Abstract
Background
Facial transplantation is a complex procedure that corrects severe facial defects due to traumas, burns, and congenital disorders. Although face transplantation has been successfully performed clinically, potential risks include tissue ischemia and necrosis. The vascular supply is typically based on the bilateral neck vessels. As it remains unclear whether perfusion can be based off a single pedicle, this study was designed to assess perfusion patterns of facial transplant allografts using near-infrared (NIR) fluorescence imaging.
Methods
Upper facial composite tissue allotransplants were created using both carotid artery and external jugular vein pedicles in Yorkshire pigs. A flap validation model was created in n = 2 pigs and a clamp occlusion model was performed in n = 3 pigs. In the clamp occlusion models, sequential clamping of the vessels was performed to assess perfusion. Animals were injected with indocyanine green (ICG) and imaged with NIR fluorescence. Quantitative metrics were assessed based on fluorescence intensity (FI).
Results
With NIR imaging, arterial perforators emitted fluorescence indicating perfusion along the surface of the skin. Isolated clamping of one vascular pedicle showed successful perfusion across the midline based on NIR fluorescence imaging. This perfusion extended into the facial allograft within 60 seconds and perfused the entire contralateral side within 5 minutes.
Conclusions
Determination of vascular perfusion is important in microsurgical constructs as complications can lead to flap loss. It is still unclear if facial transplants require both pedicles. This initial pilot study using intraoperative NIR fluorescence imaging suggests that facial flap models can be adequately perfused from a single pedicle.
Keywords: Near infrared imaging, face transplantationm, composite tissue allotransplantation
Introduction
Full-facial transplantation is a complex procedure that attempts to correct severe facial defects to near-normal proportions and function in a single operation.[1] Since the first partial human facial transplantation in 2005 [2, 3], only 18 procedures have been performed worldwide.[4] Prior to face transplantation, reconstructive options for major facial deficits were limited to multiple surgeries of autologous tissues and alloplastic materials.[5, 6] In recent years, advances in microsurgical techniques have made full-facial transplantation a clinical reality.[3, 7]
Although there have not been any reported major complications related to arterial or venous anastomosis with face transplantation, the risks of anastomotic failure still exists for facial flaps as does with any free flap with micro-vascular anastomosis. Following surgery, facial transplant recipients face many potential complications.[8, 9] These can arise from the technical difficulties of microsurgical flap surgery, as well as immunologic, metabolic, and infectious factors. There exists a growing body of literature documenting the risks and complications unique to microsurgery in the head and neck region.[10] Among these risks, vessel thrombosis is believed to be a major contributor leading to partial flap loss, fat necrosis, and even total flap loss. Full-facial transplants are typically supported with bilateral pedicles. [11] However, successful perfusion of large portions of facial flaps from a single pedicle has been demonstrated with both experimental and clinical data. [12,13] Despite these previous studies and given the diversity of composite facial flaps, it still remains unknown whether a full-facial flap can be successfully supported by a single pedicle. Vascular complications may compromise one of the pedicles supporting a full facial graft, thereby potentially placing the transplant at risk. Therefore, the purpose of this initial pilot study was to determine whether or not a unilateral pedicle was sufficient to perfuse an entire, full-facial flap.
Recently, near-infrared (NIR) fluorescence angiography was introduced to the field of reconstructive surgery for the evaluation of skin perfusion in cutaneous flaps. [14] NIR fluorescence imaging capitalizes on the deep photon penetration of near-infrared light (700–900nm) into living tissues. When coupled with the injection of a NIR fluorophore, this technology permits rapid and quantitative assessment of local tissue perfusion at depths of several millimeters. [15]
Previously our group has validated the use of the Fluorescence-Assisted Resection and Exploration (FLARE™) system along with intravenous injection of indocyanine green (ICG) for performing intraoperative NIR fluorescence angiography. We have validated its role in the identification of suitable perforators for flap design, compared the location and number of perforators identified against a gold standard (X-ray angiography), assessed its potential for monitoring perfusion of various perforator flaps, and formulated indices for the quantification of venous drainage and arterial inflow. [15–19] This modality has proven useful in identifying, monitoring, and quantifying perfusion and vascular compromise in multiple perforator flaps. In this study, we report the perfusion of an entire facial flap based on a unilateral neck pedicle in porcine, full-facial composite tissue allografts. These results bode well for the future of full-facial transplantation and suggest that human full-facial allografts can potentially survive off a single, unilateral pedicle.
Materials and Methods
Animals
Animal studies were performed under the supervision of Beth Israel Deaconess Medical Center’s Institutional Animal Care and Use Committee (IACUC) in accordance with approved institutional protocol #046-2010. Five female 35-kg Yorkshire pigs (E. M. Parsons and Sons, Hadley, MA) were used in this study. Two models were used for our study, which included a validation and an experimental clamping model. In the validation models we created two flaps in two pigs to identify a reproducible model of perfusion in a full facial flap. A combination of full-face flap elevation, pedicle clamping, and near-infrared imaging with ICG was used to identify partial and complete perfusion. Once this model was reproducible and validated with ICG, we then proceeded with occluding clamping trials. Three pigs were used for the occlusion studies: imaging was performed with a side view and top view of the face.
The pigs were induced with 4.4-mg/kg intramuscular Telazol (Fort Dodge Animal Health, Fort Dodge, IA), intubated, and maintained with 2% isoflurane (Baxter Healthcare Corp., Deerfield, IL). All animals included in this study were healthy with no prior history of allosensitization. A 16-gauge central venous catheter was inserted into the femoral vein prior to surgery. Physiological parameters (electrocardiography, heart rate, oxygen saturation, and body temperature) were monitored during all experiments.
NIR Fluorescence Imaging and Quantitative Assessment
The light source and optics of the current FLARE™ imaging system are attached to an articulated arm, which allows for positioning in 3-dimensional space while maintaining a working distance of 18 inches between the optics and the surgical field.[17–21] A light- guide coupled RF plasma light source generates > 40,000 lx of white light (400 to 650 nm), and a fiber coupled laser-diode source ≈ 10 mW/cm2 of 760-nm NIR fluorescence provides excitation light over an area of 15 × 11 cm. [22] Color and NIR fluorescence images of the surgical field are acquired simultaneously via custom-designed optics and software. [15] For NIR angiography, 1.3 mg of ICG purchased from Akorn Inc., (Decatur, IL) was injected as a rapid bolus in 10 cc of saline. As in previous studies, fluorescence intensity (FI) reached a stable level 120 seconds after injection and so was imaged at this time point. [23]
Using the FLARE™ imaging system, perfusion of the flaps can be measured using fluorescence intensity (FI). The FI was measured for 120 seconds. The formula, [{(FIt=120/FIt=0)−1} × 100], was employed to show how much the FI increased during 120 seconds from base line (0 sec).
Full Facial Flaps
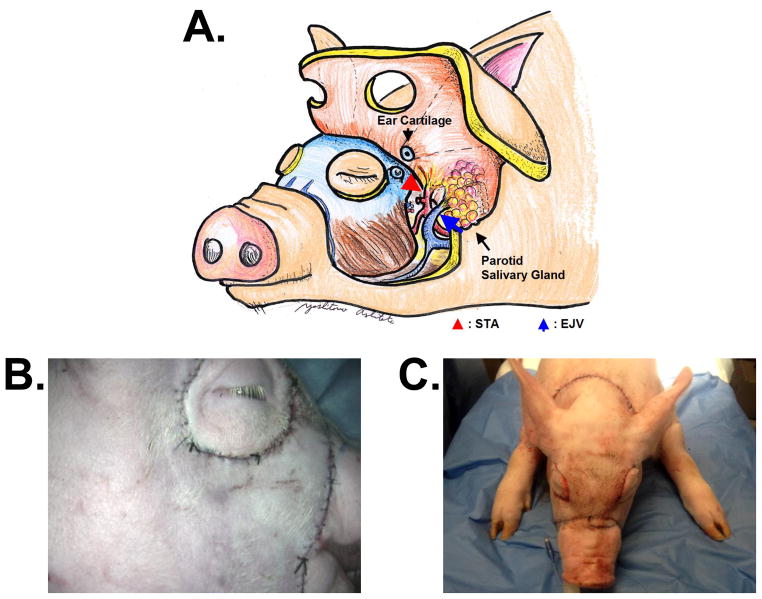
The full facial flaps on each animal were designed to include both right and left sided pedicle artery and vein, ear cartilage, and parotid salivary gland (Figure 1A). The superficial temporal artery (STA) was used for the pedicle artery and external jugular vein (EJV) was used for pedicle vein. The upper and lower eyelids were excluded from flap. The surgical procedure was similar to porcine hemi-facial flaps previously reported.[24] The skin was incised to the depth of the platysma muscle in the anterior neck and cleidooccipitalis and cleidomastoideus muscle in the posterior neck, to the depth of masseter muscles in the facial region, and above the skull in the parietal region. The flap was elevated above the trapezius, cleidooccipitalis muscle, cleidomastoideus muscle, masseter muscles, and skull. During elevation, the facial nerve and ear cartilage were transected. In the anterior neck region, once the platysma muscle, parotid salivary gland, and the tendon of the sternomastoideus muscle were transected, the EJV and common carotid artery (CCA) were exposed. After ostectomy of the paracondylar process, the external carotid artery (ECA) and the STA (one of the two terminal branches of the ECA) were exposed. The STA and EJV were preserved for pedicle vasculatures. In addition, to elevate the flap completely and to exclude peripheral blood flow, the maxillay artery and vein were ligated and transected. To preserve the blood supply of the remaining tissue, the facial artery and vein were preserved. These surgical procedures were performed on both left and right sides. After elevating the flaps completely, the skin incision was closed with 3-0 vicryl suture (Figure 1B&C).
Figure 1.
A. Schematic Drawing of a full facial flap.
B. Actual photo of a full facial flap (side view)
C. Actual photo of a full facial flap (front view)
Clamp model
After creating the flap, models were prepared to confirm transverse blood perfusion from the opposite pedicle. Three models were created in one pig. Because these models required clamping of the pedicle, a 4000 U heparin bolus was injected intravenously to prevent thrombus formation. First, pedicle artery and veins on both sides were clamped with a VASCU-STATT® II (Scanlan, St. Paul, MN) device. With bolus intravenous injection of ICG, the right-sided flap was imaged for 120 seconds (Both Pedicles Clamped Model). The clamp was removed immediately following imaging. Next, the right side flap was imaged without clamping any side following another ICG injection (Both Pedicles Opened Model). Third, pedicle artery and vein of only the right side was clamped while the left side was left patent. The right side flap was imaged with ICG injection (Opposite Side Pedicle Opened Model).
Finally, to observe perfusion across the midline to the other side of the flap, a transverse top view was acquired in another pig. In this pig, same as the Opposite Side Pedicle Opened Model, both pedicle artery and vein of only the right side were clamped while those of the left side were kept opened. Then top view was imaged with ICG [Opposite Side Pedicle Opened Model (top view)]. Perfusion was assessed using quantitation of fluorescence intensity (FI) measured in gray levels on a 12 bit (4095 levels) charge coupled device (CCD) camera and referred here as arbitrary units (A.U.).
Results
NIR Fluorescence imaging
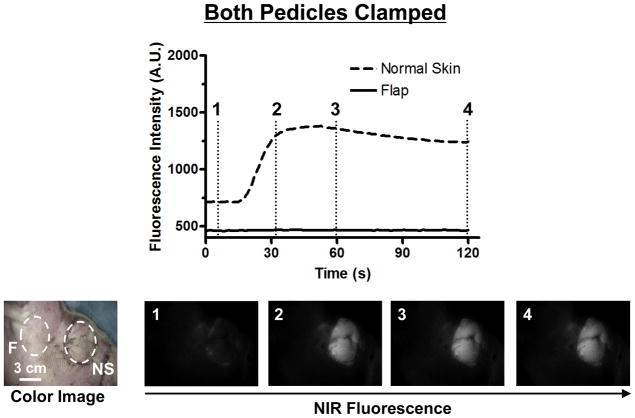
In the Both Pedicles Clamped Model, no perfusion of the flap was detected with NIR fluorescence imaging. However, the non-elevated normal skin around eyelids was illuminated (Figure 2): FI of the flap increased only 2% in 120 seconds while that of the normal skin increased 73%. This results in the complete lack of ICG entering into the partial upper face flap with contrasted illumination of adjacent non-elevated skin. Results from the Both Pedicles Clamped results correlated with both handheld Doppler ultrasound as well as with clinical judgment of flap discoloration. There was minimal detection of fluorescence in flaps as compared to normal skin, with peaks at approximately 1500 A.U. after 30 seconds.
Figure 2. Fluorescence Intensity and NIR Fluorescence Imaging (Both Pedicles Clamped Model).
1.3 mg of ICG was injected as a rapid bolus into the femoral vein. Fluorescence intensity (FI) over the normal skin (NS) and flap (F) are shown (top). Color image and NIR fluorescence images at the indicated time point (vertical dash line in top) are shown (bottom). ROIs are indicated by the dash circles on each flap.
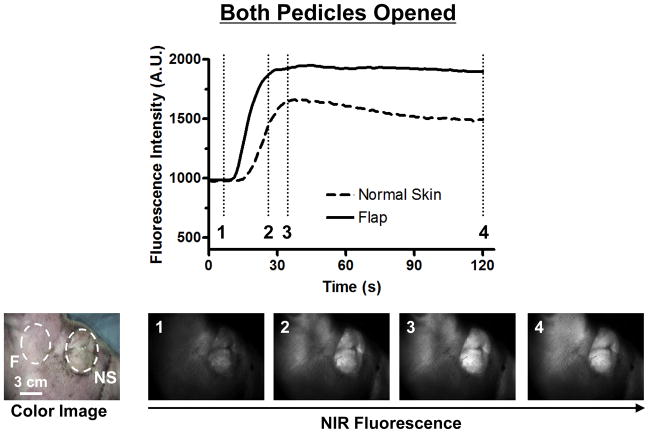
In the Both Pedicles Opened Model, NIR fluorescence imaging showed strong perfusion into the flap and normal skin (Figure 3). The FI curve showed a 92% increase in the flap, while that of the normal skin increased 53%. Both normal skin and flap fluorescence intensity rose in a similar manner, peaking at 1600 A.U and 1800 A.U., respectively after 30 seconds. Venous outflow and ICG clearance was observed in all vessels and composite flap tissue after ten minutes.
Figure 3. Fluorescence Intensity and NIR Fluorescence Imaging (Both Pedicles Opened Model).
1.3 mg of ICG was injected as a rapid bolus into the femoral vein. Fluorescence intensity (FI) over the normal skin (NS) and flap (F) are shown (top). Color image and NIR fluorescence images at the indicated time point (vertical dash line in top) are shown (bottom). ROIs are indicated by the dash circles on each flap.
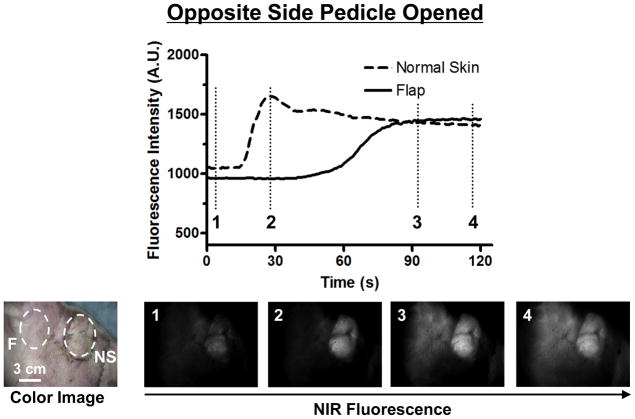
In the Opposite Side Pedicle Opened Model, NIR fluorescence imaging initially showed perfusion only on the open side (Figure 4). However, after 60 seconds, the perfusion crossed over the midline. The FI curve showed that the FI of the flap increased 51% in 120 seconds while that of the normal skin increased 34%. Although no ICG was seen initially in the contralateral side of the partial upper face flap with contrasted illumination of adjacent non-elevated skin, after 30 seconds FI in the normal skin increased acutely and peaked at 1600 A.U. In the flap, we do not see a change in FI initially, however, over time the level of fluorescence begins to rise after 60 seconds and peaking after 90 seconds to a FI level of 1500 A.U. Visually, we can see ICG entering into the flap originating superiorly from the opposite side.
Figure 4. Fluorescence Intensity and NIR Fluorescence Imaging (Opposite Side Pedicle Opened).
1.3 mg of ICG was injected as a rapid bolus into the femoral vein. Fluorescence intensity (FI) over the normal skin (NS) and flap (F) are shown (top). Color image and NIR fluorescence images at the indicated time point (vertical dash line in top) are shown (bottom). ROIs are indicated by the dash circles on each flap.
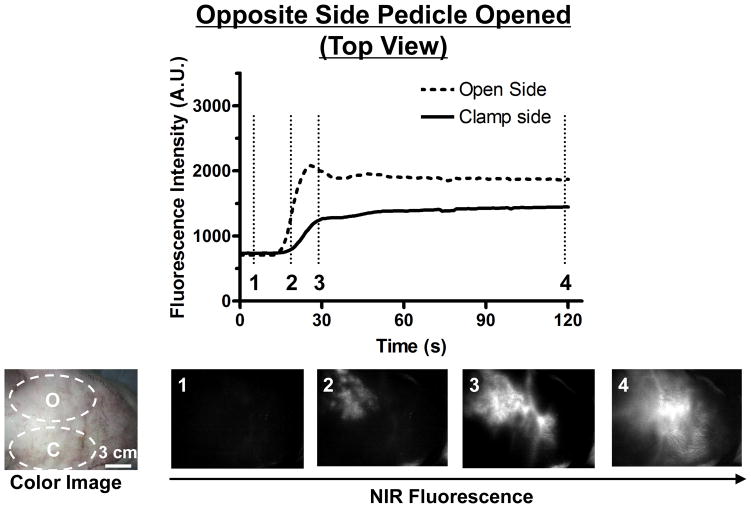
In the Opposite Side Pedicle Opened Model (top view), we can see ICG immediately entering the ipsilateral partial upper face, with the clamped contralateral side void of any contrast (Figure 5). Over time (30 sec) however, contrast from the unclamped side of the flap crossed the midline and migrated into the clamped side of the partial upper face flap. The FI curve showed that the FI of the clamped side increased 97% in 120 seconds while that of the open side increased 165%. After a longer period of time (5 min) ICG filling into the clamped side was complete. ICG transfer across the midline correlated with favorable clinical signs in the clamped side of our flap.
Figure 5. Fluorescence Intensity and NIR Fluorescence Imaging (Opposite Side Pedicle Opened; Top View).
1.3 mg of ICG was injected as a rapid bolus into the femoral vein. Fluorescence intensity (FI) over the open side (O) and the clamp side (C) are shown (top). Color image and NIR fluorescence images at the indicated time point (vertical dash line in top) are shown (bottom). ROIs are indicated by the dash circles on each flap.
Discussion
Full-facial transplantation is a complex procedure that requires rigorous screening for potential candidates and extensive preoperative planning. As facial composite tissue allotransplantation becomes more prevalent, it is increasingly important to have reliable and effective means of acquiring high-resolution arterial and venous images for preoperative vascular planning. Current methods for preoperative vascular mapping are primarily based on computed tomographic (CT) angiography and magnetic resonance (MR) angiography.
Surgeons performing facial transplantations benefit from the abundant and often redundant blood supply in the head and neck region.[24] In any microsurgical flap transfer, revascularization of the allograft following anastomosis between donor and recipient vessels is a critical step for long-term flap viability. During full-facial transplantation, vascular supply is achieved via bilateral neck pedicles. The preferred arterial anastomoses are the external carotid arteries[25] and their downstream branches, such as the facial arteries.[26,27] Additionally, the preferred venous anastomoses include the jugular and retromandibular veins.[27–29] In the current clinical experience, full- facial transplantation is accomplished with the microsurgical connection of bilateral pedicles supporting each half of a composite, full-facial transplant.
Although the vascular supply for a facial allograft typically uses bilateral neck pedicles, complications may compromise blood supply. These patients may have had burn or traumatic damage to the vessels from the initial injury. In addition, these patients often have had multiple previous surgical procedures, and often microsurgical free flaps that may have already manipulated these vessels. Given the diversity of facial allografts, it remains unclear whether a partial face allograft can be supported by a single pedicle. Although it is unclear whether full facial transplants are can survive on a single pedicle, bilateral pedicles are constructed to provide a safety net to protect an extremely valuable flap. In select candidates, it may be beneficial to perfuse a facial allograft by a single pedicle alone. The ability to design a facial transplant off of a single pedicle would substantially alter preoperative planning, and potentially simplify post-operative flap monitoring.
Our group has previously reported on the application of NIR fluorescence imaging as a novel approach for preoperative vascular planning and monitoring of tissue perfusion in porcine hemi-facial composite tissue allografts. [24] We have demonstrated that NIR fluorescence has the capability to assess perfusion of partial facial CTAs localization of perforators to the hemi facial CTA flap. In particular, an arterial and venous phase could be clearly identified. Additionally, our group has demonstrated that NIR imaging can provide real-time, intraoperative assessment of bone perfusion. [30]
In this study, we assess the perfusion pattern of face transplant models using near infrared (NIR) fluorescence imaging. As we have shown previously, NIR fluorescence angiography is highly valuable when used intraoperatively. The technology is fast, non-invasive, and can effectively examine the perfusion pattern of elevated flaps. Additionally, it can accurately identify defects in flap perfusion intra-operatively, signifying an acute failure and need for intervention.
In this study, we used traditional methods of clinical examination (skin color, turgor, and capillary refill), as well as hand-held Doppler, in conjunction with NIR imaging to assess and confirm the perfusion status of elevated facial flaps. With pre-operative NIR imaging, perforators can be localized and preserved to improve flap perfusion. ICG flow revealed patent, unoccluded facial flaps originating from the two pedicles. To further evaluate the perfusion pattern of the partial upper facial flaps, clamping trials were performed to evaluate the occlusion of both pedicles, and confirm the lack of arterial flow to the facial flap. This was critical to verify that the flap had been elevated properly and that perfusion was based solely on the isolated pedicles.
Unilateral arterial clamping was used to occlude all perfusion to one side of the flap. This was an attempt to simulate flap failure based on a single pedicle during or after transplantation, thus testing the sufficiency of a single pedicle for flap perfusion. Initially, there was a complete lack of ICG entering the ipsilateral side of the partial upper face flap with contrasted illumination of adjacent non-elevated skin. This is expected as perfusion originates only from the one unclamped pedicle. Over time, however, we observed ICG entering the contralateral flap, originating from the one open pedicle. ICG begins filling the flap superiorly and extending inferiorly into the flap, eventually completely illuminating the flap with ICG. This indicates that perfusion from the patent pedicle on the opposite side is eventually crossing over and perfusing the pedicle on the clamped side, suggesting that single pedicle perfusion is possible. More importantly, this pattern of perfusion is notably different to the original perfusion pattern seen from the pedicle.
With both pedicles patent, ICG filling begins inferiorly and migrates and extends superiorly above the ear. Over time, ICG completely fills the flap providing full perfusion to both sides, again confirming sufficiency of a single pedicle. With an overhead view, this perfusion pattern was also evident. After injection, there is initial ICG filling of the ipsilateral facial flap; however, over time ICG crosses over to the contralateral side, confirming perfusion of the opposite, clamped side.
There are however, limitations to this study. The facial flap elevated in this study is a full facial flap with our pedicle based on the external carotid artery and the external jugular vein. The structure of the pig’s skull and facial structures are somewhat different from a human as the flap is perfused mainly by the branches of the superficial temporal artery. This perfusion pattern would be consistent to a mid to upper facial flap in a human. Additional limitations include the restricted ability to monitor perfusion in a composite flap. Unfortunately, perfusion of the partial upper facial flap is based on NIR monitoring of the surface skin paddle, leaving us little information of the composite tissue below the surface. Another limitation of this technology is the slow and incomplete clearance of ICG within the time of surgery. Although ICG is a safe, widely used fluorophore (clinically approved for other indications), ICG is still present as a background following the first injection, and typically 15–20 min of clearance time is necessary between injections.
However, this new technology allows for real time evaluation of a technically challenging, microsurgical facial flap anastomosis. We were able to map perfusion of an allotransplant, and explore the possibility of basing a complex composite tissue flap on one pedicle. Are both pedicles necessary? Data shown here indicate that one might suffice.
Conclusion
NIR imaging is an emerging technology that shows promise in assessing tissue perfusion during elevation and harvest of composite flaps, in addition to monitoring perfusion after microsurgical anastomosis. This initial study employed NIR technology to define perfusion patterns of a facial flap. The results of our study suggest that a partial upper face allograft can be adequately perfused from a single pedicle.
Acknowledgments
Sources of Funding:
This study was funded by National Institutes of Health grants R01-DE-022820 and R01-CA-115296.
Footnotes
Financial Disclosure:
FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. It has been licensed to the FLARE™ Foundation, a non-profit organization focused on promoting the dissemination of medical imaging technology for research and clinical use. Dr. Frangioni is the founder and chairman of the FLARE™ Foundation. The Beth Israel Deaconess Medical Center will receive royalties for sale of FLARE™ Technology. Dr. Frangioni has elected to surrender post-market royalties to which he would otherwise be entitled as inventor, and has elected to donate pre-market proceeds to the FLARE™ Foundation. Dr. Frangioni has started three for-profit companies, Curadel, Curadel Medical Devices, and Curadel In Vivo Diagnostics, which may someday be non-exclusive sub-licensees of FLARE™ technology.
References
- 1.Gordon CR, et al. The Cleveland Clinic FACES Score: a preliminary assessment tool for identifying the optimal face transplant candidate. J Craniofac Surg. 2009;20(6):1969–74. doi: 10.1097/SCS.0b013e3181bd2c86. [DOI] [PubMed] [Google Scholar]
- 2.Devauchelle B, et al. First human face allograft: early report. Lancet. 2006;368(9531):203–9. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 3.Dubernard JM, et al. Outcomes 18 months after the first human partial face transplantation. N Engl J Med. 2007;357(24):2451–60. doi: 10.1056/NEJMoa072828. [DOI] [PubMed] [Google Scholar]
- 4.Siemionow M, Ozturk C. Face transplantation: outcomes, concerns, controversies, and future directions. J Craniofac Surg. 2012;23(1):254–9. doi: 10.1097/SCS.0b013e318241b920. [DOI] [PubMed] [Google Scholar]
- 5.Pacifico MD, Floyd D, Wood SH. Tibial stress fracture as a complication of free-fibula vascularised graft for mandibular reconstruction. Br J Plast Surg. 2003;56(8):832–4. doi: 10.1016/j.bjps.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Rogers B, et al. Computerized manufacturing of transparent face masks for the treatment of facial scarring. J Burn Care Rehabil. 2003;24(2):91–6. doi: 10.1097/01.BCR.0000054171.82007.7A. [DOI] [PubMed] [Google Scholar]
- 7.Gander B, et al. Composite tissue allotransplantation of the hand and face: a new frontier in transplant and reconstructive surgery. Transpl Int. 2006;19(11):868–80. doi: 10.1111/j.1432-2277.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 8.Lantieri LA. Face transplant: learning from the past, facing the future. Proc Am Philos Soc. 2011;155(1):23–8. [PubMed] [Google Scholar]
- 9.Meningaud JP, et al. Procurement of total human face graft for allotransplantation: a preclinical study and the first clinical case. Plast Reconstr Surg. 2010;126(4):1181–90. doi: 10.1097/PRS.0b013e3181ec2089. [DOI] [PubMed] [Google Scholar]
- 10.Wei FC, et al. The outcome of failed free flaps in head and neck and extremity reconstruction: what is next in the reconstructive ladder? Plast Reconstr Surg. 2001;108(5):1154–60. doi: 10.1097/00006534-200110000-00007. discussion 1161–2. [DOI] [PubMed] [Google Scholar]
- 11.Siemionow M, Agaoglu G. Allotransplantation of the face: how close are we? Clin Plast Surg. 2005;32(3):401–9. vii. doi: 10.1016/j.cps.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Siemionow MZ, et al. First U.S. near-total human face transplantation: a paradigm shift for massive complex injuries. Plast Reconstr Surg. 2010;125(1):111–22. doi: 10.1097/PRS.0b013e3181c15c4c. [DOI] [PubMed] [Google Scholar]
- 13.Meningaud JP, et al. Face transplant graft procurement: a preclinical and clinical study. Plast Reconstr Surg. 2008;122(5):1383–9. doi: 10.1097/PRS.0b013e3181882146. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelmi BJ, et al. First successful replantation of face and scalp with single-artery repair: model for face and scalp transplantation. Ann Plast Surg. 2003;50(5):535–40. doi: 10.1097/01.SAP.0000037875.69379.56. [DOI] [PubMed] [Google Scholar]
- 15.Rubben A, et al. Infrared videoangiofluorography of the skin with indocyanine green--rat random cutaneous flap model and results in man. Microvasc Res. 1994;47(2):240–51. doi: 10.1006/mvre.1994.1018. [DOI] [PubMed] [Google Scholar]
- 16.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Molecular Imaging. 2010;9(5):237–55. [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BT, et al. Intraoperative near-infrared fluorescence imaging in perforator flap reconstruction: current research and early clinical experience. J Reconstr Microsurg. 2010;26(1):59–65. doi: 10.1055/s-0029-1244805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui A, et al. Image-guided perforator flap design using invisible near-infrared light and validation with x-ray angiography. Ann Plast Surg. 2009;63(3):327–30. doi: 10.1097/SAP.0b013e318193493d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui A, et al. Submental perforator flap design with a near-infrared fluorescence imaging system: the relationship among number of perforators, flap perfusion, and venous drainage. Plast Reconstr Surg. 2009;124(4):1098–104. doi: 10.1097/PRS.0b013e3181b5a44c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui A, et al. Real-time intraoperative near-infrared fluorescence angiography for perforator identification and flap design. Plast Reconstr Surg. 2009;123(3):125e–7e. doi: 10.1097/PRS.0b013e31819a3617. [DOI] [PubMed] [Google Scholar]
- 21.Lee BT, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plast Reconstr Surg. 2010;126(5):1472–81. doi: 10.1097/PRS.0b013e3181f059c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui A, et al. Quantitative assessment of perfusion and vascular compromise in perforator flaps using a near-infrared fluorescence-guided imaging system. Plast Reconstr Surg. 2009;124(2):451–60. doi: 10.1097/PRS.0b013e3181adcf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gioux S, et al. First-in-human pilot study of a spatial frequency domain oxygenation imaging system. J Biomed Opt. 2011;16(8):086015. doi: 10.1117/1.3614566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen JT, et al. Face transplant perfusion assessment using near-infrared fluorescence imaging. J Surg Res. 2012;177(2):e83–8. doi: 10.1016/j.jss.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houseman ND, Taylor GI, Pan WR. The angiosomes of the head and neck: anatomic study and clinical applications. Plast Reconstr Surg. 2000;105(7):2287–313. doi: 10.1097/00006534-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Lantieri L, et al. Repair of the lower and middle parts of the face by composite tissue allotransplantation in a patient with massive plexiform neurofibroma: a 1-year follow-up study. Lancet. 2008;372(9639):639–45. doi: 10.1016/S0140-6736(08)61277-5. [DOI] [PubMed] [Google Scholar]
- 27.Pomahac B, et al. Evaluation of appearance transfer and persistence in central face transplantation: a computer simulation analysis. J Plast Reconstr Aesthet Surg. 2010;63(5):733–8. doi: 10.1016/j.bjps.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 28.Pomahac B, et al. Novel surgical technique for full face transplantation. Plast Reconstr Surg. 2012;130(3):549–55. doi: 10.1097/PRS.0b013e31825dc25c. [DOI] [PubMed] [Google Scholar]
- 29.Alam DS, et al. The technical and anatomical aspects of the World’s first near-total human face and maxilla transplant. Arch Facial Plast Surg. 2009;11(6):369–77. doi: 10.1001/archfacial.2009.80. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen JT, et al. Bone flap perfusion assesment using near-infrared fluorescence imaging. J Surg Res. 2012;178(2):e43–50. doi: 10.1016/j.jss.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]