Abstract
Although the role of the erythropoietin (Epo) receptor (EpoR) in erythropoiesis has been known for decades, its role in non-hematopoietic tissues is still not well defined. Klotho has been shown and Epo has been suggested to protect against acute ischemia-reperfusion injury in the kidney. Here we found in rat kidney and in a rat renal tubular epithelial cell line (NRK cells) EpoR transcript and antigen, and EpoR activity signified as Epo-induced phosphorylation of Jak2, ErK, Akt, and Stat5 indicating the presence of functional EpoR. Transgenic overexpression of Klotho or addition of exogenous recombinant Klotho increased kidney EpoR protein and transcript. In NRK cells, Klotho increased EpoR protein, enhanced Epo-triggered phosphorylation of Jak2 and Stat5, the nuclear translocation of phospho-Stat5, and protected NRK cells from hydrogen peroxide cytotoxicity. Knock-down of endogenous EpoR rendered NRK cells more vulnerable, and overexpression of EpoR more resistant to peroxide-induced cytotoxicity, indicating that EpoR mitigates oxidative damage. Knock-down of EpoR by siRNA abolished Epo-induced Jak2, and Stat5 phosphorylation, and blunted the protective effect of Klotho against peroxide-induced cytotoxicity. Thus in the kidney, EpoR and its activity are downstream effectors of Klotho enabling it to function as cytoprotective protein against oxidative injury.
Keywords: Cytotoxicity, Erythropoietin, Erythropoietin receptor, Kidney cell line, Klotho, Oxidative stress, Jak2, NRK cell, Stat5
INTRODUCTION
Erythropoietin (EPO) is a glycoprotein hormone originally characterized as the principal regulator of erythropoiesis by inhibiting apoptosis and stimulating proliferation and differentiation of erythroid precursor cells.1,2 EPO production is from the liver in the fetus,2 overlaps with mesonephros and metanephros, and completely switches to the kidney postnatally.1-3 EPO transduces its signaling via the EPO receptor (EpoR) in hematopoietic cells to regulate erythropoiesis as a complex multi-step process of differentiation of early erythroid progenitors to enucleated red blood cells.4In vitro and in vivo experiments suggests that EPO/EpoR cell signaling may have beneficial effect on ischemia or hypoxia-induced tissue injury in brain,5,6 heart,7-10 and kidney.11-13 However, the literature at this stage is far from uniform. At least in the kidney, there are also studies that showed no or even adverse effects of EPO administration on acute ischemia injury.14-16 Even if exogenous EPO or erythropoiesis-stimulating agents (ESA) indeed confer tissue protection as shown in some studies,17,18 the mechanisms of their actions remain elusive. Further understanding along this line will help resolve the controversy and decipher whether there is a therapeutic application in the horizon.
There is emerging and convincing evidence that EpoR is expressed in non-hematopoietic tissues19 such as the brain,20 heart, lung,21 kidney,22,23 vascular endothelium,24 smooth muscle cells,25 and skeletal muscle cells.26 The paracrine and autocrine EPO/EpoR axis has been proposed to participate in a myriad of biologic processes including cell proliferation, apoptosis, angiogenesis, organogenesis, cytoprotection against ischemia, tissue repair, and carcinogenesis.27 Deletion of EpoR leads to severe tissue damage, slow tissue regeneration, and reduced angiogenesis after ischemia in mice.28,29 An alternative and contrary paradigm has been proposed where functional EpoR is restricted absolutely and exclusively to the erythropoietic lineage and extra-erythropoietic EpoR's are all non-functional.30-33 This discrepancy is not yet resolved and the mechanism of EPO effect on non-erythropoietic tissues needs to be defined.
Klotho was originally touted as an anti-aging protein but since has been discovered to exert a host of biologic effects on multiple systems.34 Klotho is a single-pass transmembrane protein but a secreted soluble form of Klotho can be generated by alternative splicing or proteolytic cleavage from membrane Klotho and be released into blood thus functioning as a circulating substance35,36 to exert multiple systemic biological actions on distant organs.37 Klotho is principally synthesized in kidney and brain, but it is expressed in multiple organs.34,37 Recent studies suggest that either overexpression of transmembrane or administration of secreted Klotho exert protective effects against ischemia-reperfusion-induced acute kidney injury.38,39
We inquired whether the protective effects of Klotho in the kidney have any relationship with EpoR. The main goals of the present study are: 1) To provide an independent determination of whether there is EpoR protein and activity in the kidney and cultured kidney cells; 2) To establish a cell culture model to study EpoR function; 3) To test if the protective effect of Klotho against oxidative cytotoxicity involves the EpoR. We showed that EpoR mRNA, protein, and activity are present in the kidney and kidney cells, and that Klotho acutely and chronically increases EpoR transcript and protein, and EpoR-dependent signal transduction. In addition, knock-down of EpoR enhances, and overexpression decreases, susceptibility to oxidative injury. Finally, the protective effect of Klotho against H2O2-induced cytotoxicity is partially abrogated by deletion of endogenous EpoR. In concert, the data suggests that EpoR is a downstream signaling component involved in Klotho's cytoprotective effect.
RESULTS
Klotho modulates the expression of EpoR transcript, protein and function in kidney
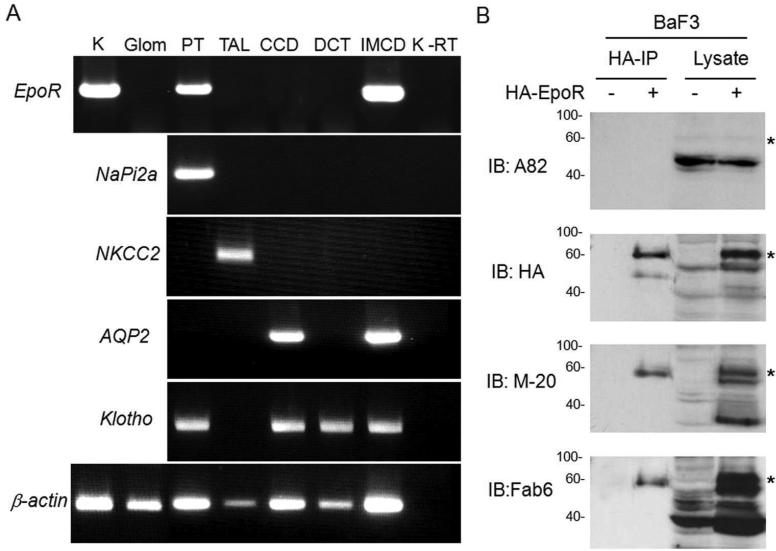
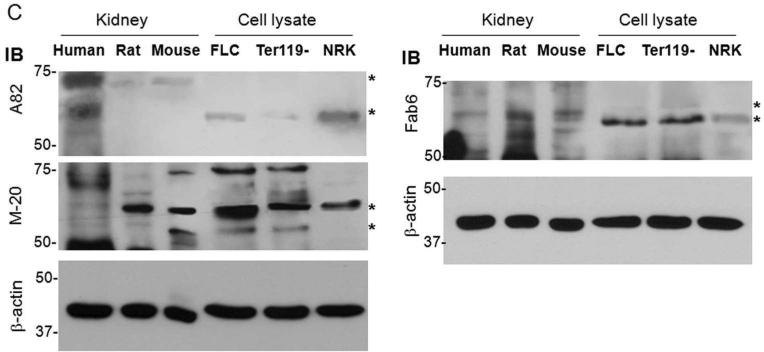
Numerous studies suggested that EpoR is widely expressed in non-hematopoietic tissues and cells. We strived to further confirm this in the kidney. First, we found unequivocal evidence of EpoR mRNA in adult rat kidney (Figure 1A). Microdissected structures from rat kidneys further provide confirmation of EpoR location. Figure 1A reveals that proximal tubules and inner medullary collecting duct express EpoR mRNA, but other segments have low or undetectable EpoR mRNA expression, which is consistent with the findings of De Beuf and coworkers.40 We next explored whether EpoR mRNA is accompanied by EpoR protein expression in the kidney. We first used BaF3 cells stably expressing HA murine EpoR and probed both lysates and HA immunoprecipitated complex with different EpoR antibodies to confirm these reagents do indeed label the EpoR. Similar to HA antibody, two bands of ~60 and ~50 kDa were detected with M-20 and Fab 6 antibodies in both cell lysate. The ~60 kDa band was detected in the HA-immunoprecipitates (Figure 1B). These bands are indeed the EpoR, because they were not present in lysates and immunoprecipitates from non-transfected cells. A82 which is human-specific31 is weakly cross-reactive or non-reactive with rodent EpoR.30,32 We next examined EpoR protein in the mouse, rat and human kidney and compared its expression to known EpoR expressing cells (Ter119-cells and mouse fetal liver cells) using several antibodies against EpoR (Figure 1C). While each antibody may have off-target labeling, it is unlikely that all react with non-specific proteins with similar gel mobility as the EpoR. With every antibody, there are multiple bands within the expected range of mobility of EpoR from 50-70 kDa, possibly from existence of different glycosylated EpoR41 and possibly from some processed EpoR polypeptides. In addition, based on semi-quantitative immunoblots by M-20 and Fab 6 antibodies (Figure 1C), EpoR protein abundance in rat or mouse kidney is less than half that of fetal liver cells (FLC).
Figure 1. Expression of EpoR protein and mRNA in rat kidney.
(A) EpoR mRNA expression in the rat kidney or microdissected glomeruli and renal tubules and from normal adult rats at age of 3 months old by RT-PCT. Total RNA was extracted, and complimentary DNA (cDNA) generated with Oligo dT. Specific target genes were examined by PCR with rat specific primers (shown in method section). AQP2: aquaporin-2; CCD: cortical collecting duct; DCT: distal convaluted tubules; Glo: glomeruli; IMCD: inner medullary collecting duct; K: Rat kidney tissue containing cortex and medullar; NaPi-2a: Na-Pi dependent cotransporter-2a; NKCC2: Na-K-2Cl cotransporter; PT: proximal tubules; TAL: thick ascending limb; K-RT: kidney sample with reverse transcriptase omitted; (B) EpoR protein expression in BaF3 cell transfected with HA-tagged mouse EpoR or empty vector. Total lysates from native BaF3 and BaF3-HA-EpoR cells were subjected to immunoprecipitation by HA-Resin followed by immunobloting for EpoR with several anti-EpoR antibodies: A82, HA, M-20, Fab 6. Asterisks depict the specific HA-EpoR band. (C) Total proteins were extracted from human and rodent kidneys and murine FLC and Ter119-cells, and subjected to immunobloting EpoR with several antibodies against EpoR. A82 is monoclonal rabbit antibody kindly provided by Dr. Steve Elliot; M-20 is polyclonal antibody purchased from Santa Cruz Biotech. Fab 6 is a synthetic human Fab against human EpoR isolated from a phage-displayed library. Asterisks indicate multiple forms of glycosylated EpoR or EpoR fragments. Hu: human; Mu: mouse; FLC: fetal liver cells at E13.5
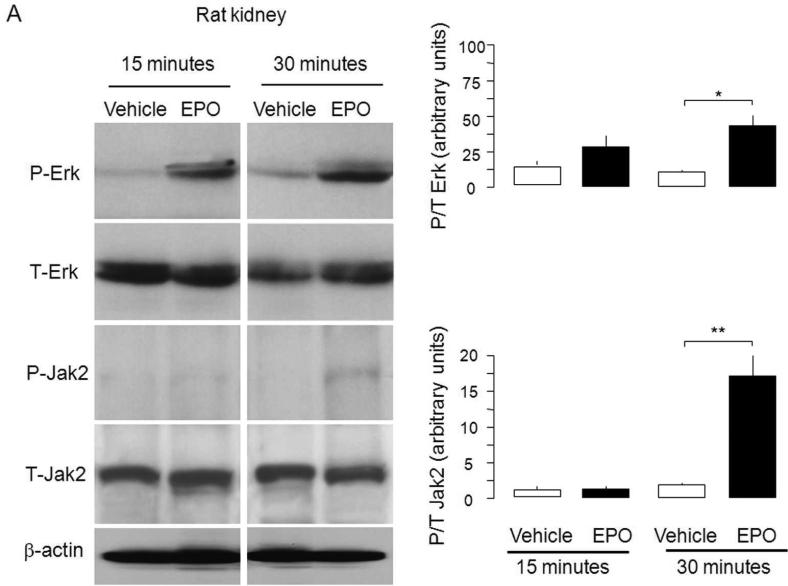
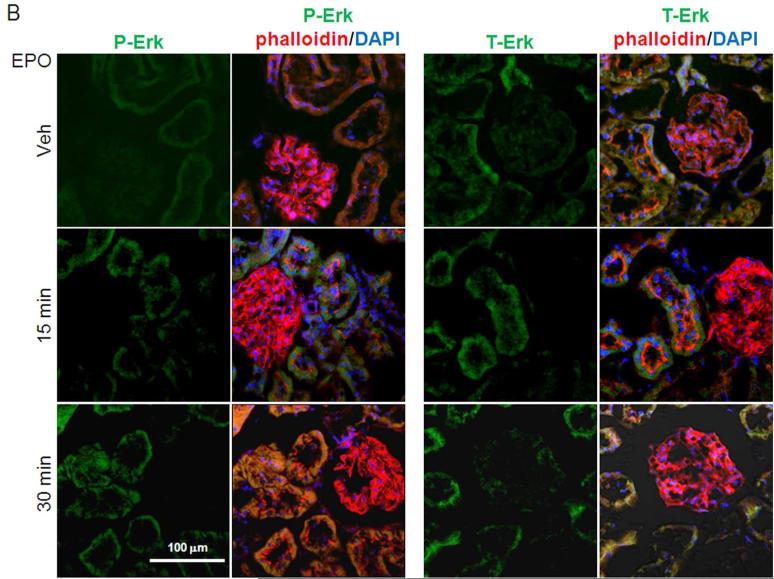
Since proponents of the erythrocentric-restricted expression of EpoR speculated that presence of extra-erythropoietic EpoR antigen represents non-functional receptors of unknown significance,30,32 we further tested our findings with EpoR signaling assays. We applied the criterion proposed by Elliot and coworkers30 which states that presence of antigen needed to be confirmed by assay of functional EpoR activity. We injected EPO (100 IU/ml) through the renal artery to normal rat kidney and measured downstream EpoR signaling. As shown in Figure 2A-C, activation of Jak2 and ERK were readily detected upon EPO stimulation. Therefore, the renal EpoR antigen that we detected represents fully functional receptors. Injection of lower concentrations of EPO (50 IU/ml and 10 IU/ml) also lead to similar changes but the magnitude is smaller (not shown).
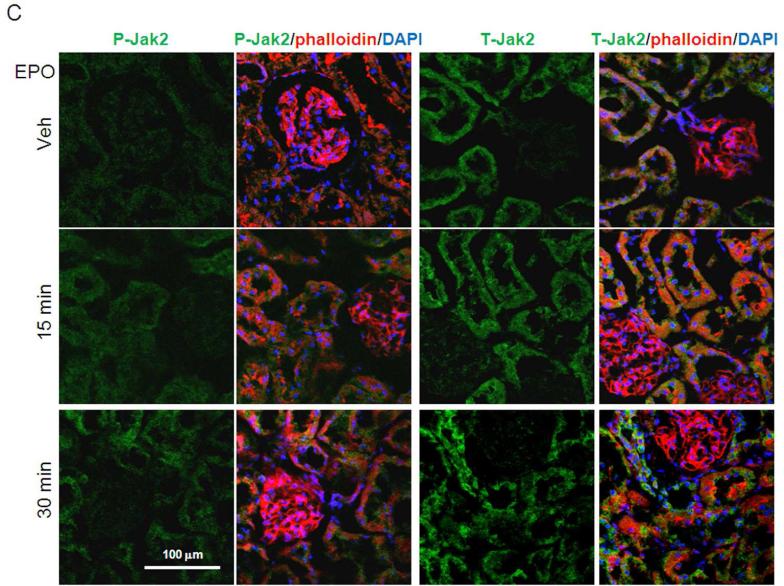
Figure 2. Functional EpoR in rat kidney. EpoR activity was assessed by EPO-induced activation of downstream effectors in rat kidney assayed by determination of phosphorylation of Erk and Jak2.
Renal artery was surgically isolated and EPO in oxygenated 1640 culture media (40 ml of 100 IU/ml) was continuously perfused for 15 minutes with Intravenous infusion system (Instech Laboratories, Inc, Plymouth Meeting, PA). After 15 minutes, kidneys were harvested for further study. (A) Left panel shows representative blots for phospho-Erk (P-Erk) and total Erk (T-Erk), and phospho-Jak2 (P-Jak2) and total Jak2 (T-Jak2). Summarized data (means ± SD) are shown in the right panel. (B) Representative immunohistochemistry for P-Erk and T-Erk in the kidney. (C) Representative immunohistochemistry for P-Jak2 and T-Jak2 in the kidney.
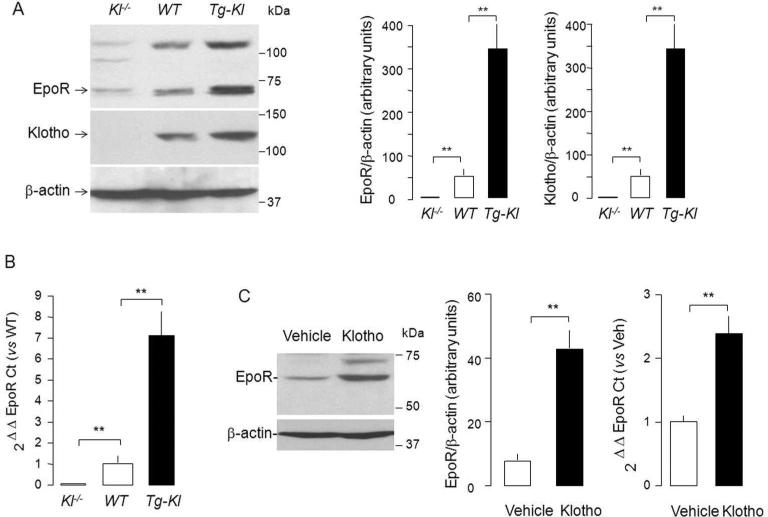
We next examined the correlation of EpoR protein levels with renal Klotho protein levels using genetic manipulation of Klotho (Figure 3A). Transgenic mice overexpressing Klotho (Tg-Kl)38,42,43 have higher; and Klotho hypomorph's (Kl-/-) have lower levels of EpoR protein in the kidney compared to WT mice (Figure 3A). The change of EpoR mRNA was parallel to that of protein (Figure 3B), suggesting that chronic modulation of EpoR by Klotho is at least at the transcript level.
Figure 3. Genetic levels of Klotho correlates with levels of EpoR protein and mRNA expression in the kidney.
(A) sixty mg protein of total kidney Lysate was loaded onto each lane, subjected to SDS-PAGL, and immunoblotted for EpoR and Klotho. EpoR and Klotho protein expression in mouse kidney (detected with KM206 and M-20 antibody respectively). Kl-/-= Klotho knock-out mice; WT=Wild type mice; Tg-Kl= transgenic Klotho overexpressing mice. Left panel: Typical blot. Right: Summarized data as means ± SD. (B) EpoR transcript level in mouse kidney by quantitative RT-PCR (qRT-PCR) expressed relative to wild type mouse and summarized as means ± SD. (C) Acute Klotho injection up-regulates EpoR protein and mRNA expression in rat kidney. Full length extracellular domain of recombinant mouse Klotho protein (rMKl) was injected intraperitoneally 0.01 mg/Kg body weight) once into rats and kidneys were harvested 24 hours later. EpoR protein was examined by immunoblot with M-20 and EpoR mRNA by qRT-PCR respectively. Typical immunoblot of EpoR protein (left panel), summarized data of EpoR protein (middle panel) and EpoR transcript by qRT-PCR (right panel). All bars and error bars are mean and SD. *p<0.05, **p<0.01 ANOVA followed by Student-Newman-Keuls test.
To rule out secondary effects from chronic genetic manipulations, we examined the short-term effect of exogenous soluble recombinant Klotho on expression of EpoR. Normal SD rats were injected intraperitoneally with recombinant mouse Klotho (rMKl) and the expression of EpoR protein and mRNA in the kidneys was examined one day after Klotho administration. The abundance of both EpoR protein and transcript was appreciably increased in the kidney of rats treated with Klotho compared with vehicle (Figure 3C), suggesting that EpoR in the kidney is indeed regulated by Klotho.
To further define whether Klotho regulates erythropoiesis, we measured hematocrit, plasma EPO and renal EPO transcripts and did not see any significant difference in WT and Tg-Kl mice (Table 1), indicating that Klotho overexpression does not induce polycythemia and the up-regulation of EpoR is not universal in all organs.
Table 1.
Hematocrit and EPO concentration in WT and Tg-Kl mice
| WT | Tg-Kl | T test | |
|---|---|---|---|
| HCT (%) | 46.3±3.7 (9) | 46.5±3.2 (12) | >0.005 |
| Plasma EPO (pg/ml) | 121.7±16.7 (6) | 131.1±33.0 (5) | >0.05 |
| Kidney EPO transcript (2-ΔΔCt) | 1.0±0.1 (4) | 1.2±0.2 (5) | >0.05 |
HCT: Hematocrit; EPO: erythropoietin; Relative EPO transcript was analyzed by normalizing cyclophilin followed by comparing with WT mice. Data are shown Means ± SD. Statistical differences were analyzed by unpaired Student-t test, and statistical significance accepted when p<0.05 between WTand Tg-Kl groups.
Klotho increases EpoR protein and mRNA expression in NRK cells
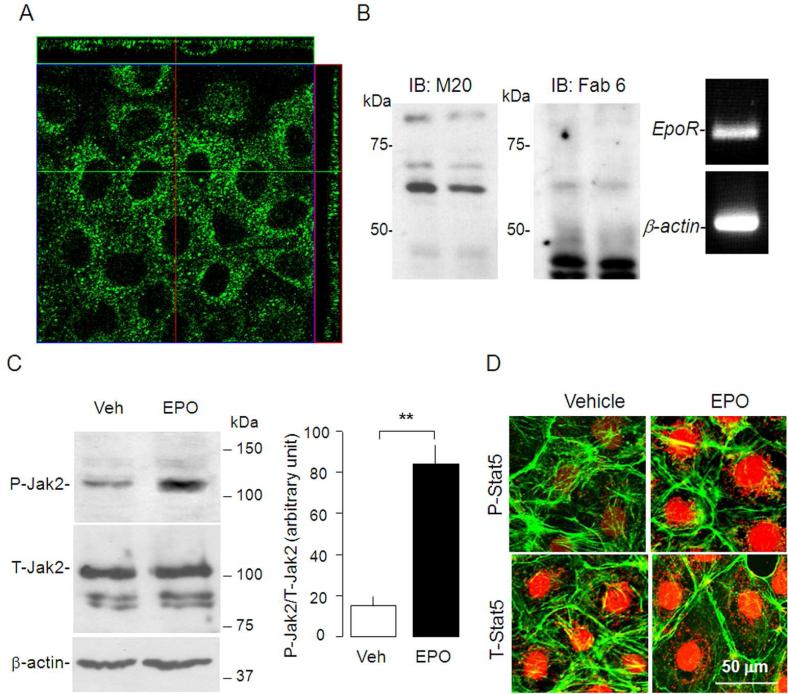
The in vivo experiments above lend strong support that Klotho regulates EpoR expression, but definitive proof of a direct effect mandates an in vitro system. We used NRK cells as our in vitro model because NRK cells are polarized renal epithelial cells with both proximal44 and distal tubule characteristics.45 We first tested whether the NRK cell line is a reliable cell culture model for studying regulation of Klotho on EpoR. Immunocytochemistry revealed EpoR expression in both apical and basolateral membranes in addition to intracellular location in NRK cells (Figure 4A). Immunobloting with the M-20 antibody revealed a ~65 KDa band in total cell lysate from NRK cells (Figure 4B left panel) which was confirmed by another antibody (Fab 6) (Figure 4B right panel).31EpoR mRNA is also abundant in NRK cells (Figure 4C). To further examine if the EpoR in NRK is functional, we stimulated NRK cells with EPO (100 IU/ml) for 30 minutes to test whether EPO is able to activate Jak2 and Stat5, two known downstream effectors of EPO/EpoR signaling pathway46 that are implicated in EPO-induced cytoprotection from radiation and cytotoxic agent.47 Exogenous EPO induces JAK2 activation, assayed by antibodies specifically recognizing the phosphorylated form of JAK2 (P-Jak2/T-Jak2) compared to vehicle (P-Jak2/T-Jak2) (Figure 4D). In addition, the levels of phosphorylated-Stat5 was increased in nucleus following EPO stimulation (Figure 4E), indicating that functional EpoR exists in NRK cells.
Figure 4. Expression of EpoR in NRK cells.
(A) Immunocytochemistry for EpoR in NRK cells. Confocal fluorescent images in xy, yz, xz planes. (B) Left and middle panel: Representative immunoblots for EpoR with M-20 (left panel) and with Fab 6 (middle panel) in total lysate of NRK cells. Right panel: RT–PCR of EpoR transcript in NRK cells. (C) NRK cells incubated with culture media containing 10% FBS and treated with EPO (100 IU/ml) or vehicle (Veh) for 10 minutes and total lysates were immunobloted for phosphorylated Jak2 (P-Jak2) and total Jak2 (T-Jak2) (left panel). Summarized data as mean ± SD (right panel) (D) Representative immunocytochemistry for phospho-Stat5 (P-Stat5) and total Stat5 (T-Stat5) (red) in NRK cells treated with EPO or vehicle (Veh). β-actin (green) served as counterstain.
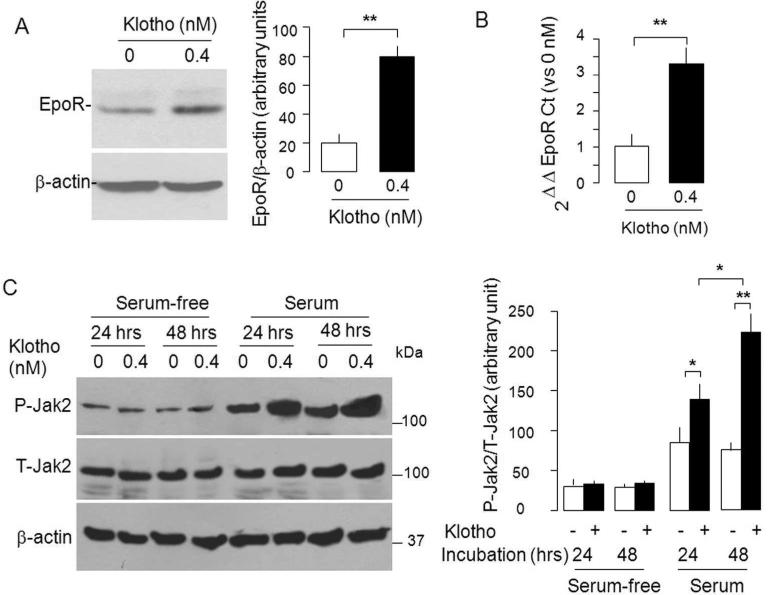
Next, we tested if Klotho directly modulates EpoR in the cell line; a conclusion we cannot made from the in vivo experiment. Twenty-four hours after addition of recombinant mouse Klotho (rMKl),43 NRK cells showed significantly increase in both EpoR transcript (Figure 5A) and protein (Figure 5B). The increase detected by M-20 was confirmed by blotting with our anti-EpoR Fab. To define whether the up-regulation of EpoR by Klotho is functional, we examined the effect of rMKl on downstream EpoR effectors. Figure 5C shows that in the presence of fetal bovine serum (FBS), Klotho augmented Jak2 activation without an appreciable increase in total Jak2 in NRK cells; whereas this effect of rMKl on Jak2 activation was abolished without FBS which renders the medium devoid of EPO;48 indicating that Klotho-induced Jak2 activation (Figure 5C) is dependent upon the presence of EPO. It is also important to indicate that there are a lot more factors in FBS other than EPO which may also contribute to the effect.
Figure 5. Klotho regulates EpoR protein, mRNA, and signaling in NRK cells.
NRK cells were treated with Klotho in cell culturere medium containing 10% FBS for 24 hours. (A) Typical immunoblot for EpoR (left panel) and data summarized as means ± SD (right panel). (B). EpoR mRNA by qPCR. Cyclophilin served as control. Means ± SD of 4 independent experiments. **p< 0.01 unpaired Student t-test. (C).NRK cells were incubated with Klotho protein in medium containing 10% FBS for 24 and 48 hours. Total lysates were immunoblotted for phosphorylated (P-Jak2) and total Jak2 (T-Jak2) (left panel). Summarized data as means ± SD (right panel) of 4 independent experiments. *p<0.05, **p<0.01 ANOVA followed by Student-Newman-Keuls test.
EpoR is involved in cytoprotection of EPO against H2O2 cytotoxicity
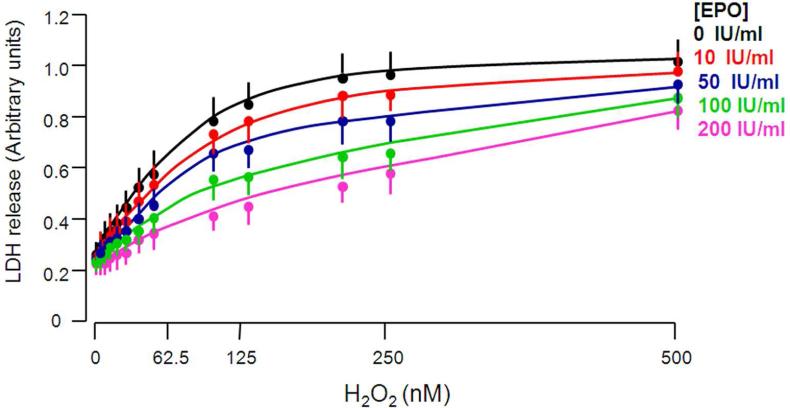
EPO has been shown to induce or enhance cells’ resistance to hypoxia,49 oxidative stress,50 cytokine-induced apoptosis,49 radiation,47 and cytotoxic agents.47 To examine if EPO protects NRK cells from H2O2 cytotoxicity, we treated NRK cells with different concentrations of EPO and measured LDH released into culture medium from NRK cells exposed to H2O2. As shown in Figure 6, H2O2 increased LDH release from NRK cells. EPO mitigated H2O2-induced LDH release from NRK cells in a dose-dependent manner (Figure 6), suggesting that EPO protects NRK cells against oxidative stress-induced cytotoxicity triggered by H2O2
Figure 6. EPO protects NRK cells from H2O2-induced cytotoxicity.
NRK cells were treated with different concentrations of EPO and H2O2 as shown in regular cell culture medium containing 10% FBS for 24 hours and supernatants were collected for LDH assay.
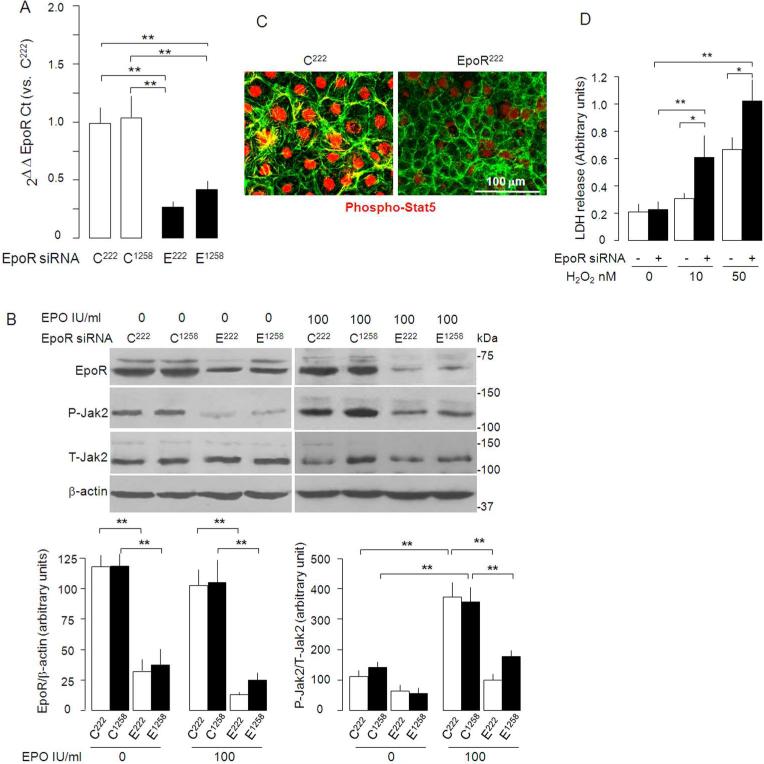
To examine whether the expression of EpoR in NRK is required for EPO to exert cytoprotection, we first silenced endogenous EpoR in NRK to study if EpoR-deficient NRK cells are more vulnerable to oxidative stress. We tested the efficiency of EpoR knock-down by two small interfering RNA (siRNA) at 222nd (EpoR222) or at 1258th (EpoR1258) nucleotide of EpoR. EpoR222 siRNA was more potent than EpoR1258 in knocking down EpoR mRNA (Figure 7A) and protein (Figure 7B), blocking EPO induced Jak2 activation (Figure 7B), and EPO-induced translocation of phospho-Stat5 into the nucleus (Figure 7C). Therefore, EpoR222 siRNA was used throughout this study. EpoR222 siRNA significantly augmented LDH released from NRK induced by H2O2 treatment (Figure 7D), indicating that EpoR deficiency renders NRK cells more susceptible to H2O2-induced cytotoxicity.
Figure 7. EpoR is required for EPO cytoprotection against H2O2.
Knockdown of endogenous EpoR by siRNA was confirmed by qRT-PCR to quantify EpoR mRNA shown as means ± SD from 4 independent experiments (A). E222 and E1258 are EpoR siRNA's at indicated sequences locations, C222 and C1258 are as corresponding scrambled controls C222 and C1258). EpoR222 siRNA knocked down EpoR mRNA by 76.3 ± 5.0%, while EpoR1258 siRNA by 56.9 ± 3.9%. (B) EpoR protein and Jak2 phosphorylation in NRK cells incubated with culture medium containing 10% FBS and treated with EpoR or control siRNA. Representative blot (left panel) and summarized data as percentage of C222 (right panel). (C) Effect of EpoR knockdown on phosphorylation and nuclear translocation of Stat5 (red). (D) Effect of EpoR knockdown on susceptibility of NRK cells to H2O2. Means±SD from 4 independent experiments *p<0.05, **P<0.01 by ANOVA followed by Student-Newman-Keuls test. E222=EopR222, E1258=EpoR1258, C222=Control222, C1258=Control1258.
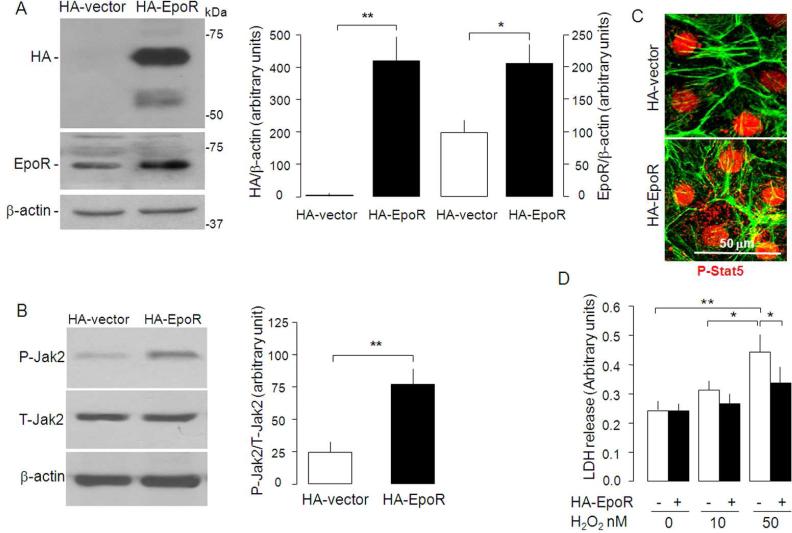
To acquire more direct evidence that EpoR contributes to cytoprotection in the kidneys, we increased EpoR expression in NRK by transfecting full length mouse EpoR into NRK cells (Figure 8A). Increase in EpoR elevated phosph-Jak2 (Figure 8B) and phosph-Stat5 (Figure 8C), indicating that the augmented EpoR is functional and could enhance EPO/EpoR signal transduction. Furthermore, overexpressing EpoR significantly reduced LDH release from NRK exposed to H2O2 (Figure 8D). Taken in concert, EpoR in NRK cells constitutes one of the cellular components to combat against oxidation-induced cytotoxicity.
Figure 8. Effect of overexpression of EpoR on H2O2 toxicity in NRK cells.
(A) NRK cells were transfected with HA-tagged EpoR and growth in cell culture medium containing 10% FBS. Total cell lysates were subjected to SDS-PAGE followed by immunobloting with anti-HA and anti-EpoR. Typical blot (left panel) and summarized data as means ± SD (right panel). *p<0.05 **p<0.01 by Student t test. (B) HA-EpoR overexpression and EPO-induced phosphorylation of Jak2. Typical blot (left panel) and summarized data as means ± SD (right panel). **p<0.01 by Student t test. (C) Effect of HA-EpoR overexpression on phosphorylation and translocation of Stat5. P-Stat5=phosph-Stat5. (D) Effect of EpoR overexpression on H2O2 cytotoxicity in NRK cell's. Means ± SD from 4 independent experiments *p<0.01 by ANOVA followed by Student-Newman-Keuls test.
Klotho protects NKR cells from H2O2 cytotoxicity via EpoR
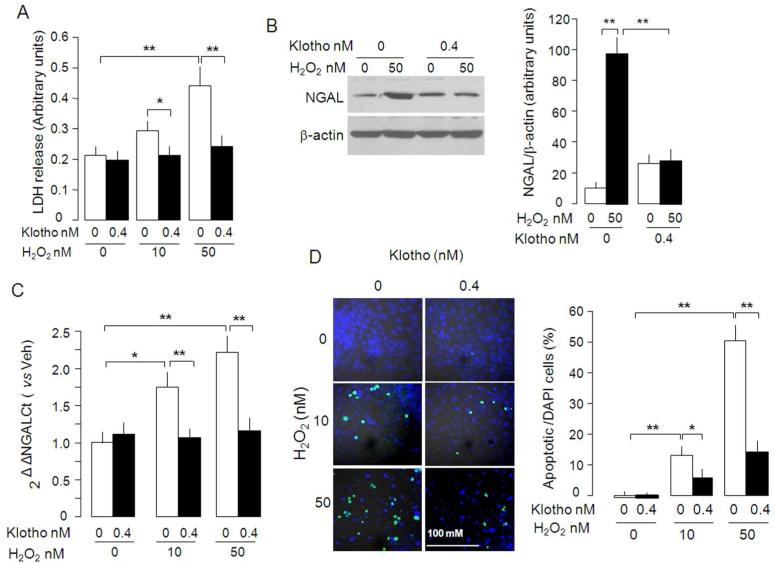
We previously showed that Klotho protects the kidney from ischemia-reperfusion induced acute injury in rodents,38 but the mechanism of Klotho's cytoprotection was not delineated. To examine the role of Klotho in cytoprotection, we incubated NRK cells with H2O2 and measured LDH in culture medium when Klotho protein was added. Klotho protein blunted LDH release triggered by H2O2 (Figure 9A) and inhibited NGAL protein, an early marker of acute kidney damage (Figure 9B), and mRNA induction (Figure 9C) by H2O2. Moreover, Klotho also reduced H2O2-induced apoptosis (Figure 9D), which further supports that Klotho directly protects NKR cells from H2O2 cytotoxicity.
Figure 9. Effect of soluble Klotho protein on H2O2-induced cytotoxicity in NRK cells.
(A) NRK cells were treated with H2O2 in culture medium containing 10% FBS with or without Klotho (0.4 nM) for 1 day. The culture media were collected for LDH release assay. (B) Cell lysates were immunoblotted for NGAL protein (left panel) and summarized data as mean ± SD from 4 independent experiments. (C) NGAL mRNA quantified by RT-qPCR’ relative to no Klotho and no H2O2. (D). Apoptotic cells detected by TUNEL assay (left panel). The ratio of apoptotic cells (TUNEL+) over total cells (DAPI+) (right panel). Means ± SD from 4 independent experiments. Differences between groups were statistically analyzed by ANOVA followed by Student-Newman-Keuls test. *p<0.05, **p<0.01.
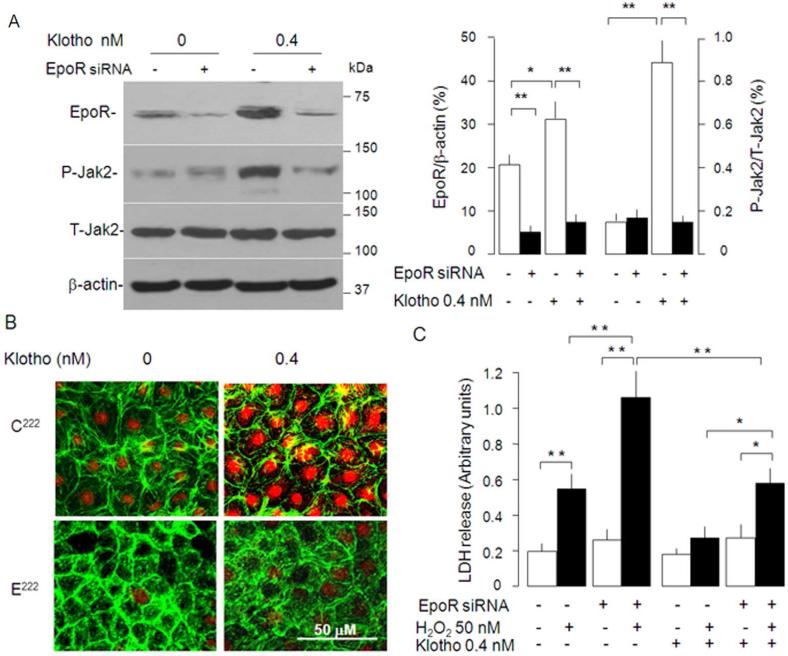
To test if EpoR is required for Klotho to exert its cytoprotective effect, we tested the Klotho effect in NRK cells with EpoR-knockdown. In the presence of 10% FBS, NRK cells with lower EpoR protein have lower phospho-Jak2 compared to NRK cells with normal levels of EpoR (Figure 10A). Klotho's ability to activate Jak2 (Figure 10A) and Stat5 (Figure 10B) in NRK cells was much attenuated in EpoR-knockdown cells, suggesting that the response to Klotho is reduced in NRK cells when EpoR was silenced.
Figure 10. Effect of knock-down of EpoR on the cytoprotection by Klotho.
(A) NRK cells with or without EpoR knockdown was treated with Klotho in cell culture medium containing 10% FBS and EpoR signaling was assayed as phospho-Jak. Typical blot (left panel) and means ± SD (right panel) (B). NRK cells with and without EpoR knock-down were treated with Klotho and EpoR signaling was measured as phosphorylation and translocation of Stat5. (C) Effect of EpoR knock-down on H2O2-induced cytotoxicity in NRK cells. Means ± SD from 4 independent experiments. Differences between groups statistically analyzed by ANOVA followed by Student-Newman-Keuls test. *p<0.05, **p<0.01.
Next, we tested if failure to induce phospho-Jak2 and phospho-Stat5 due to knock-down of endogenous EpoR is associated with escalated susceptibility to H2O2. Consistent with our previous results, LDH release from NRK cells with normal levels of endogenous EpoR was much less than that from EpoR-knockdown NRK cells in the presence of Klotho (Figure 7D and 10C). Importantly, the cytoprotection conferred by Klotho is partially blunted in NRK cells deficient of EpoR (Figure 10C), indicating that the cytoprotection of Klotho is partially dependent upon the presence of EpoR.
DISCUSSION
EPO, a member of the type 1 cytokine superfamily, was first identified as an endocrine erythropoietic factor produced primarily by the kidney and required for maintenance of erythrocyte production51 by functioning as a ligand through its receptor (EpoR). EpoR belongs to the cytokine receptor super-family.52 At the cell surface, engagement of EPO results in activation of Jak2 kinase activity,53,54 which phosphorylates and translocates Stat5 into the nucleus to trigger transcription of specific target genes.55 Thus, phospho-Erk, phospho-Jak2, and phospho-Stat5 and its nuclear translocation are reliable indices for activation of EPO/EpoR signal transduction.56
Recent data suggest that EpoR is expressed in extra-hematopoietic tissues or cells including normal brain cells and tumor cells;57 retina;58 cardiomyocytes of ventricle and atrium;18,59 tumor cells such as endometrial adenocarcinoma,60 melanoma,61 breast cancer;62 cervical cancer,63 prostatic cancer,64 papillary thyroid cancer,65 Merkel cell cancer,66 and renal cancer;67 capillary endothelial cells or cell lines;24 arteriovenous fistulae in patients on hemodialysis;68 normal lung tissue, lung endothelial cells and lung cancer;21,69,70 white adipose tissue;71 dental pulps;72 glomerular mesangial cells and renal tubules;22 and renal cysts.67 This suggests that EpoR likely mediates a wide variety of extra-erythropoietic actions.73,74 An alternative view presented by one group of investigators favors an extremely restricted distribution and function of the EpoR to only erythropoietic tissues.30-32,75 These investigators submitted a three-point fallacy of the existence of EpoR transcripts that are likely not translated, non-specific labeling by commercial antibodies, and finally genuine expression of EpoR protein that are non-functional.30-32 However, this view remains to be proven. To unequivocally establish the presence of functional EpoR in kidney, we followed the criteria set forth by this school and demonstrated the presence of EpoR transcript by RT-PCR, protein labeling not by one but by multiple antisera, and activation of not one but multiple EpoR downstream signaling molecules upon EPO addition, and reduction of EpoR protein and signaling in cells by EpoR siRNA. This dataset allows us to conclude with confidence about the presence of functional EpoR in the kidney. However, it is important to note that problems with suboptimal antibody reagents remains. Is not known why different antibody detects EpoR bands of slightly different mobility. M-20 has been shown to identifies both EpoR and heat shock protein-70, thus M-20 definitely should not be used for immunohistochemistry.76 There is comparable pattern of labeling of EpoR in FLC, NRK cells, rat and mouse kidneys (Figure 1C) by M-20 and the anti-EpoR Fab. EpoR protein is barely detectable by A82 in rodent kidneys and weakly in FLC and NRK cells by due to its specificity for human EpoR.31
EpoR transcripts are prominently expressed in rat renal tubules but not in rat glomeruli, which is agreement of finding shown by De Beuf et al.40 Furthermore, our data is in agreement with published results from other independent laboratories showing that functional EpoR is present in non- hematopoietic cells because knockdown of EpoR abrogates EPO-induced cell proliferation in cancer cells,77 and constitutive activation of EpoR by mutation of EpoR protects renal tubules from ischemic injury53. In addition, Cassis et al. recently showed EpoR expression in the kidney by immunohistochemistry.78 De Beuf and colleagues revealed that EpoR expression is responsive to oxidative stress, because H2O2 up-regulated EpoR expression and induced accumulation of EpoR in the plasma membrane.50 More recently Doleschel and colleagues used fluorescent EPO as a probe and examined EpoR expression in murine organs by fluorescence-mediated tomography.79 The in vivo results showed that exogenous EPO is accumulated in the kidney and in vitro results validated the specific binding of labeled EPO to EpoR.79 Now, our findings provided proof of functional EpoR in the kidney.
The cytoprotection conferred by EPO is associated with anti-apoptosis and anti-oxidation.80-82 In cultured immortalized pig renal tubular cells and mouse mesangial cells, EPO protects against oxidative, cytotoxic or hypoxic injury81 but there was no direct evidence that the protective effect is mediated by EpoR. Our study shows that presence of EpoR in NRK is required for EPO to signal; knock-down of endogenous EpoR renders NRK cells more susceptible, and over-expression of EpoR more resistant to H2O2 toxicity. The non-erythropoietic effects of EPO have been proposed to be mediated by the common beta receptor (βcR), which heterodimerizes with EpoR.73 At present, it is not known whether the EpoR-dependent cytoprotection in NRK cells involves βcR.
Other studies have shown biologic effects of EPO on primary kidney cell culture or kidney cell lines.23,50,81,83,84 EPO stimulates EpoR-operated Ca2+ channels in rat mesangial cells.83 EPO binds to murine proximal tubules (Kd ~96 pM), human proximal tubules (Kd~1.1 nM), and human medullary collecting duct cells (Kd~1.3 nM) and stimulates DNA synthesis and cell proliferation in murine proximal tubular cells.23 Darbepoetin protects pig tubular and mouse mesangial cells against apoptosis stimulated by toxic and hypoxic insults81 and recombinant human EPO protects primary cultured human proximal tubule cells from hypoxic induced apoptosis and decreased cisplatin-induced apoptosis in human renal epithelial cell line.84 Epoetin-δ protects primary human renal tubular epithelial cells from oxidative stress induced by glucose oxidase.50 Administration of continuous erythropoietin receptor activator protects kidney from sepsis-induced acute injury.85 These findings are very compelling in concert but they do not demonstrate dependence on EpoR in the kidney. We furnish data that supports the notion that these experiments conducted with EPO may be acting directly on renal epithelium.
In addition to its original anti-aging function,34 Klotho has vast pleiotropic actions.37 In the context of acute kidney injury, Klotho confers renoprotection and promotes renal regeneration based on a variety of animal and cell models. Klotho upregulates endogenous antioxidants,86 inhibits apoptosis,39 mitigates cell senescence,87 increases cell resistance to oxidative injury by up-regulating HSP70,39 increases endogenous NGAL,38 maintains stem cells,87 promotes angiogenesis88,89 and endothelial function,89,90 and suppresses fibrogenesis.91 The present study provides a novel mechanism of Klotho action which is up-regulation of EpoR.
In NRK cells, Klotho increases EpoR expression and activates well established canonical components along the EPO/EpoR signaling cascade. The presence of EPO or fresh serum in cell culture medium is a prerequisite for Klotho to enhance EPO/EpoR signaling activity. So far, the mechanism by which serum-containing media affect Klotho-regulated EpoR signal transduction is not clear. The effect of fresh serum may be mediated by EPO but the current reagents do not allow us to quantify bovine. It is entirely possible that the serum effect is not due to EPO alone. By calculation (not measurement), 10% serum should lower than 50 IU/ml EPO which is required for protection of NRK cells against oxidative stress (Figure 6). It is likely that other factors in serum sensitize the Klotho effect. Regardless of how many factors are involved in conferring cytoprotection, knock-down of endogenous EpoR in NRK cells partially abolished Klotho's cytoprotection, further supporting the hypothesis that Klotho exerts its cytoprotective function at least in part by up-regulating EpoR signal transduction. In addition, Klotho also plays cytoprotection above and beyond EpoR-independent signal pathway.
In conclusion, Klotho exerts beneficial effects in acute kidney damage induced by oxidative stress.38 Our in vivo and in vitro studies show that Klotho upregulates EpoR expression in the kidney and a kidney cell line, amplifies the EPO-triggered signaling pathways, and protects NRK cells from oxidative injury in an EpoR-dependent fashion. These results provide novel insights into the mechanisms of Klotho action, and support an extra-erythropoietic role of the EpoR in renal tubular epithelia.
MATERIALS AND METHODS
Human study
The study was approved by the Institutional Review Board at the University of Texas (UT) Southwestern Medical Center. The human kidney samples were dissected from normal kidney tissues adjacent to the tumor tissue whose kidneys were surgically removed due to kidney carcinoma in affiliated hospital with UT Southwestern. Recruited subjects gave informed consent.
Animal study
Transgenic mice over-expressing Klotho (Tg-Kl; EFmKL46 line)43 and Klotho hypo-morphic mice (Kl-/-)34 were maintained at the Animal Research Center at the University of Texas Southwestern Medical Center. Wild type littermates were used as controls. Normal Sprague-Dawley (SD) rats (220-250 gm body weight) were intraperitoneally injected once with full extracellular domain of recombinant mouse Klotho protein (rMKl)43 at a dose of 0.01 mg/Kg BW.38 Mouse plasma EPO was determined using ELISA with mouse erythropoietin quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer's instruction. All animal work was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. Nephron segments and collecting ducts were dissected using established methods.92,93 Accuracy and specificity of dissection was verified by RT-PCR of segment-specific markers (Figure 1). Detailed protocols of animal study were shown in supplementary materials.
Isolated kidney infusion of EPO
SD rats were anesthetized with intraperitoneal injection of Ketamine:Xylazine:Acepromazine (50/5/1 mg/Kg). Kidneys were isolated and the renal arteries were cannulated with a blunted hypodermic needle. Once the needle was securely in the artery, the preparation was flushed with 10 ml of 1640 culture media (Invitrogen) until the kidney was blanched. A ligature was then tightened to secure the needle in the artery. The kidneys were transferred to a humidified petri dish at 37°C and perfused continuously with gassed (95% O2, 5% CO2) 1640 culture media containing EPO in (100 IU/ml) in a non-recirculating system under conditions of constant flow at a rate of 3.0 ml/min by means of a peristaltic pump (Instech Laboratories, Inc, Plymouth Meeting, PA) for 15 minutes. In total, around 40 ml of 100 IU/ml EPO were infused. Control solution did not contain EPO. After a 15-minute infusion, kidneys were harvested for further study.
Generation of synthetic anti-EpoR Fab
The synthetic human Fab was isolated from a phage-displayed library (Library F constructed by Sidhu Laboratory) with diversity introduced into the three heavy-chain complementarity-determining regions (CDRs) and the third light chain CDR. Biopanning, phage ELISAs and Fab protein purification were performed as described.94,95 Using competitive phage ELISAs, we estimated the affinity of Fab-6 for the EpoR-ECD to be in the single-digit nanomolar range (data not shown). Detailed protocol for generation of Fab antibody was shown in supplementary materials.
Cell culture
BaF3 cell line, a murine interleukin 3 (IL-3)-dependent hematopoietic pro-B-cell line, and normal rat kidney (NRK cells), a polarized renal tubular epithelial cell line with mixed proximal and distal characteristics, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). BaF3-HA-EpoR cell line was generated by stably expressing HA-tagged murine full length EpoR via a retroviral vector.96 Mouse fetal liver cells are isolated and purified from WT embryos at E13.5. Ter119 negative cells (Ter119-cells) are derived from murine fetal liver cells at E13.5 and lost of Ter119 antigen. Both cells are erythroid progenitors enriched EpoR.97 Detailed protocols regarding cell culture study were shown in supplementary materials.
RNA interference
Small interfering RNA (siRNA) of EpoR duplexes were designed by using the web-based BLOCK-iT RNAi Designer software (Invitrogen, Carlsbad, CA). Knockdown was achieved by cotransfection of two siRNA's per gene with Lipofectamine 2000. The efficiency of EpoR mRNA and protein knockdown was evaluated by RT-qPCR and immunoblot respectively to optimize experimental conditions (dose of siRNA and time after transfection). The information about siRNA oligonucleotides are shown in supplementary materials. EpoR-siNRA222 and its control222 were eventually selected as tool to knock down endogenous EpoR in NRK cells.
RNA extraction, RT-PCR and real time PCR
Total RNA was extracted using RNAeasy kit (Qiagen, Germantown, MD) from NRK cells or rodent kidney tissues as described.38,42 Complementary DNA (cDNA) was generated with oligo-dT primers using SuperScript III First Strand Synthesis System (Invitrogen, Carlsbad, CA) according to manufacturer's protocol. Primers used for detection of EpoR, NaPi-2a, NKCC2, AQP2, Klotho and β-actin were described in our previous publications.92,93 and shown in supplementary materials. PCR products were analyzed by 2% agarose ethidium bromide gel electrophoresis. Primers used for qPCR to detect EpoR and cyclophilin were described in supplementary materials.
LDH and TUNEL assays and immunocytochemistry
NRK cells were seeded in 12 well-plates and rendered quiescent overnight after 100% confluence. Next day, cells were treated with different concentration of H2O2 with or without rMKl Klotho. Supernatants were harvest for measurement of LDH release with LDH cytotoxicity detection kit (Clontech Laboratories Inc., Mountain View, CA). The apoptotic cells were detected with in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN). For immunocytochemisry, NRK cells were stained with primary antibodies (described in supplementary materials) followed by incubation with FITC-conjugated secondary antibodies (Invitrogen, Carlsbad, CA). Images were visualized with a Zeiss LSM-510 confocal microscope (Carl Zeiss, Advanced Imaging Microscopy, Germany).
Kidney immunohistochemistry
Four μm sections of paraffin embedded kidneys were made and subjected to immunohistochemistry. Primary antibodies used in this study were described in supplementary materials.
Immunoprecipitation and immunoblot
Immunoprecipitation of HA-EpoR protein from BaF-HA-EpoR cell lysates and immunoblotting was performed as previously38,92 and described in supplementary materials. The detailed method of preparation of kidney lysate is described in supplementary materials.
Statistical analyses
Data are expressed as the means ± SD. Statistical analysis was performed using unpaired Student's t-test, or analysis of variance (ANOVA) followed by Student-Newman-Keuls test whenever appropriate. A P value of ≤ 0.05 was considered statistically significant. Unless otherwise stated, representative figures reflect the results in a minimum of three independent experiments.
Supplementary Material
ACKNOWLEDGEMENTS
This work was in part supported by the National Institutes of Health (R21-HL096862, R01-DK092461, R01-091392, R01-HL089966), O'Brien Kidney Research Center (NIH P30DK-07938), American Heart Foundation Western Affiliate Beginning-Grant-in-Aid (0865235F), the Simmons Family Foundation, the Charles and Jane Pak Foundation, and an investigator-initiated GRIP grant from Genzyme Corporation. The authors are grateful to Dr. Steven Elliot (Amgen, Thousand Oaks, CA) for providing the anti-human EpoR antibody (A82); and to Kierste Miller, Jean Paek, and Yue Ma for technical assistance.
Footnotes
DISCLOSURE
All the authors declare no competing interests.
REFERENCES
- 1.David RB, Lim GB, Moritz KM, et al. Quantitation of the mRNA levels of Epo and EpoR in various tissues in the ovine fetus. Mol Cell Endocrinol. 2002;188:207–218. doi: 10.1016/s0303-7207(01)00718-3. [DOI] [PubMed] [Google Scholar]
- 2.Moritz KM, Lim GB, Wintour EM. Developmental regulation of erythropoietin and erythropoiesis. Am J Physiol. 1997;273:R1829–1844. doi: 10.1152/ajpregu.1997.273.6.R1829. [DOI] [PubMed] [Google Scholar]
- 3.Wintour EM, Butkus A, Earnest L, et al. The erythropoietin gene is expressed strongly in the mammalian mesonephric kidney. Blood. 1996;88:3349–3353. [PubMed] [Google Scholar]
- 4.Wu H, Liu X, Jaenisch R, et al. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 5.Bartesaghi S, Marinovich M, Corsini E, et al. Erythropoietin: a novel neuroprotective cytokine. Neurotoxicology. 2005;26:923–928. doi: 10.1016/j.neuro.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang YD, Hasan F, Giordano FJ, et al. Effects of recombinant human erythropoietin on platelet activation in acute myocardial infarction: results of a double-blind, placebo-controlled, randomized trial. Am Heart J. 2009;158:941–947. doi: 10.1016/j.ahj.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith KJ, Bleyer AJ, Little WC, et al. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59:538–548. doi: 10.1016/s0008-6363(03)00468-1. [DOI] [PubMed] [Google Scholar]
- 9.Calvillo L, Latini R, Kajstura J, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Takemura G, Li Y, et al. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–543. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DW, Pat B, Vesey DA, et al. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006;69:1806–1813. doi: 10.1038/sj.ki.5000356. [DOI] [PubMed] [Google Scholar]
- 12.Bi B, Guo J, Marlier A, et al. Erythropoietin expands a stromal cell population that can mediate renoprotection. Am J Physiol Renal Physiol. 2008;295:F1017–1022. doi: 10.1152/ajprenal.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahlmann FH, Fliser D. Erythropoietin and renoprotection. Curr Opin Nephrol Hypertens. 2009;18:15–20. doi: 10.1097/MNH.0b013e32831a9dde. [DOI] [PubMed] [Google Scholar]
- 14.Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharples EJ, Thiemermann C, Yaqoob MM. Mechanisms of disease: Cell death in acute renal failure and emerging evidence for a protective role of erythropoietin. Nat Clin Pract Nephrol. 2005;1:87–97. doi: 10.1038/ncpneph0042. [DOI] [PubMed] [Google Scholar]
- 16.Imamura R, Moriyama T, Isaka Y, et al. Erythropoietin protects the kidneys against ischemia reperfusion injury by activating hypoxia inducible factor-1alpha. Transplantation. 2007;83:1371–1379. doi: 10.1097/01.tp.0000264200.38926.70. [DOI] [PubMed] [Google Scholar]
- 17.Brines M. The therapeutic potential of erythropoiesis-stimulating agents for tissue protection: a tale of two receptors. Blood Purif. 2010;29:86–92. doi: 10.1159/000245630. [DOI] [PubMed] [Google Scholar]
- 18.Von Salisch S, Klar M, Thurisch B, et al. Gata4 and Sp1 regulate expression of the erythropoietin receptor in cardiomyocytes. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Shen K, Liu Z, et al. Regulated human erythropoietin receptor expression in mouse brain. J Biol Chem. 1997;272:32395–32400. doi: 10.1074/jbc.272.51.32395. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Zhang J, Moe OW, et al. Synergistic upregulation of erythropoietin receptor (EPO-R) expression by sense and antisense EPO-R transcripts in the canine lung. Proc Natl Acad Sci U S A. 2008;105:7612–7617. doi: 10.1073/pnas.0802467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echigoya MH, Obikane K, Nakashima T, et al. Glomerular localization of erythropoietin receptor mRNA and protein in neonatal and mature mouse kidney. Nephron Exp Nephrol. 2005;100:e21–29. doi: 10.1159/000084109. [DOI] [PubMed] [Google Scholar]
- 23.Westenfelder C, Biddle DL, Baranowski RL. Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int. 1999;55:808–820. doi: 10.1046/j.1523-1755.1999.055003808.x. [DOI] [PubMed] [Google Scholar]
- 24.Anagnostou A, Liu Z, Steiner M, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ammarguellat F, Gogusev J, Drueke TB. Direct effect of erythropoietin on rat vascular smooth-muscle cell via a putative erythropoietin receptor. Nephrol Dial Transplant. 1996;11:687–692. doi: 10.1093/oxfordjournals.ndt.a027361. [DOI] [PubMed] [Google Scholar]
- 26.Rundqvist H, Rullman E, Sundberg CJ, et al. Activation of the erythropoietin receptor in human skeletal muscle. Eur J Endocrinol. 2009;161:427–434. doi: 10.1530/EJE-09-0342. [DOI] [PubMed] [Google Scholar]
- 27.Jelkmann W, Bohlius J, Hallek M, et al. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol. 2008;67:39–61. doi: 10.1016/j.critrevonc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Nakano M, Satoh K, Fukumoto Y, et al. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res. 2007;100:662–669. doi: 10.1161/01.RES.0000260179.43672.fe. [DOI] [PubMed] [Google Scholar]
- 29.Tsai PT, Ohab JJ, Kertesz N, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinclair AM, Coxon A, McCaffery I, et al. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood. 2010;115:4264–4272. doi: 10.1182/blood-2009-10-248666. [DOI] [PubMed] [Google Scholar]
- 31.Elliott S, Busse L, McCaffery I, et al. Identification of a sensitive anti-erythropoietin receptor monoclonal antibody allows detection of low levels of EpoR in cells. J Immunol Methods. 2010;352:126–139. doi: 10.1016/j.jim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Swift S, Ellison AR, Kassner P, et al. Absence of functional EpoR expression in human tumor cell lines. Blood. 2010;115:4254–4263. doi: 10.1182/blood-2009-10-248674. [DOI] [PubMed] [Google Scholar]
- 33.Sinclair AM, Rogers N, Busse L, et al. Erythropoietin receptor transcription is neither elevated nor predictive of surface expression in human tumour cells. Br J Cancer. 2008;98:1059–1067. doi: 10.1038/sj.bjc.6604220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 35.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta-and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu M- C, Shiizaki K, Kuro-o M, et al. FIBROBLAST GROWTH FACTOR 23 AND KLOTHO: Physiology and Pathophysiology of an Endocrine Network of Mineral Metabolism. Ann Rev Physiol. 2013:75. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugiura H, Yoshida T, Mitobe M, et al. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant. 2010;25:60–68. doi: 10.1093/ndt/gfp451. [DOI] [PubMed] [Google Scholar]
- 40.De Beuf A, Verhulst A, Helbert MF, et al. Tubular erhtyropoietin receptor expression mediates erythropoietin-induced renoprotection. Hematology Journal. 2009;3:1–10. [Google Scholar]
- 41.Sawyer ST, Hankins WD. The functional form of the erythropoietin receptor is a 78-kDa protein: correlation with cell surface expression, endocytosis, and phosphorylation. Proc Natl Acad Sci U S A. 1993;90:6849–6853. doi: 10.1073/pnas.90.14.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lash LH, Putt DA, Matherly LH. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. J Pharmacol Exp Ther. 2002;303:476–486. doi: 10.1124/jpet.102.040220. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Bobulescu IA, Goyal S, et al. Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F761–766. doi: 10.1152/ajprenal.00117.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L, Dong H, Zhang CC, et al. A JAK2 interdomain linker relays Epo receptor engagement signals to kinase activation. J Biol Chem. 2009;284:26988–26998. doi: 10.1074/jbc.M109.011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belenkov AI, Shenouda G, Rizhevskaya E, et al. Erythropoietin induces cancer cell resistance to ionizing radiation and to cisplatin. Mol Cancer Ther. 2004;3:1525–1532. [PubMed] [Google Scholar]
- 48.Seong SR, Lee JW, Lee YK, et al. Stimulation of cell growth by erythropoietin in RAW264.7 cells: association with AP-1 activation. Arch Pharm Res. 2006;29:218–223. doi: 10.1007/BF02969397. [DOI] [PubMed] [Google Scholar]
- 49.Wenker SD, Chamorro ME, Vota DM, et al. Differential antiapoptotic effect of erythropoietin on undifferentiated and retinoic acid-differentiated SH-SY5Y cells. J Cell Biochem. 2010;110:151–161. doi: 10.1002/jcb.22521. [DOI] [PubMed] [Google Scholar]
- 50.De Beuf A, Hou XH, D'Haese PC, et al. Epoetin delta reduces oxidative stress in primary human renal tubular cells. J Biomed Biotechnol. 2010;2010:395785. doi: 10.1155/2010/395785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldwasser E. Erythropoietin and the differentiation of red blood cells. Fed Proc. 1975;34:2285–2292. [PubMed] [Google Scholar]
- 52.Longmore GD, Watowich SS, Hilton DJ, et al. The erythropoietin receptor: its role in hematopoiesis and myeloproliferative diseases. J Cell Biol. 1993;123:1305–1308. doi: 10.1083/jcb.123.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breggia AC, Wojchowski DM, Himmelfarb J. JAK2/Y343/STAT5 signaling axis is required for erythropoietin-mediated protection against ischemic injury in primary renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;295:F1689–1695. doi: 10.1152/ajprenal.90333.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 55.Huang LJ, Shen YM, Bulut GB. Advances in understanding the pathogenesis of primary familial and congenital polycythaemia. Br J Haematol. 2010;148:844–852. doi: 10.1111/j.1365-2141.2009.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kondyli M, Gatzounis G, Kyritsis A, et al. Immunohistochemical detection of phosphorylated JAK-2 and STAT-5 proteins and correlation with erythropoietin receptor (EpoR) expression status in human brain tumors. J Neurooncol. 2010 doi: 10.1007/s11060-010-0156-2. [DOI] [PubMed] [Google Scholar]
- 57.Marti HH, Wenger RH, Rivas LA, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim HW, Kim JL, Lee HK, et al. Enalapril alters expression of key growth factors in experimental diabetic retinopathy. Curr Eye Res. 2009;34:976–987. doi: 10.3109/02713680903249913. [DOI] [PubMed] [Google Scholar]
- 59.Wright GL, Hanlon P, Amin K, et al. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. Faseb J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 60.Giatromanolaki A, Fiska A, Pitsiava D, et al. Erythropoietin receptors in endometrial carcinoma as related to HIF1{alpha} and VEGF expression. In Vivo. 2009;23:699–703. [PubMed] [Google Scholar]
- 61.Selzer E, Wacheck V, Kodym R, et al. Erythropoietin receptor expression in human melanoma cells. Melanoma Res. 2000;10:421–426. doi: 10.1097/00008390-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Sfacteria A, Mazzullo G, Bertani C, et al. Erythropoietin receptor expression in canine mammary tumor: an immunohistochemical study. Vet Pathol. 2005;42:837–840. doi: 10.1354/vp.42-6-837. [DOI] [PubMed] [Google Scholar]
- 63.Leo C, Horn LC, Rauscher C, et al. Expression of erythropoietin and erythropoietin receptor in cervical cancer and relationship to survival, hypoxia, and apoptosis. Clin Cancer Res. 2006;12:6894–6900. doi: 10.1158/1078-0432.CCR-06-1285. [DOI] [PubMed] [Google Scholar]
- 64.Arcasoy MO, Amin K, Vollmer RT, et al. Erythropoietin and erythropoietin receptor expression in human prostate cancer. Mod Pathol. 2005;18:421–430. doi: 10.1038/modpathol.3800288. [DOI] [PubMed] [Google Scholar]
- 65.Eccles TG, Patel A, Verma A, et al. Erythropoietin and the erythropoietin receptor are expressed by papillary thyroid carcinoma from children and adolescents. Expression of erythropoietin receptor might be a favorable prognostic indicator. Ann Clin Lab Sci. 2003;33:411–422. [PubMed] [Google Scholar]
- 66.Kase S, Yoshida K, Osaki M, et al. Expression of erythropoietin receptor in human Merkel cell carcinoma of the eyelid. Anticancer Res. 2006;26:4535–4537. [PubMed] [Google Scholar]
- 67.Lee YS, Vortmeyer AO, Lubensky IA, et al. Coexpression of erythropoietin and erythropoietin receptor in von Hippel-Lindau disease-associated renal cysts and renal cell carcinoma. Clin Cancer Res. 2005;11:1059–1064. [PubMed] [Google Scholar]
- 68.Ikegaya N, Yamamoto T, Takeshita A, et al. Elevated erythropoietin receptor and transforming growth factor-beta1 expression in stenotic arteriovenous fistulae used for hemodialysis. J Am Soc Nephrol. 2000;11:928–935. doi: 10.1681/ASN.V115928. [DOI] [PubMed] [Google Scholar]
- 69.Foster DJ, Moe OW, Hsia CC. Upregulation of erythropoietin receptor during postnatal and postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1107–1115. doi: 10.1152/ajplung.00119.2004. [DOI] [PubMed] [Google Scholar]
- 70.Beleslin-Cokic BB, Cokic VP, Wang L, et al. Erythropoietin and hypoxia increase erythropoietin receptor and nitric oxide levels in lung microvascular endothelial cells. Cytokine. 2011;54:129–135. doi: 10.1016/j.cyto.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teng R, Gavrilova O, Suzuki N, et al. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nature communications. 2011;2:520. doi: 10.1038/ncomms1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gong Q, Jiang H, Wei X, et al. Expression of erythropoietin and erythropoietin receptor in human dental pulp. J Endod. 2010;36:1972–1977. doi: 10.1016/j.joen.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 73.Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–250. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 75.Laugsch M, Metzen E, Svensson T, et al. Lack of functional erythropoietin receptors of cancer cell lines. Int J Cancer. 2008;122:1005–1011. doi: 10.1002/ijc.23201. [DOI] [PubMed] [Google Scholar]
- 76.Elliott S, Busse L, Bass MB, et al. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:1892–1895. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- 77.Paragh G, Kumar SM, Rakosy Z, et al. RNA interference-mediated inhibition of erythropoietin receptor expression suppresses tumor growth and invasiveness in A2780 human ovarian carcinoma cells. Am J Pathol. 2009;174:1504–1514. doi: 10.2353/ajpath.2009.080592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cassis P, Gallon L, Benigni A, et al. Erythropoietin, but not the correction of anemia alone, protects from chronic kidney allograft injury. Kidney Int. 2012;81:903–918. doi: 10.1038/ki.2011.473. [DOI] [PubMed] [Google Scholar]
- 79.Doleschel D, Mundigl O, Wessner A, et al. Targeted near-infrared imaging of the erythropoietin receptor in human lung cancer xenografts. J Nucl Med. 2012;53:304–311. doi: 10.2967/jnumed.111.091124. [DOI] [PubMed] [Google Scholar]
- 80.Jie KE, Verhaar MC, Cramer MJ, et al. Erythropoietin and the cardiorenal syndrome: cellular mechanisms on the cardiorenal connectors. Am J Physiol Renal Physiol. 2006;291:F932–944. doi: 10.1152/ajprenal.00200.2006. [DOI] [PubMed] [Google Scholar]
- 81.Fishbane S, Ragolia L, Palaia T, et al. Cytoprotection by darbepoetin/epoetin alfa in pig tubular and mouse mesangial cells. Kidney Int. 2004;65:452–458. doi: 10.1111/j.1523-1755.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 82.Pallet N, Bouvier N, Legendre C, et al. Antiapoptotic properties of recombinant human erythropoietin protects against tubular cyclosporine toxicity. Pharmacol Res. 2010;61:71–75. doi: 10.1016/j.phrs.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 83.Marrero MB, Venema RC, Ma H, et al. Erythropoietin receptor-operated Ca2+ channels: activation by phospholipase C-gamma 1. Kidney Int. 1998;53:1259–1268. doi: 10.1046/j.1523-1755.1998.00887.x. [DOI] [PubMed] [Google Scholar]
- 84.Li J, Vesey DA, Johnson DW, et al. Erythropoietin Reduces Cisplatin-Induced Apoptosis in Renal Carcinoma Cells via a PKC Dependent Pathway. Cancer Biol Ther. 2007:6. doi: 10.4161/cbt.6.12.4975. [DOI] [PubMed] [Google Scholar]
- 85.Rodrigues CE, Sanches TR, Volpini RA, et al. Effects of continuous erythropoietin receptor activator in sepsis-induced acute kidney injury and multi-organ dysfunction. PLoS ONE. 2012;7:e29893. doi: 10.1371/journal.pone.0029893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 88.Shimada T, Takeshita Y, Murohara T, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 89.Kusaba T, Okigaki M, Matui A, et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U S A. 2010;107:19308–19313. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saito Y, Nakamura T, Ohyama Y, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 91.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–2450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuster DG, Zhang J, Shi M, et al. Characterization of the sodium/hydrogen exchanger NHA2. J Am Soc Nephrol. 2008;19:1547–1556. doi: 10.1681/ASN.2007111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.F.A. F, Sidhu SS. Making antibodies in bacteria. In: Howard GC, Kaser E MR, editors. Making and using antibodies. CRC Press; 2007. pp. 157–180. [Google Scholar]
- 95.Fellouse FA, Esaki K, Birtalan S, et al. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J Mol Biol. 2007;373:924–940. doi: 10.1016/j.jmb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Bulut GB, Sulahian R, Ma Y, et al. Ubiquitination regulates the internalization, endolysosomal sorting, and signaling of the erythropoietin receptor. J Biol Chem. 2011;286:6449–6457. doi: 10.1074/jbc.M110.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sulahian R, Cleaver O, Huang LJ. Ligand-induced EpoR internalization is mediated by JAK2 and p85 and is impaired by mutations responsible for primary familial and congenital polycythemia. Blood. 2009;113:5287–5297. doi: 10.1182/blood-2008-09-179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.