal dysfunction, n (%) | 33 (3) | 18 (2) | 0.04 | 15(3) | 11(2) | 0.42 |
| Charlson comorbidity index | ||||||
| 0 | 245(25) | 394(41) | <0.00 | 16(27) | 266(45) | <0.001 |
| 1–2 | 476(50) | 444(46) | 1 | 300(50) | 260(44) | |
| ≥3 | 240(25) | 123(13) | 135(23) | 70(12) | ||
Heart Failure and Subsequent Cancer Risk
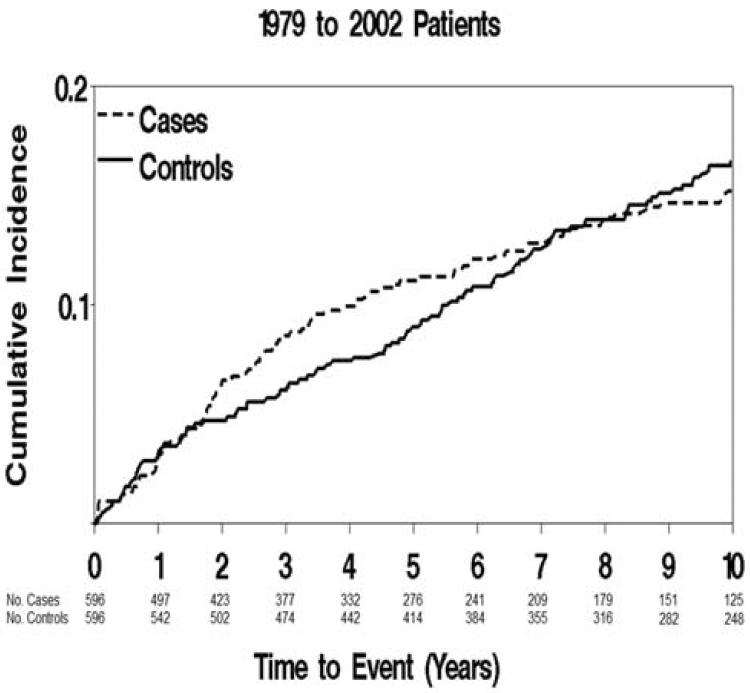
To investigate the incidence of cancer among HF patients, we excluded case-control pairs where either the case or control had a prior cancer diagnosis, resulting in 596 pairs in the cohort analysis. Their characteristics (Table 1) were similar to the initial case-control groups. During 9,203 person-years of follow-up (mean [SD] follow-up: 7.7 [6.4] years), 244 new cancer cases were identified (102 among HF patients and 142 among controls). The cumulative incidence of cancer among HF patients and matched controls is shown in Figure 1. The incidence of cancer between HF patients and controls was similar initially, but diverged after 2 years of follow-up, with higher rates among the HF patients. Of the 244 cancers, 48 were digestive system cancers; 46 male reproductive; 39 hematologic; 24 breast; 20 respiratory; 19 urinary; 7 female reproductive; 7 skin and 34 other cancers.
Figure 1. Incidence of Cancer among HF Patients and Controls.
Cumulative incidence of cancer among HF patients and matched controls with death considered a competing event.
Patients with HF had a 60% higher risk of developing incident cancer (HR 1.60; 95% CI 1.14–2.26) compared to controls and accounting for the matching variables. This remained unchanged after further adjustment for BMI, smoking, and Charlson comorbidity index (HR 1.68; 95% CI 1.13–2.50). Adding former smoking to the model did not change the HR estimate (1.67; 95% CI 1.12– 2.50), and no smoking×HF status interaction was detected (p=0.63). A similar association between HF and cancer risk was found after adjusting for BMI, smoking, diabetes, prior myocardial infarction, hypertension, peripheral vascular disease, dementia and chronic obstructive pulmonary disease (HR 1.64; 95% CI 1.07–2.51).
We further investigated the association of HF and incident cancer in subgroups. Comparing men and women, there was no statistically significant difference in the associations (P for HF×sex interaction 0.74). Men with HF had a 55% increased risk of developing incident cancer compared to men without HF (HR 1.55; 95% CI 0.85–2.80), while women with HF had a 71% greater risk (HR 1.71; 95% CI 0.99–2.95) after adjustment for BMI, smoking and Charlson comorbidity index. Regarding age, there was no statistically significant difference in the associations (P for HF×age group interaction 0.22). Subjects ≤ 75 years of age with HF had more than a two-fold increased risk in developing incident cancer than their HF-free counterparts (HR 2.06; 95% CI 1.15–3.71) while subjects > 75 years of age had a 30% higher risk (HR 1.29; 95% CI 0.73–2.29).
In analyses restricted to HF patients, the association between left ventricular function and incident cancer was examined. Ejection fraction was recorded in 368 HF patients; 44% had preserved EF. Among the patients with available EF evaluation, 70 cancer cases were identified during follow-up. Cancer was not associated with preserved vs. reduced EF (HR 0.80; 95% CI 0.49–1.31) after adjustment for age, sex, and index year. Further adjustment for the Charlson comorbidity index yielded similar results (HR 0.78; 95% CI 0.48–1.29).
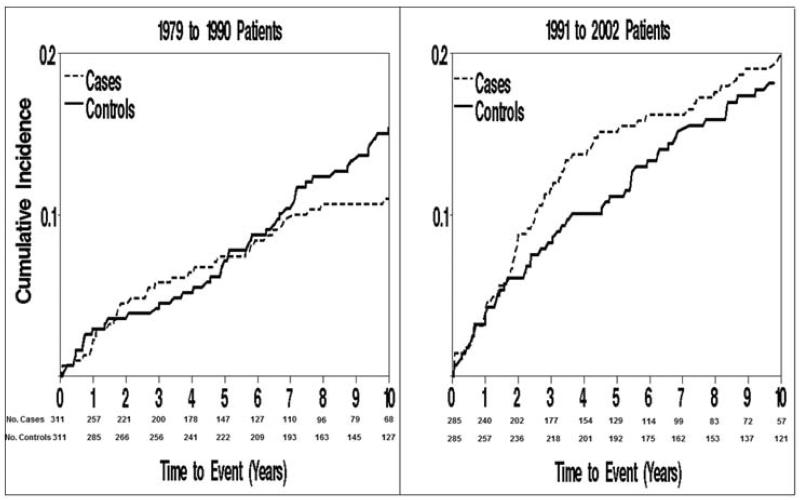
The competing risk-adjusted cumulative incidence rates of cancer among HF patients and matched controls by time period are shown in Figure 2, illustrating an increased cancer risk associated with HF during the later time period. Indeed, from the stratified proportional hazards regression model, P for HF×year interaction was 0.075. For HF patients diagnosed between 1979–1990, there was a 48% increased risk of incident cancer in HF patients compared to HF-free patients (HR1.48; 95% CI 0.79–2.78), while HF patients diagnosed between 1991–2002 had an 86% increased risk (HR 1.86; 95% CI 1.10–3.15) after adjustment for BMI, smoking and Charlson comorbidity index.
Figure 2. Incidence of Cancer among HF Patients and Controls by Year Groups.
Cumulative incidence of cancer among HF patients and matched controls with death considered a competing event, stratified by year groups.
To minimize the possibility of co-occurrence of HF and cancer, we repeated the analysis examining the association of HF and cancer while excluding the first 5 years of follow-up after HF diagnosis. The results from the stratified proportional hazards regression models were similar to those obtained for the entire follow-up period (HR 1.77; 95% CI 0.97–3.20 in the model accounting for the matching variables and HR 1.63; 95% CI 0.83–3.21 in the model further adjusted for BMI, smoking and Charlson comorbidity index).
Mortality
Mortality was high in the HF cases. The 5-year survival estimate for HF cases was 53% (95% CI 49%– 57%). Incident cancer was associated with a large excess risk of death (HR 1.68; 95% CI 1.33–2.14). This association persisted after adjustment for age, sex, index year and Charlson comorbidity index (HR 1.56; 95% CI 1.22–1.99). Among the non-HF controls, the 5-year survival estimate was 77% (95% CI 73%–80%). Incident cancer was associated with a large increased risk of death (HR 2.55; 95% CI 2.03–3.22), which persisted after adjustment for age, sex, index year and Charlson comorbidity index (HR 1.93; 95% CI 1.51–2.46). There was no difference in the in the association between cancer and mortality for cases vs. controls (P for HF×cancer interaction 0.18).
Discussion
In this community population, we have shown that while the prevalence of cancer in newly diagnosed HF patients is similar to controls, the incidence of subsequent cancer diagnosis is approximately 70% higher among patients with HF. The risk of cancer after HF is increasing over time and is associated with increased mortality.
Incident Cancer in Heart Failure Patients
As the finding of an increased risk of cancer in HF is novel, studies will be needed to examine the mechanisms of this association. Our findings could reflect detection bias related to the intensified medical evaluation due to the diagnosis of HF (e.g., evaluation for transplant). This is unlikely since, in the present study, none of the HF patients with reduced EF who were diagnosed with cancer had a transplant work-up. Additionally, the timing of cancer diagnosis in our study suggests that detection bias is unlikely to operate. In our experience, most diagnostic evaluations and corresponding costs (14), occur in the first year after HF diagnosis, while the present findings indicate increased incident cancer after the second year. Furthermore, the association persisted after excluding individuals who developed cancer within 5 years of HF diagnosis. The increased risk of cancer may relate to shared risk factors between HF and cancer. For example, patients with chronic obstructive pulmonary disease have an increased risk for both HF and lung cancer (15), and chronic kidney disease is associated with HF and with an increased risk of cancer in elderly men (16). This seems unlikely in the present study, however, as adjustment for comorbidities yielded similar results and renal dysfunction was rare and not significantly different between cases and controls. Further, the risk of cancer tended to be greater in younger patients arguing against a major role of measured and unmeasured comorbidities as these increase in prevalence with age. Other hypothetical explanations include the possibility that stress from chronic illness (17) or mechanisms associated directly with the physiology of HF may also be operating in cancer such as inflammation, tissue hypoxia and hormonal (erythropoietin) activation (18). None of these are currently established.
Radiation from medical imaging is a concern (19,20); however, the risks associated with radiation exposure are typically long-term with cancer developing after 10–15 years, which is longer than the average survival after HF diagnosis. Some cardiovascular medications may contribute to the occurrence of malignancy (21). Calcium channel blockers (11) and digoxin (22) seem unlikely culprits as none of these agents occupies a central role in the treatment of HF, and their use has declined over time while the risk of cancer has been increasing (23,24). Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) stimulate angiogenesis and thus, in theory, may increase cancer risk (25). Their use has increased over time, and we observed that the increased risk of cancer associated with HF tended to be stronger in the later time period (1991–2002). While these parallel trends are concerning, they should be viewed as hypothesis generating rather than reflecting causality. Furthermore, alternative mechanisms such as increased risk of frailty in the context of improved survival should be investigated in future studies.
Mortality
Cancer was independently associated with an increased risk of death in this cohort as well as in previously reported population registries (26). This is important because the clinical implications of cancer diagnosis in a patient already diagnosed with HF are not fully defined, and it may be argued that outcomes will be determined by HF. Our analysis does not support the latter view since patients with HF who were later diagnosed with cancer had a 56% higher risk of death compared with HF patients who did not develop cancer. This information is relevant to clinical decision making in HF.
Limitations, Strengths and Clinical Implications
Some limitations of our study should be acknowledged. Data on healthcare utilization were not available, so detection bias cannot be ruled out. Our sample size did not afford analysis of specific cancer types or cause-specific death. Estimation of left ventricular function was not available in all patients, and the analysis of the association between EF and incident cancer should be interpreted with caution. No medication data were available for controls and thus could not be included in the analysis. Lastly, as is applicable to any observational study, the observed associations could reflect residual confounding where the effect of a confounder is not completely removed through the modeling strategy. Our findings should be interpreted as reflecting an association rather than causality.
Our study also has notable strengths. We utilized the comprehensive data resources of the Rochester Epidemiology Project to examine cancer diagnoses occurring before and after HF. We assembled a population-based incident HF cohort with diagnosis confirmed by standardized criteria (2). The controls were randomly selected from an enumeration of the Olmsted County population, and thus should be representative of the community (9). Furthermore, clinical characteristics were recorded prior to knowledge of cancer outcomes, and longitudinal follow-up allowed the gain of novel insights on clinical outcomes in HF.
There are several clinical implications of our findings. The increased incidence of cancer among HF patients who already have an excess mortality underscores the importance of cancer surveillance in this population. The emergence of a greater risk in more recent years will require particular watchfulness. These findings also illustrate the importance of multi-morbidity among patients living with chronic diseases and support the concept of providing holistic rather than disease-based care (27).
Conclusions
This community study indicates that patients with HF experience a large excess risk of subsequent malignancies. This excess risk appears to increase over time. Cancer markedly increases mortality in HF underscoring the importance of non-cardiac morbidity and of cancer surveillance in the management of HF patients.
Acknowledgements
We thank Kay A. Traverse, RN and Ellen E. Koepsell, RN for assistance with data collection, Ruoxiang Jiang for assistance with data analysis and Deborah S. Russell for secretarial assistance.
Sources of Funding Research reported in this publication was supported by the National Institutes of Health under National Heart, Lung and Blood Institute Award Number R01HL72435 and the National Institute on Aging Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- HF
Heart Failure
- HR
Hazard Ratio
- BMI
Body Mass Index
- EF
Ejection Fraction
- ACE
Angiotensin – Converting Enzyme
- ARBs
Angiotensin – Receptor Blockers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL. The heart failure epidemic. Int J Environ Res Public Health. 2010;7:1807–30. doi: 10.3390/ijerph7041807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 8.McKelvie RS, Moe GW, Cheung A, et al. The 2011 Canadian Cardiovascular Society heart failure management guidelines update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol. 2011;27:319–38. doi: 10.1016/j.cjca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 10.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 11.Pahor M, Guralnik JM, Ferrucci L, et al. Calcium-channel blockade and incidence of cancer in aged populations. Lancet. 1996;348:493–7. doi: 10.1016/S0140-6736(96)04277-8. [DOI] [PubMed] [Google Scholar]
- 12.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 13.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez LA, Wallander MA, Martin-Merino E, Johansson S. Heart failure, myocardial infarction, lung cancer and death in COPD patients: a UK primary care study. Respiratory Medicine. 2010;104:1691–9. doi: 10.1016/j.rmed.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20:1341–50. doi: 10.1681/ASN.2008090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtney JG, Longnecker MP, Theorell T, Gerhardsson de Verdier M. Stressful life events and the risk of colorectal cancer. Epidemiology. 1993;4:407–14. doi: 10.1097/00001648-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Schroten NF, van der Putten K, Rutten FH, Diepenbroek A, Mosterd A, Gaillard CA. High cumulative incidence of cancer in patients with cardio-renal-anaemia syndrome. Eur J Heart Fail. 2010;12:855–60. doi: 10.1093/eurjhf/hfq078. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg MJ, Afilalo J, Lawler PR, Abrahamowicz M, Richard H, Pilote L. Cancer risk related to low-dose ionizing radiation from cardiac imaging in patients after acute myocardial infarction. CMAJ. 2011;183:430–6. doi: 10.1503/cmaj.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849–57. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ocampo NV, Tafreshi J, Hauschild CL, Pai RG. Cardiovascular medications and risk of cancer. Am J Cardiol. 2011;108:1045–51. doi: 10.1016/j.amjcard.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 22.Biggar RJ, Wohlfahrt J, Oudin A, Hjuler T, Melbye M. Digoxin use and the risk of breast cancer in women. J Clin Oncol. 2011;29:2165–70. doi: 10.1200/JCO.2010.32.8146. [DOI] [PubMed] [Google Scholar]
- 23.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg RJ, Darling C, Joseph B, et al. Epidemiology of decompensated heart failure in a single community in the northeastern United States. Am J Cardiol. 2009;104:377–82. doi: 10.1016/j.amjcard.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–36. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart S, Ekman I, Ekman T, Oden A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1 162 309 hospital cases in Sweden (1988 to 2004) Circ Cardiovasc Qual Outcomes. 2010;3:573–80. doi: 10.1161/CIRCOUTCOMES.110.957571. [DOI] [PubMed] [Google Scholar]
- 27.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition-multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]