Abstract
Worldwide, acute kidney injury (AKI) is associated with poor patient outcomes. Over the last few years, collaborative efforts, enabled by a common definition of AKI, have provided a description of the epidemiology, natural history and outcomes of this disease and improved our understanding of the pathophysiology. There is increased recognition that AKI is encountered in multiple settings and in all age groups, and that its course and outcomes are influenced by the severity and duration of the event. The effect of AKI on an individual patient and the resulting societal burden that ensues from the long term effects of the disease, including development of chronic kidney disease (CKD) and end stage renal disease (ESRD), is attracting increasing scrutiny. There is evidence of marked variation in the management of AKI which is, to a large extent, due to a lack of awareness and an absence of standards for prevention, early recognition and intervention. These emerging data point to an urgent need for a global effort to highlight that AKI is preventable, its course modifiable, and its treatment can improve outcomes. In this article, we provide a framework of reference and propose specific strategies to raise awareness of AKI globally, with the goal to ultimately improve outcomes from this devastating disease.
Introduction
AKI is now well established as a common, often under-recognized disorder, which is associated with a high risk for mortality, development of chronic kidney disease (CKD) and other organ dysfunction. This condition has both short and long-term effects on functional status, and leads to increased resource utilization (1–3). Several studies have confirmed that while the course of AKI is variable based on the setting where it occurs, the severity and duration of AKI determines outcomes, including dialysis requirement, renal functional recovery and survival (4, 5). There is increasing recognition both of the effect of AKI on the individual patient, and the resulting societal burden ensuing from its long term effects, including development of CKD and end stage renal disease (ESRD) requiring dialysis or transplantation(6).
Data derived from high-income countries (HI), using standardized definitions for diagnosis and staging of AKI, have facilitated comparisons of incidence and outcomes in different clinical settings (7). Conversely, a paucity of information on the prevalence, course and outcomes of AKI in low and middle income (LMI) countries contributes to a somewhat biased view of AKI as a disease of hospitalized patients. In LMI countries, while the majority of urban cases occur in the context of acute illness, usually in association with hypovolemia and sepsis, AKI occurring in the community (e.g. diarrheal states, malaria) is under-recognized(8, 9).
Despite emerging data and increasing interest in AKI as a major contributor to adverse outcomes, there is still considerable lack of understanding of the condition among physicians, allied personnel and the lay public. As a result, systematic efforts have been limited and few resources have been allocated to inform health care professionals and the public of the importance of AKI as a preventable and treatable disease. Recent publications have highlighted deficiencies and wide variation in the care of AKI patients worldwide (10, 11). These reports have demonstrated missed opportunities to prevent and detect AKI, and suboptimal management.
AKI is encountered in multiple settings and is commonly first encountered by nonspecialized health care providers, either in the community or the hospital setting. Since AKI is not associated with any specific symptoms and the diagnosis is largely based on measurement of lab parameters, it is essential that caregivers be educated on the risks for AKI and equipped with the knowledge for early recognition, timely intervention and effective follow up.
This article provides a rationale and a proposal for a calibrated approach to raise awareness of the incidence and consequences of AKI to all potential stakeholders, including patients. Our ultimate goal is to improve the recognition and timely management of this silent killer, and to emphasize the need for collaboration among all those involved in the care of these patients
Why do we need to raise awareness of the importance of acute kidney injury?
a. AKI is common worldwide and is encountered in multiple settings but remains poorly recognized
The incidence of AKI worldwide varies widely across different studies and is largely dependent on the setting (hospital (h-AKI) vs. community-acquired (c-AKI)) and the at-risk populations investigated. It affects between 7 and 18% of hospital inpatients (12–15) and ranges from 20–200 per million population in the community (Tables 1 and 2)(16). Hospital-acquired AKI (h-AKI) is more common in the elderly; patients presenting with community acquired AKI (c-AKI) are usually younger and healthier (17). In HI countries, while mild AKI developing in the general hospital ward has become less common, more severe forms in the intensive care unit (ICU) have become predominant. Among critically ill patients, the incidence of AKI varies between 30 and 70%; approximately 5% of ICU admissions require renal replacement therapy (RRT) (18). In LMI countries, clinical presentation of AKI is dependent on location: while in larger cities, ICU-acquired h-AKI predominates, in rural areas, c-AKI is more common.
Table 1.
Reported incidence of AKI in high-income (HI) and low- and middle-income (LMI) countries. (Modified from (8)
| Community Acquired | Change In Incidence | Hospital Acquired | Change In Incidence | |
|---|---|---|---|---|
| HI Countries | 200 PMP | 51 To 62% | 60–288/100,000 Pop | 6.8 Times Increase; 11%/Year Increase |
| LMI Countries | 20 PMP | No Significant Change | 5.4/100,000 Pop | 1.06 Increase Over 5 Years |
POP: population; PMP: per million population
Table 2.
Incidence of hospital and community acquired AKI in the world (Modified from (8)
| Reference | Location | Study features | Incidence | Change in incidence over time |
|---|---|---|---|---|
| Hospital acquired AKI in the developed world | ||||
| Hou et al (1983)(99) | US | 2,262 adult admissions | 4.9% of admissions | N/A |
| Nash et al (2002)(12) | US | 4,622 adult admissions | 7.2% of admissions | N/A |
| Waikar et al (2006)(100) | US | Nationwide sample of 5,563,381 adults discharged 1988 to 2002 | 61–288 per 100,000 head of population | 4.7 times increase |
| Waikar et al (2006)(100) | US | Nationwide sample of 5,563,381 adults who required dialysis discharged 1988 to 2002 | 4–27 per 100,000 head of population | 6.8 times increase |
| Xue et al (2006)(19) | US | 5,403,015 adults discharged between 1992–2001 from Medicare database | 23.8 per 1,000 hospital discharges | 11% per year increase |
| Uchino et al (2005)(18) | International | Multicenter study 1,738/29,269 ICU patients in 23 countries between 2000–2001 | 5–6% of ICU admissions; 80% received dialysis | N/A |
| Hospital acquired AKI in LMI countries | ||||
| Abraham et al (1989)(101) | Kuwait (400,000 inhabitants) | 77 adults admitted university hospital 1984–1986 | 5.4 per 100,000 head of population per year | N/A |
| Jha et al (1992)(23, 102) | North India | 190 of 29,503 adults presenting to a referral center 1 year period | 6.4 per 1,000 admissions per year | N/A |
| Noronha et al (1997)(23) | Sao Paulo, Brazil | Review of adult inpatients | 7.9 per 1,000 admissions | N/A |
| Thomas et al (2000)(103) | Trinidad and Tobago | AKI post 205 cardiac surgeries 1993–1997 | 21 per 1,000 surgeries | N/A |
| Al-Homrany et al (2003)(104) | Saudi Arabia | 26,000 adults 2 year period | 3.7 per 1,000 admissions | N/A |
| Kohli et al (2007)(105) | Chandigarh, India | 294/33,301 admissions large urban center 1 year period | 2.1 per 1,000 admissions | N/A |
| Wang et al (2005 and 2007)(106, 107) | Peking, China | Retrospective review 225,000 inpatients university center 1994–2003 | 0.36 per 1,000 admissions | 1.06 times increase over 5 years |
| Community acquired AKI in the developed world | ||||
| Kaufman et al (1991)(108) | US | 100 adults, increase SCr> 2 mg/dl or A/CKD | 1% hospital admissions | N/A |
| Feest et al (1993)(109) | UK | 125/444,971 adults, SCr>5.5 mg/dl. Requiring dialysis |
172 PMP 22 PMP |
N/A |
| Chanard et al (1994)(110) | France | Adults who completed a questionnaire 1991 | 104 PMP | N/A |
| Liaño and Pascual (1996)(111) | Madrid, Spain | Multicenter; 665/4.2 million adults SCr>2 mg/dl or 50% increase in CKD Adults, 36% required dialysis |
209 PMP 75 PMP |
N/A |
| Khan et al (1997)(84) | Scotland | 311 adults, increased SCr or A/CKD 52/311 severe AKI |
620 PMP/year (50 PMP/yr. requiring dialysis) 102 PMP |
N/A |
| Stevens et al (2001)(30) | UK | 288/593,000 adults | 486 PMP/year | N/A |
| Metcalfe et al (2002)(112) | Scotland | Census assessment; 375 adults PMP dialyzed | 203 PMP/year | N/A |
| Obialo et al (2000)(113) | US | Hospital discharges 1994–1996, 100 African-American; SCr>2.0 mg/dl; de novo AKI | Community acquired AKI 0.55%; hospital acquired 0.15% | N/A |
| Prescott et al (2007)(114) | Aberdeen, Scotland | Adults requiring dialysis 2002 | 282 PMP/year | N/A |
| Ali et al (2007)(115) | Scotland | 523,390 adults in 2003; AKI and A/CKD RIFLE criteria | 1,811 PMP AKI AND 336 PMP A/CKD | N/A |
| Hsu et al (2007)(16) | US | Kaiser Permanente California; 61,269 adults no dialysis AKI 1996–2003 3,885 adults dialysis AKI 1996–2003 |
Increase from 322.7 to 522.4 per 100,000 person-years Increase from 19.5 to 29.5 per 100,000 person-years |
62% increase 51% increase |
| Community-acquired AKI in LMI countries | ||||
| Abraham et al (1989)(101) | Kuwait | 77 adults presenting to university hospital | 4.1 per 100,000 head of population/year | N/A |
| Seedat and Nathoo (1993)(59) | Durban, South Africa | Adults, 1986–1988 | 20 PMP | No change 1980–1990 |
| Anochie and Eke (2005)(116) | Nigeria | Children presenting referral center | 11.7 per million children/year | N/A |
| Al-Homray (2003)(104) | Saudi Arabia | 26,000 adults during 2 years observation | 2.3 per 1,000 admissions | N/A |
| Vukusich et al (2004)(117) | Santiago, Chile | 10 urban centers, 114 adults requiring dialysis 6-month period | 0.31 per 1,000 discharges | N/A |
| Kohli et al (2007)(105) | Chandigarh, India | 294 of 33,301 admissions large urban medical center 1 year period | 6.6 of 1,000 admissions | N/A |
| Wang et al (2005/2007)(106, 107) | Peking, China | Retrospective review 225,000 patients university center 1994–2003 | 0.54 per 1,000 admissions | N/A |
The etiology of h-AKI in urban areas of both HI and LMI countries is similar, but treatment resources differ widely (18–20). Hospital-acquired AKI develops in the ICU, in the context of multiple organ failure, post-cardiovascular procedures or sepsis, or as a complication of nephrotoxic medications. Patients affected are generally elderly, obese and diabetic. Conversely, the etiology of c-AKI differs considerably between HI and LMI countries. In LMI countries, AKI is often a disease of the young, caused by specific infections or toxins resulting in diarrhea and hypovolemia. Etiology is dependent on geographical location and may be secondary to infectious agents such as malaria, leptospirosis, dengue, envenomation (snakes or arthropods), and be subject to seasonal variation. In those regions, HIV disease, obstetric complications (e.g. septic abortion), or intoxications (often caused by traditional herbs or household products) are prevalent (21–27).
A common factor across HI and LMI countries is that most cases of AKI are managed by non-nephrologists, who maybe unfamiliar with the risk factors and early manifestations of the disease. This contributes to delayed recognition and suboptimal management. For instance, fluid accumulation decreases of serum creatinine concentration by increasing total body water, thus contributing to delayed diagnosis and underestimation of the severity of AKI (28, 29).
The quality of care delivered to hospitalized patients with AKI has been recently reviewed in different studies and revealed large gaps in the care including delayed recognition, inadequate investigations, deficient monitoring, delayed and often flawed management and lack of follow up (30),(31). These findings were encountered even when nephrologists were available and illustrate the clear need for educating caregivers in all disciplines regarding this disorder.
In LMI countries, insufficient and late recognition is even more problematic both in the community and in the hospital setting. Late recognition leads to delayed management, by which time associated morbidity and mortality have worsened considerably. Late recognition may account for the apparently 10-fold lower incidence in LMI countries: it is likely that AKI cases are underreported due to several factors, including access to appropriate medical care, lack of knowledge, and non-availability of standard tests that are considered routine in the developed world (e.g. serum creatinine). As an example, a recent report describes an “epidemic” of kidney disease in Central America that is blamed for several deaths annually in impoverished sugar cane workers across Nicaragua, Costa Rica, El Salvador and Guatemala. It is believed that the condition results from repetitive episodes of AKI secondary to dehydration. Standard work-days of 12–14 hours, with double shifts during the summer planting season when temperatures top 40° C likely cause heat stroke, rhabdomyolysis and AKI. Alternative mechanisms including environmental toxins and pesticides are being evaluated (32). Survivors develop a disproportionate degree of CKD. Limited resources make chronic dialysis treatment unlikely, thus making the condition quite deadly.
b. AKI contributes to adverse outcomes including Chronic Kidney Disease and Mortality
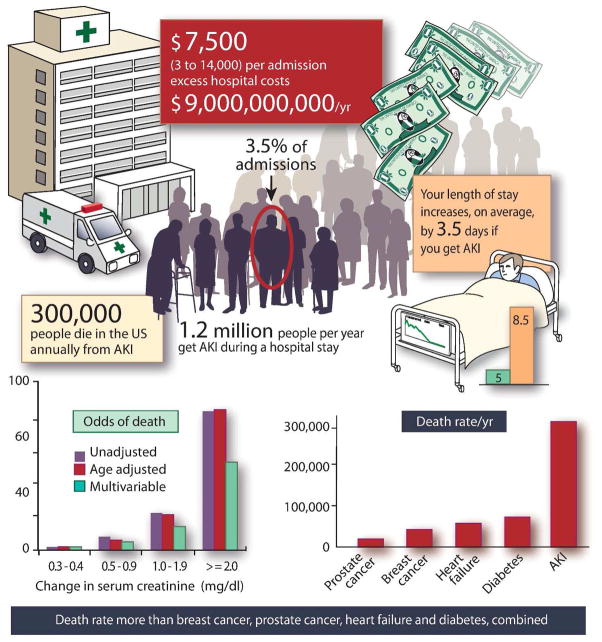
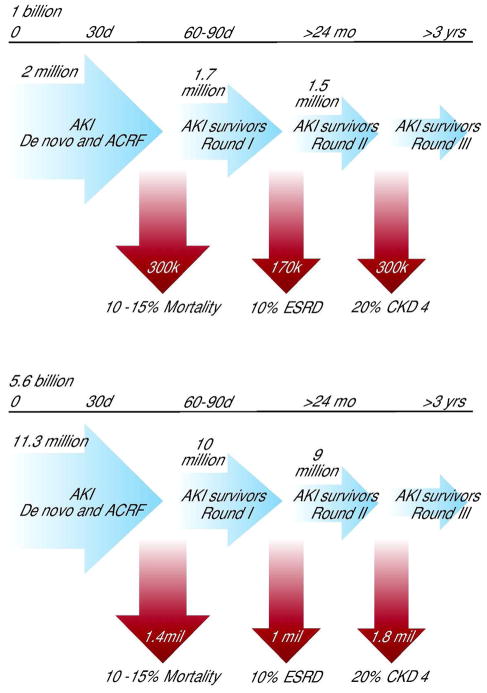
Several epidemiological studies have demonstrated the independent association of AKI with a higher risk of death. As severity increases so does mortality, which is highest among patients requiring renal replacement therapy (50–60%) (Figure 1) (8). Duration and severity of AKI predict progression to CKD and contribute to the unabated increase in the number of ESRD patients (4, 5). Older patients are at higher risk of AKI, as they have less renal functional reserve and are affected by multiple co-morbidities. After the acute episode, significant increase in cardiovascular risk associated with CKD creates new demand for follow-up and treatment (33–39). Although a direct causal relationship for AKI leading to CKD has not been clearly established(40), there is considerable evidence from both animal and human studies supporting a strong association of AKI with CKD (41). For instance, a fourfold increase in the incidence of AKI over the last 15 years, and the higher proportion of severe AKI requiring dialysis (which has nearly doubled), have been implicated as important determinants to the increase in the incidence of CKD (42–44). Figures 2a and b illustrate the burden of cases of AKI, deaths and progression to CKD in HI and LMI countries. In the latter, calculations were made assuming a similar incidence as in HI countries; actual data is, as discussed, currently unavailable.
Figure 1.
The global burden of AKI (Modified from (13)
Figure 2.
FIGURE 2A: HIGH INCOME COUNTRIES
FIGURE 2B: LOW AND MIDDLE INCOME COUNTRIES
Figures 2A and 2B illustrate the burden of cases of AKI, deaths and progression to CKD in HI and LMI countries. In the latter, calculations were made assuming a similar incidence as in HI countries; actual data is unavailable. (Figures modified from (118)
Most of the data on AKI outcomes are derived from studies in HI countries: in LMI countries, the long-term impact of AKI is almost completely unknown. The limited available evidence suggests that AKI may represent one of the main causes of CKD and ESRD in those countries, especially among children (45, 46), equally as important as the increasing incidence of obesity and diabetes. In El Salvador, end stage renal disease is the leading cause of hospital deaths in adults, the second cause of death in men and the fifth leading cause of death in adults of both sexes in the general population (32).
c. AKI is preventable, treatable and reversible
Although intuitively true, evidence that prevention is indeed associated with better outcomes is hindered for two main reasons. In HI countries, diversity of definitions and heterogeneous management hamper interpretation of whether prevention is associated with benefit. Preventive treatment has been demonstrated for only a few etiologies of AKI, including radio-contrast-induced and post-cardiac surgery, but there is persistent controversy on the best methods of prevention (47). When AKI develops in the context of multiple organ failure and sepsis, late diagnosis thwarts preventive treatment. Results of the ongoing Surviving Sepsis Campaign large randomized controlled trials may in the future clarify this point. Recent studies addressing the impact of early nephrology consultation (31, 48, 49) leading to simple preventive and management measures (e.g. providing proper volume assessment and adjusting medication regimens to prevent further hemodynamic or toxic kidney injury) have shown a decrease in the incidence and severity of h-AKI. Given the observational nature of those studies, residual confounding may explain the association with timely nephrology consultation. (31). Advances in electronic medical records and e-Alerts are emerging as potential approaches for identifying high-risk patients and modifying the use of nephrotoxic medications (15) (15, 50, 51).
In LMI countries c-AKI is often preventable by simple measures such as rehydration of volume-contracted patients with cholera, or use of bed netting to avert severe malaria (52–55). This area is nevertheless not free of contention, as recent evidence in Sub-Saharan Africa suggesting that vigorous volume expansion may be damaging in children primarily affected by malaria-induced AKI (56).
d. AKI causes a societal burden
The proportion of patients with renal failure who receive treatment is a reflection of each country’s economic status(8). There is a clear correlation between the number of nephrologists, dialysis facilities, number of patients on chronic dialysis and a country’s gross domestic product (GDP) (57). In LMI countries, hemodialysis is a rare and expensive resource. Peritoneal dialysis is often a better and less costly alternative but it is generally available for only a limited period of time, to support the patient through the acute phase. Concerns have been raised on the adequacy of peritoneal dialysis therapy for the treatment of hypercatabolic AKI (58–61), but recent studies (22, 62–66) have demonstrated good results. Patients who fail to recover kidney function are unlikely to receive long-term RRT(67). Lack of healthcare workers in LMI countries has been a major constraint in limiting progress in initiatives such as the HIV “3 by 5” and Millennium Development Goals. Lack of human and financial resources in LMI has hampered nephrology programs both in detection and prevention of CKD and the ability of doctors, nurses and other personnel to provide acute and chronic dialysis and transplantation(68). In HI countries outcomes post AKI may be dismal in the median- and long-term, with a large number of patients ending their lives in nursing homes or disabled (69, 70). Given the increasing burden of CKD, even HI countries will soon become unable to appropriately afford the increasing costs of a growing ESRD population.
e. AKI is poorly managed
Recent reports and anecdotal experience demonstrate that it is necessary to improve the recognition and response to patients developing AKI and to improve the management of the condition once it has occurred. The UK National Confidential Enquiry into Patient Outcomes and Death (NCEPOD) Adding Insult to Injury AKI Study, reported in 2009 that only 50% of patients who died from a diagnosis of AKI received good care (71). There were deficiencies in the recognition and management of patients who developed AKI. A further NCEPOD report (72), confirmed that a significant proportion of patients over the age of 80 who died within 30 days of surgery, developed AKI. It was recommended that there should be improvements in general medical care and risk assessment of AKI in this patient group. Failure to recognize the condition results in a delay in treatment and appropriate referral. Aitken et al (73) showed in a cohort of over 1500 patients in a single hospital in Glasgow that AKI was unrecognized in 23.5% of patients, of which 2/3 were discharged without resolution of renal function. Significant weaknesses were found in the management including poorly kept fluid balance charts (48.2%), failure to adjust nephrotoxic drugs (38.2%) and failure to act on abnormal biochemistry results (41%). The recent outbreak of hemolytic uremic syndrome (HUS) associated with serotoxin E. Coli in Germany highlights the importance of having well-established AKI patient pathways to cope with significant outbreaks of AKI associated with food poisoning (74).
Cultural and political differences often impact on the incidence and management of AKI. Thus, policies on abortion and obstetric complications have a clear impact on the incidence of septic AKI or pre-eclamptic complications in pregnant women with poor prenatal care in both LMI and HI countries.(8, 75–77) In LMI countries, cultural barriers often impair seeking treatment of AKI, and patients prefer the help of traditional healers.(77, 78) Practical geographical and transportation barriers impair the early management of AKI and emphasize the need of early prevention measures. Gender, age and income discrimination impact the outcome of AKI(8). Thus, being a female child is a risk factor for worse AKI survival in Africa and India, and being poor and Black worsens the survival of disease in Africa and in America(8, 59, 79–83).
It must not be forgotten that although the majority of cases of AKI in HI countries result from sepsis and shock, a significant proportion of cases still develop AKI due to inappropriate medications or rarer causes such as vasculitis and obstruction that require prompt referral to nephrologists or urologists. Delays in recognizing AKI in these specific settings will potentially lead to irreversible injury. This reinforces the need to provide prompt and appropriate therapy to patients developing AKI wherever it is diagnosed. In many cases, radiology services for diagnostic ultrasound or interventional procedures are not guaranteed 24 hours, seven days a week. Hospitals need to consider how to provide such specialized services when not available on-site.
e. Healthcare workers are not well informed about the disease and its consequences
AKI is ubiquitous; given the myriad of factors contributing to its development, it is essential that healthcare workers and the public be aware of the risk factors for AKI and the key concepts in prevention and management. For instance, c-AKI may result from the use of non-steroidal drugs to control pain after a dental procedure in a patient with underlying CKD, if patient and prescriber are unaware of the risks. Similarly, although public awareness of CKD has improved considerably with concerted educational efforts across the world, emphasis has been on controlling hypertension and diabetes as potential causes, without much discussion on preventing AKI as a potential modifiable risk factor. Often, the earliest reversible phases of AKI may be missed because of lack of awareness.
Who is the target audience?
Given the relative paucity of nephrologists worldwide in relation to the prevalence of AKI (approximately 40,000 nephrologist worldwide for 7 billion population) only a fraction of patients who get AKI are seen by nephrologists, and in most instances a range of other healthcare professionals provide initial management. In the UK, only 22 to 31% of AKI patients were referred to nephrologists (71, 84), with a bias against referring older and more comorbid patients (84). Additionally, in LMI countries availability for specialty care may be further limited e.g. in India there are approximately 1200 nephrologists to care for a 1.2 billion population. As AKI will most commonly initially present to non-nephrologists, it is essential to identify other healthcare professionals who can make a significant difference to the prevention, detection and management of AKI. First and foremost, physicians in primary care and specialists are essential targets as they are already knowledgeable about kidney disease but may not be as familiar with the recent advances in AKI. Providing them with the evidence that small changes in renal function contribute to often severely adverse outcomes, and updating their knowledge for risk assessment, prevention, diagnosis and management, will be key. Professionals who directly care for patients (e.g. dentists, chiropractors) will similarly need to be engaged, as they are very likely to encounter patients and prescribe potentially nephrotoxic medications.
Allied health professionals delivering patient care are a second major group that needs to be involved, in the community as well as in the hospital. Nursing plays a key role as an integral part of the patient care team. In this sense, care may be quite different based on available local resources. In HI countries, nurses play a central role in the care of hospitalized patients where standards of care are well established. Credentialing is required and ongoing educational programs are mandated to maintain nursing competence. In contrast, in LMI countries there is wider variation in the level of nursing care. Care is often provided in small private clinics where standards for nursing care may be highly variable and educational activities may not be as well defined.
In LMI countries, the role of allied health personnel in rural environments cannot be overemphasized. In those regions, where physicians are generally unavailable, most of the initial preventive strategies can and should be implemented by nurses and allied health professionals under nursing supervision. Many of those initiatives have to do with basic sanitation and the application of well-defined treatment algorithms (use of bed nets, rehydration in cholera), which will need to be applied by well-trained primary health care providers with basic levels of education, such as members of the community, students, and housewives. Educators and teachers have a crucial role, and such collaboration is frequently the most effective way to break down cultural and religious barriers to effective prevention and treatment of AKI(8).
Laboratory personnel, clinical biochemists, pharmacists, and dieticians will also play important roles. Clinical biochemists will be essential in developing potential electronic alert systems for patients who are identified as having rising serum creatinine and also for the future implementation of new biomarkers (85–87). Thorough monitoring of drug prescriptions, whether paper-based or electronically delivered, and close pharmacist involvement will be crucial. Given the high prevalence of medication-induced AKI, pharmacists have a central role in reviewing medications and advising on dosing for patients with CKD. Similarly, nutritional management is also extremely important in patients with AKI, for whom it is necessary to identify appropriate nutritional support.
Professional societies and organizations able to disseminate information effectively to their membership are a key component of AKI awareness initiatives. In this sense, the involvement of professional organizations such as the International Society of Nephrology (ISN) and the American Society of Nephrology (ASN), fostering AKI research and education agendas, plays a critical role. The advisory commissions of each organization on AKI are already generating documents and initiatives to address the problem in joint fashion. Networks such as the Acute Kidney Injury Network (AKIN), the Acute Dialysis Quality Initiative (ADQI) and Kidney Disease: Improving Global Outcomes (KDIGO) can be used to engage other societies and expand the reach to other specialists. The National Kidney Foundation (NKF) and the International Federation of Kidney Foundations (IFKF) will be important grass-roots organizations to promote these initiatives. Reaching out and collaborating with nursing and pharmacy professionals will allow widespread development and implementation of these activities.
Funding agencies are an important target: resources are required to create education tool kits and to support ongoing and future research endeavors.
Current models of increased awareness and intervention in underserved areas, joining the efforts of international private foundations and local resources, have demonstrated excellent results (88). Similar models must be developed to deal with AKI in LMI regions of the world, where the management of large problems (such as malaria) will necessarily have a strong impact on its renal complications.
Industry can play an important role in these initiatives by leveraging their distribution networks and sales force to disseminate information. New drugs to prevent and treat AKI and newer technologies for renal replacement therapy can be developed via collaborative multidisciplinary efforts. Newer diagnostic tools will permit earlier diagnosis of AKI, at stages where early intervention permits better patient outcomes.
If we are to make a significant and long-lasting difference to the care of patients at risk of or who develop AKI, there must be engagement with those who hold “the purse strings”. This will include hospital managers at the local level; health administrators and politicians at the national level; and members of international organizations such as the WHO and the United Nations (UN). To achieve this goal, it will be crucial to accrue more data on the epidemiology of AKI and its outcomes, not only in HI countries but also in LMI countries. Health administrators and politicians are perennially plagued by conflicting priorities and budget constraints; therefore, raising the awareness of the problem and achieving data on the quantitative impact of AKI is crucial to achieve these goals.
Fundamental to leveraging support across all these organizations will be patients and their families. The patients’ voice must be heard and their stories relayed, as this is what makes the problem real and tangible. Politicians will respond to the problem only if they think it is sufficiently important, or if there is a groundswell of support for its solution and convergence of goals. Patient-supported initiatives can be implemented via regional or international societies such as the National Kidney Foundation, or the International Federation of Kidney Foundations.
How do we raise the awareness of acute kidney injury?
The multiple and very diverse stakeholders on the management of AKI can be likened to the story of The Elephant in the Village of the Blind of the Indian subcontinent tradition, which illustrates the need for communication and respect for different perspectives when overall knowledge is difficult to achieve all at once. Thus, it is necessary to design a common framework that is anchored on evidence, and that builds on a rapidly expanding wealth of knowledge. The current conceptual framework for AKI recognizes that the disease is a process that evolves from early injury through severe damage, resulting in kidney failure and the need for renal replacement therapy. The natural course can vary from complete renal recovery to dialysis dependency or death. Individuals transition from one state to another during the course of the disease (Fig 3). This conceptual framework also recognizes that AKI can occur in individuals who have normal kidney function or have pre-existing kidney damage, thus allowing risk assessment. Based on this conceptual framework, we propose a strategy for raising awareness that emphasizes 5 areas of focus; Risk assessment, Recognition, Response, Renal support and Rehabilitation (Table 3). Each of these areas has specific components that can be adapted to develop an educational strategy and to target specific stakeholders, with an appropriate emphasis in each particular domain. While physicians and nursing personnel would need to be aware of the overall concept, risk assessment and early recognition could be emphasized for allied health professionals.
Fig 3.
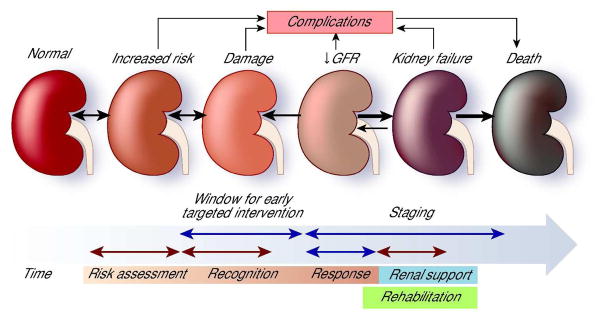
Conceptual framework and targeted approach for raising awareness of AKI (modified from ref (119)
Table 3.
5R’s strategy for educating care givers on AKI adapted from NHS
| Category | Component | Areas of Focus |
|---|---|---|
| Risk assessment | ||
| Susceptibility | Genetic, Clinical risk scores | |
| Surveillance | E-Alerts, Drug dosing modifications | |
| Primary prevention | High risk patients and situations e.g. contrast exposure | |
| Recognition | ||
| Diagnosis | Functional changes (urine output), biomarkers | |
| Staging | AKIN, KDIGO, Duration of AKI | |
| Response | ||
| Reversible factors | Hydration, Hemodynamics, Relieve obstruction remove nephrotoxic medications | |
| Avoid nephrotoxins | Drug dose adjustments | |
| Referral | Nephrology consultation in high risk patients and at recognition | |
| Therapy | Emerging molecules targeting different pathways | |
| Renal support | ||
| Dialytic modalities, | Dosing, duration, timing of initiation and withdrawal | |
| Rehabilitation | ||
| Follow–Up | Team approach (primary care, specialist, nursing, social worker, patient family) | |
| Recovery | Targeted interventions e.g. hypertension management | |
| Functional assessment | Quality of Life |
Based on the principles described above, we recommend a multipronged strategy (Table 4).
Table 4.
A global agenda to raise awareness and improve patient care
| Category | Components |
|---|---|
| Education - The five R’s | |
| Risk assessment | |
| Recognition | |
| Response | |
| Renal Support | |
| Rehabilitation | |
| Research Agenda | |
| Epidemiological studies (outcomes, comparative effectiveness) | |
| Prevention studies | |
| Treatment studies | |
| Audits and Quality improvement | |
| Funding strategy | |
| Governmental | |
| Non-Government organization | |
| International organizations- Leverage of ongoing worldwide strategies | |
| Development of a tool kit for AKI | |
| Essential toolkit for recognition and management of AKI | |
| Emphasis on early recognition and management | |
| Utilize KDIGO guidelines on diagnosis and management | |
| Identify knowledge gaps and educate | |
| Appropriate to each region | |
| Community vs. hospital acquired AKI | |
| Culturally sensitive | |
| Avoidance of the problems of discrimination by income, gender, religion | |
| Appropriate for the resources available | |
First, it is essential to improve education on AKI at both undergraduate and postgraduate levels for all healthcare professionals, and to emphasize the importance of identifying patients at risk of AKI. The Academy of Royal Medical Colleges has published a core competency for AKI (89). This document provides a pragmatic approach relevant to each specific healthcare professional. It defines the knowledge, skills and behaviors required for safe and effective patient care along the Chain of Response as described by National Institute of Health and Clinical Excellence (NICE) (90). The Chain of Response reflects escalating levels of intervention in the care of an acute patient with input from staff with a variety of different backgrounds and skills. The health care team must have the competencies to record patient information and vital signs recognize abnormal values and Institute intervention at level appropriate to the patient’s clinical condition. Five levels of competency are recognized which are: Recorder, Recognizer, and Primary, Secondary and Tertiary Responder. It is hoped that the implementation of the AKI Core Competencies framework will assist in improving the care that patients with AKI receive.
Second, physicians should be provided specific guidance for evaluating and managing patients with AKI based on the 5R approach. The recently published AKI Clinical Practice Guidelines in 2012 from Kidney Disease: Improving Global Outcomes (KDIGO) has provided a strong platform for this purpose (91). This international guideline has harmonized the definitions previously proposed by the by the Acute Dialysis Quality Initiative (RIFLE) and the Acute Kidney Injury Network (92–94) and provides guidelines for prevention, assessment and management of AKI that are designed to be applicable globally. These guidelines provide an opportunity to raise the awareness of AKI around a clearly characterized disease condition that has previously in poorly defined. We propose utilizing these guidelines as a common component to educate stakeholders in raising awareness of AKI.
Third, hospital administrators and quality control personnel should be made aware of the evidence for including AKI as a core measure of general medical care and as a key factor determining outcomes. Hospitals should be encouraged to adopt audit measures around the care of patients that develop AKI as proposed by NCEPOD and other guideline bodies (71). Episodes of avoidable AKI should be used as a benchmark for the care of acutely ill patients. This will provide an incentive to improve patient safety and reduce the number of episodes of AKI that are potentially avoidable. Specific AKI guidelines should be developed for community-based healthcare professionals and systems put in place to help detect patients developing AKI at an earlier stage in the disease process. The utilization of e-alerts in electronic medical records should be encouraged to provide real time feedback to care givers for recognizing high risk patients, early diagnosis and timely intervention including referral and follow up.
Fourthly, an AKI toolkit should be developed to be used globally including a checklist of simple measures that can be instituted to reduce the risk of AKI and how to manage it if it occurs. These checklists may be specific to individual groups of patients in a variety of different settings to take into account contrasting causes of AKI that occur in the developed and the developing world. For certain groups of healthcare professionals it will be essential to provide clear referral criteria. Patient education needs to be improved with the provision of information on websites. Patients who have suffered an episode of AKI should be provided with information regarding the causes of the episode and the need for long-term follow-up.
Finally, there are opportunities to raise the awareness of AKI as a complication of other disease processes in the developing world and mobilize local resources to manage it at an early phase. The World Health Organization (WHO)/United Nations UNDP Millennium Project and the Campaign to Eradicate Malaria deal with the main root causes of community disease in developing countries (95–97). The Millennium Project attempts to eradicate extreme poverty and hunger, achieve universal primary education, promote gender equality and empower women, improve maternal health and reduce child mortality, combat HIV/AIDS, malaria and other diseases, ensure environmental sustainability and promote a global partnership for development. All these issues are intimately related to AKI in developing countries. Malaria is a main cause of AKI in Sub-Saharan Africa and South-East Asia, obstetrical complications constitute a large cause of fatal AKI, and hemolytic uremic syndrome causes AKI frequently resulting in ESRD in children(8, 98). Furthermore, it is important to recognize that in many cases, gender and social/economical discrimination is at the cause root of the problem. International and regional initiatives must make all efforts to address that important aspect of the problem. The recognition and management of AKI in developing countries should be leveraged on such international initiatives, with the development of databases to demonstrate the scale of the problem.
Conclusions
Acute kidney injury is a common worldwide problem, which imposes a so far unrecognized global burden. It is necessary to raise awareness of AKI and to equip caregivers and patients with knowledge and tools to identify and adequately manage patients at risk. Efforts to address the problem are hindered by a fractured and sometimes ineffective approach. To a large extent, this is due to late recognition, inappropriate application of new knowledge, and lack of coordination among caregivers and institutions. In many cases, implementation of simple, actionable measures may go a long way to decrease incidence, severity, and death. Positive change will be dependent on progress at all levels, from the health care worker in the African village to the prominent politician making public health policy. Such an agenda must contain specific recommendations and be adaptable to different contexts:
At the practical level, we propose to develop a toolkit containing simple, immediately applicable measures to ensure early detection and fast action whenever AKI develops.
At the policy level, we propose a series of efforts destined to implement coordinated actions among government and non-government agencies, societies and institutions. Such initiatives should ensure continuity and coordination of such efforts, in the context of larger programs for the management of closely related conditions. For example, there is urgent need to address AKI in the context of ongoing programs combating increasingly prevalent, severe malaria in LMI regions of the world. Raising the awareness of this silent killer will be achieved only by coordinated efforts, involving the global nephrology community and the multiple components of the healthcare system in each region of the world.
Footnotes
Disclosure: For this article the authors declare no conflict of interest
References
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012 Aug 25;380(9843):756–66. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011 Nov;6(11):2567–72. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008 May;3(3):844–61. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 4.Coca SG, King JT, Jr, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010 Nov;78(9):926–33. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011 Jun;79(12):1361–9. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009 Jun;53(6):961–73. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerda J, Bagga A, Kher V, Chakravarthi RM. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008 Mar;4(3):138–53. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 9.Cerda J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008 May;3(3):881–6. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JFG, Smith N, Kelly K, Mason M. Adding insult to injury: a review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure) A report by the National Confidential Enquiry into Patient Outcome and Death. 2009 http://wwwncepodorguk/2009akihtm2009.
- 11.Foley RN, Collins AJ. The USRDS: What You Need to Know about What It Can and Can’t Tell Us about ESRD. Clin J Am Soc Nephrol. 2012 Nov 2; doi: 10.2215/CJN.06840712. [DOI] [PubMed] [Google Scholar]
- 12.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 14.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006 Jul;34(7):1913–7. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 15.Thomas M, Sitch A, Dowswell G. The initial development and assessment of an automatic alert warning of acute kidney injury. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 Jul;26(7):2161–8. doi: 10.1093/ndt/gfq710. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007 Jul;72(2):208–12. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz R, Erdem Y. Acute kidney injury in the elderly population. International urology and nephrology. 2010 Mar;42(1):259–71. doi: 10.1007/s11255-009-9629-7. [DOI] [PubMed] [Google Scholar]
- 18.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005 Aug 17;294(7):813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 19.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006 Apr;17(4):1135–42. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 20.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006 Jul;2(7):364–77. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 21.Daher E, Zanetta DM, Cavalcante MB, Abdulkader RC. Risk factors for death and changing patterns in leptospirosis acute renal failure. Am J Trop Med Hyg. 1999;61(4):630–4. doi: 10.4269/ajtmh.1999.61.630. [DOI] [PubMed] [Google Scholar]
- 22.Muthusethupathi MA, Shivakumar S, Suguna R, Jayakumar M, Vijayakumar R, Everard CO, et al. Leptospirosis in Madras--a clinical and serological study. J Assoc Physicians India. 1995;43(7):456–8. [PubMed] [Google Scholar]
- 23.Noronha IL, Schor N, Coelho SN, Jorgetti V, Romao JE, Junior, Zatz R, et al. Nephrology, dialysis and transplantation in Brazil. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1997;12(11):2234–43. doi: 10.1093/ndt/12.11.2234. [DOI] [PubMed] [Google Scholar]
- 24.Yang CW, Pan MJ, Wu MS, Chen YM, Tsen YT, Lin CL, et al. Leptospirosis: an ignored cause of acute renal failure in Taiwan. Am J Kidney Dis. 1997;30(6):840–5. doi: 10.1016/s0272-6386(97)90091-3. [DOI] [PubMed] [Google Scholar]
- 25.Gotuzzo E, Cieza J, Estremadoyro L, Seas C. Cholera. Lessons from the epidemic in Peru. Infect Dis Clin North Am. 1994;8(1):183–205. [PubMed] [Google Scholar]
- 26.Eko FO, Udo SM, Antia-Obong OE. Epidemiology and spectrum of vibrio diarrheas in the lower cross river basin of Nigeria. Cent Eur J Public Health. 1994;2(1):37–41. [PubMed] [Google Scholar]
- 27.Prakash J, Gupta A, Kumar O, Rout SB, Malhotra V, Srivastava PK. Acute renal failure in falciparum malaria--increasing prevalence in some areas of India--a need for awareness. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(12):2414–6. doi: 10.1093/oxfordjournals.ndt.a027206. [DOI] [PubMed] [Google Scholar]
- 28.Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14(3):R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011 Dec;39(12):2665–71. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens PE, Tamimi NA, Al-Hasani MK, Mikhail AI, Kearney E, Lapworth R, et al. Non-specialist management of acute renal failure. Qjm. 2001;94(10):533–40. doi: 10.1093/qjmed/94.10.533. [DOI] [PubMed] [Google Scholar]
- 31.Mehta RL, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual MT, et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002 Oct 15;113(6):456–61. doi: 10.1016/s0002-9343(02)01230-5. [DOI] [PubMed] [Google Scholar]
- 32.Orantes CM, Herrera R, Almaguer M, Brizuela EG, Hernandez CE, Bayarre H, et al. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC review. 2011 Oct;13(4):14–22. doi: 10.37757/MR2011V13.N4.5. [DOI] [PubMed] [Google Scholar]
- 33.Hajhosseiny R, Khavandi K, Goldsmith DJ. Cardiovascular disease in chronic kidney disease: untying the Gordian knot. International journal of clinical practice. 2012 Jul 11; doi: 10.1111/j.1742-1241.2012.02954.x. [DOI] [PubMed] [Google Scholar]
- 34.House AA. Cardio-renal syndrome type 4: epidemiology, pathophysiology and treatment. Seminars in nephrology. 2012 Jan;32(1):40–8. doi: 10.1016/j.semnephrol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Jablonski KL, Chonchol M. Cardiovascular disease: Should statin therapy be expanded in patients with CKD? Nature reviews Nephrology. 2012;8(8):440–1. doi: 10.1038/nrneph.2012.142. [DOI] [PubMed] [Google Scholar]
- 36.Jurkovitz CT, Elliott D, Li S, Saab G, Bomback AS, Norris KC, et al. Physician utilization, risk-factor control, and CKD progression among participants in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2012 Mar;59(3 Suppl 2):S24–33. doi: 10.1053/j.ajkd.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J. Cardiovascular risk in chronic kidney disease: role of the sympathetic nervous system. Cardiology research and practice. 2012;2012:319432. doi: 10.1155/2012/319432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman M, Ford CE, Cutler JA, Davis BR, Piller LB, Whelton PK, et al. Long-term renal and cardiovascular outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol. 2012 Jun;7(6):989–1002. doi: 10.2215/CJN.07800811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shara NM, Wang H, Mete M, Al-Balha YR, Azalddin N, Lee ET, et al. Estimated GFR and Incident Cardiovascular Disease Events in American Indians: The Strong Heart Study. Am J Kidney Dis. 2012 Jul 25; doi: 10.1053/j.ajkd.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. 2012 Jun;23(6):979–84. doi: 10.1681/ASN.2011121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nature reviews Nephrology. 2013 Feb;9(2):77–85. doi: 10.1038/nrneph.2012.280. [DOI] [PubMed] [Google Scholar]
- 42.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009 Nov;76(10):1089–97. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 43.Hsu CY. Linking the population epidemiology of acute renal failure, chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2007 May;16(3):221–6. doi: 10.1097/MNH.0b013e3280895ad9. [DOI] [PubMed] [Google Scholar]
- 44.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011 Feb 14;171(3):226–33. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava RN, Bagga A, Moudgil A. Acute renal failure in north Indian children. Indian J Med Res. 1990;92:404–8. [PubMed] [Google Scholar]
- 46.Sinha R, Nandi M, Tullus K, Marks SD, Taraphder A. Ten-year follow-up of children after acute renal failure from a developing country. Nephrol Dial Transplant. 2009 Mar;24(3):829–33. doi: 10.1093/ndt/gfn539. [DOI] [PubMed] [Google Scholar]
- 47.KDIGO Clinical Practice Guideline for Acute Kidney Injury: Summary of Recommendation Statements. Kidney International. 2012 Mar 1;(Suppl 2 1):8–12. doi: 10.1038/kisup.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Valdivieso JR, Bes-Rastrollo M, Monedero P, de Irala J, Lavilla FJ. Prognosis and serum creatinine levels in acute renal failure at the time of nephrology consultation: an observational cohort study. BMC Nephrol. 2007;8:14. doi: 10.1186/1471-2369-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balasubramanian G, Al-Aly Z, Moiz A, Rauchman M, Zhang Z, Gopalakrishnan R, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis. 2011 Feb;57(2):228–34. doi: 10.1053/j.ajkd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 50.McCoy AB, Cox ZL, Neal EB, Waitman LR, Peterson NB, Bhave G, et al. Realtime pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: a randomized, controlled trial. Appl Clin Inform. 2012 Jan 1;3(2):221–38. doi: 10.4338/ACI-2012-03-RA-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colpaert K, Hoste EA, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med. 2012 Apr;40(4):1164–70. doi: 10.1097/CCM.0b013e3182387a6b. [DOI] [PubMed] [Google Scholar]
- 52.Grand rounds: The opportunity for and challenges to malaria eradication. MMWR Morbidity and mortality weekly report. 2011 Apr 22;60(15):476–80. [PubMed] [Google Scholar]
- 53.Keating J, Eisele TP. Epidemiology of malaria morbidity after control scale-up. The Lancet infectious diseases. 2011 Dec;11(12):891–2. doi: 10.1016/S1473-3099(11)70212-2. [DOI] [PubMed] [Google Scholar]
- 54.Korenromp EL. Lives saved from malaria prevention in Africa--evidence to sustain cost-effective gains. Malaria journal. 2012;11:94. doi: 10.1186/1475-2875-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Y, Patton E, Kilian A, Dovey S, Eckert E. Can universal insecticide-treated net campaigns achieve equity in coverage and use? the case of northern Nigeria. Malaria journal. 2012;11:32. doi: 10.1186/1475-2875-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. The New England journal of medicine. 2011 Jun 30;364(26):2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 57.Hooi LS, Wong HS, Morad Z. Prevention of renal failure: the Malaysian experience. Kidney Int Suppl. 2005;(94):S70–4. doi: 10.1111/j.1523-1755.2005.09418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seedat YK. Acute renal failure in the black population of South Africa. Int J Artif Organs. 1993;16(12):801–2. [PubMed] [Google Scholar]
- 59.Seedat YK, Nathoo BC. Acute renal failure in blacks and Indians in South Africa--comparison after 10 years. Nephron. 1993;64(2):198–201. doi: 10.1159/000187314. [DOI] [PubMed] [Google Scholar]
- 60.Mehta KS, Halankar AR, Makwana PD, Torane PP, Satija PS, Shah VB. Severe acute renal failure in malaria. J Postgrad Med. 2001;47(1):24–6. [PubMed] [Google Scholar]
- 61.Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. The New England journal of medicine. 2002;347(12):895–902. doi: 10.1056/NEJMoa020074. [DOI] [PubMed] [Google Scholar]
- 62.Chitalia VC, Almeida AF, Rai H, Bapat M, Chitalia KV, Acharya VN, et al. Is peritoneal dialysis adequate for hypercatabolic acute renal failure in developing countries? Kidney Int. 2002;61(2):747–57. doi: 10.1046/j.1523-1755.2002.00177.x. [DOI] [PubMed] [Google Scholar]
- 63.Muthusethupathi MA, Shivakumar S, Vijayakumar R, Jayakumar M. Renal involvement in leptospirosis--our experience in Madras City. J Postgrad Med. 1994;40(3):127–31. [PubMed] [Google Scholar]
- 64.Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int Suppl. 2008 Apr;(108):S87–93. doi: 10.1038/sj.ki.5002608. [DOI] [PubMed] [Google Scholar]
- 65.Gabriel DP, Caramori JT, Martin LC, Barretti P, Balbi AL. Continuous peritoneal dialysis compared with daily hemodialysis in patients with acute kidney injury. Perit Dial Int. 2009 Feb;29(Suppl 2):S62–71. [PubMed] [Google Scholar]
- 66.Arogundade FA, Ishola DA, Jr, Sanusi AA, Akinsola A. An analysis of the effectiveness and benefits of peritoneal dialysis and haemodialysis using Nigerian made PD fluids. African journal of medicine and medical sciences. 2005 Sep;34(3):227–33. [PubMed] [Google Scholar]
- 67.Arije A, Kadiri S, Akinkugbe OO. The viability of hemodialysis as a treatment option for renal failure in a developing economy. African journal of medicine and medical sciences. 2000;29(3–4):311–4. [PubMed] [Google Scholar]
- 68.Naicker S, Eastwood JB, Plange-Rhule J, Tutt RC. Shortage of healthcare workers in sub-Saharan Africa: a nephrological perspective. Clinical nephrology. 2010 Nov;74(Suppl 1):S129–33. doi: 10.5414/cnp74s129. [DOI] [PubMed] [Google Scholar]
- 69.Joyce VR, Smith MW, Johansen KL, Unruh ML, Siroka AM, O’Connor TZ, et al. Health-Related Quality of Life as a Predictor of Mortality among Survivors of AKI. Clin J Am Soc Nephrol. 2012 Jul;7(7):1063–70. doi: 10.2215/CJN.00450112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palevsky PM. Chronic-on-acute kidney injury. Kidney Int. 2012 Mar;81(5):430–1. doi: 10.1038/ki.2011.435. [DOI] [PubMed] [Google Scholar]
- 71.Stewart JA. Adding insult to injury: care of patients with acute kidney injury. Br J Hosp Med (Lond) 2009 Jul;70(7):372–3. doi: 10.12968/hmed.2009.70.7.43116. [DOI] [PubMed] [Google Scholar]
- 72.NCEPOD. The National Confidential Enquiry into Patient Outcome and Death Elective & Emergency Surgery in the Elderly An Age Old Problem. 2010 wwwncepodorguk/2010eesehtm.
- 73.Aitken E, Carruthers C, Gall L, Kerr L, Geddes C, Kingsmore D. Acute kidney injury: outcomes and quality of care. Qjm. 2013 Jan 22; doi: 10.1093/qjmed/hcs237. [DOI] [PubMed] [Google Scholar]
- 74.The German 2011 epidemic of Shiga toxin-producing E . Coli--the nephrological view. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 Sep;26(9):2723–6. doi: 10.1093/ndt/gfr462. [DOI] [PubMed] [Google Scholar]
- 75.Ujah IA, Aisien OA, Mutihir JT, Vanderjagt DJ, Glew RH, Uguru VE. Factors contributing to maternal mortality in north-central Nigeria: a seventeen-year review. Afr J Reprod Health. 2005;9(3):27–40. [PubMed] [Google Scholar]
- 76.Unuigbe JA, Oronsaye AU, Orhue AA. Abortion-related morbidity and mortality in Benin City, Nigeria: 1973–1985. Int J Gynaecol Obstet. 1988;26(3):435–9. doi: 10.1016/0020-7292(88)90342-6. [DOI] [PubMed] [Google Scholar]
- 77.Jha V, Chugh KS. Nephropathy associated with animal, plant, and chemical toxins in the tropics. Semin Nephrol. 2003;23(1):49–65. doi: 10.1053/snep.2003.50003. [DOI] [PubMed] [Google Scholar]
- 78.Mabina MH, Moodley J, Pitsoe SB. The use of traditional herbal medication during pregnancy. Trop Doct. 1997;27(2):84–6. doi: 10.1177/004947559702700212. [DOI] [PubMed] [Google Scholar]
- 79.Dirks JH, Levin NW. Dialysis rationing in South Africa: a global message. Kidney Int. 2006 Sep;70(6):982–4. doi: 10.1038/sj.ki.5001798. [DOI] [PubMed] [Google Scholar]
- 80.Moosa MR, Kidd M. The dangers of rationing dialysis treatment: the dilemma facing a developing country. Kidney Int. 2006 Sep;70(6):1107–14. doi: 10.1038/sj.ki.5001750. [DOI] [PubMed] [Google Scholar]
- 81.Martins D, Agodoa L, Norris K. Kidney disease in disadvantaged populations. International journal of nephrology. 2012;2012:469265. doi: 10.1155/2012/469265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney international. 2005 Sep;68(3):914–24. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 83.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. Journal of the American Society of Nephrology : JASN. 2007 Apr;18(4):1299–306. doi: 10.1681/ASN.2006050524. [DOI] [PubMed] [Google Scholar]
- 84.Khan IH, Catto GR, Edward N, Macleod AM. Acute renal failure: factors influencing nephrology referral and outcome. Qjm. 1997;90(12):781–5. doi: 10.1093/qjmed/90.12.781. [DOI] [PubMed] [Google Scholar]
- 85.Chawla LS, Kellum JA. Acute kidney injury in 2011: Biomarkers are transforming our understanding of AKI. Nature reviews Nephrology. 2012 Feb;8(2):68–70. doi: 10.1038/nrneph.2011.216. [DOI] [PubMed] [Google Scholar]
- 86.Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004 Dec;10(6):476–82. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- 87.Lewington AJ, Sayed A. Acute kidney injury: how do we define it? Ann Clin Biochem. 2010 Jan;47(Pt 1):4–7. doi: 10.1258/acb.2009.009249. [DOI] [PubMed] [Google Scholar]
- 88.Pronyk PM, Muniz M, Nemser B, Somers MA, McClellan L, Palm CA, et al. The effect of an integrated multisector model for achieving the Millennium Development Goals and improving child survival in rural sub-Saharan Africa: a non-randomised controlled assessment. Lancet. 2012 Jun 9;379(9832):2179–88. doi: 10.1016/S0140-6736(12)60207-4. [DOI] [PubMed] [Google Scholar]
- 89.Finlay S. Defining the role of the clinician. London: Academy of Medical Royal Colleges; 2011. Acute kidney injury: a competency framework. [Google Scholar]
- 90.Excellence. NIfHaC. National Institute for Health and Clinical Excellence. Clinical guideline. Vol. 50. London: NICE; 2007. Acutely ill patients in hospital: Recognition of and response to acute illness in adults in hospital. [PubMed] [Google Scholar]
- 91.Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clinical practice. 2012 Aug 7;120(4):179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 92.Kellum JA, Mehta RL, Levin A, Molitoris BA, Warnock DG, Shah SV, et al. Development of a clinical research agenda for acute kidney injury using an international, interdisciplinary, three-step modified Delphi process. Clin J Am Soc Nephrol. 2008 May;3(3):887–94. doi: 10.2215/CJN.04891107. [DOI] [PubMed] [Google Scholar]
- 93.Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol. 2003;14(8):2178–87. doi: 10.1097/01.asn.0000079042.13465.1a. [DOI] [PubMed] [Google Scholar]
- 94.Molitoris BA, Levin A, Warnock DG, Joannidis M, Mehta RL, Kellum JA, et al. Improving outcomes of acute kidney injury: report of an initiative. Nat Clin Pract Nephrol. 2007 Aug;3(8):439–42. doi: 10.1038/ncpneph0551. [DOI] [PubMed] [Google Scholar]
- 95.Campbell CC. Halting the toll of malaria in Africa. Am J Trop Med Hyg. 2008 Jun;78(6):851–3. [PubMed] [Google Scholar]
- 96.Sachs JD, McArthur JW. The Millennium Project: a plan for meeting the Millennium Development Goals. Lancet. 2005 Jan 22–28;365(9456):347–53. doi: 10.1016/S0140-6736(05)17791-5. [DOI] [PubMed] [Google Scholar]
- 97.Teklehaimanot A, McCord GC, Sachs JD. Scaling up malaria control in Africa: an economic and epidemiological assessment. Am J Trop Med Hyg. 2007 Dec;77(6 Suppl):138–44. [PubMed] [Google Scholar]
- 98.Srivastava RN, Moudgil A, Bagga A, Vasudev AS. Hemolytic uremic syndrome in children in northern India. Pediatr Nephrol. 1991;5(3):284–8. doi: 10.1007/BF00867477. [DOI] [PubMed] [Google Scholar]
- 99.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–8. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 100.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, et al. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688–94. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 101.Abraham G, Gupta RK, Senthilselvan A, van der Meulen J, Johny KV. Cause and prognosis of acute renal failure in Kuwait: a 2-year prospective study. J Trop Med Hyg. 1989;92(5):325–9. [PubMed] [Google Scholar]
- 102.Jha V, Malhotra HS, Sakhuja V, Chugh KS. Spectrum of hospital-acquired acute renal failure in the developing countries--Chandigarh study. Q J Med. 1992;83(303):497–505. [PubMed] [Google Scholar]
- 103.Thomas CN, Brann SH, Douglas AR, Thomas JM, Daniel SC, Posthoff C, et al. Coronary artery bypass graft outcome: the Trinidad and Tobago experience. West Indian Med J. 2000;49(4):290–3. [PubMed] [Google Scholar]
- 104.Al-Homrany M. Epidemiology of acute renal failure in hospitalized patients: experience from southern Saudi Arabia. East Mediterr Health J. 2003;9(5–6):1061–7. [PubMed] [Google Scholar]
- 105.Kohli HS, Bhat A, Jairam A, Aravindan AN, Sud K, Jha V, et al. Predictors of mortality in acute renal failure in a developing country: a prospective study. Ren Fail. 2007;29(4):463–9. doi: 10.1080/08860220701260651. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Cui Z, Fan M. Hospital-acquired and community-acquired acute renal failure in hospitalized Chinese: a ten-year review. Ren Fail. 2007;29(2):163–8. doi: 10.1080/08860220601095918. [DOI] [PubMed] [Google Scholar]
- 107.Wang IK, Wang ST, Chang HY, Lin CL, Kuo HL, Chen TC, et al. Prognostic value of acute physiology and chronic health evaluation II and organ system failure in patients with acute renal failure requiring dialysis. Ren Fail. 2005;27(6):663–9. doi: 10.1080/08860220500234881. [DOI] [PubMed] [Google Scholar]
- 108.Kaufman J, Dhakal M, Patel B, Hamburger R. Community-acquired acute renal failure. Am J Kidney Dis. 1991;17(2):191–8. doi: 10.1016/s0272-6386(12)81128-0. [DOI] [PubMed] [Google Scholar]
- 109.Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. Bmj. 1993;306(6876):481–3. doi: 10.1136/bmj.306.6876.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chanard J, Wynckel A, Canivet E, Jolly D. Evaluation of the frequency of acute renal insufficiency and therapeutic modalities in the nephrological milieu. Nephrologie. 1994;15(1):13–6. [PubMed] [Google Scholar]
- 111.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50(3):811–8. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 112.Metcalfe W, Simpson M, Khan IH, Prescott GJ, Simpson K, Smith WC, et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. Qjm. 2002;95(9):579–83. doi: 10.1093/qjmed/95.9.579. [DOI] [PubMed] [Google Scholar]
- 113.Obialo CI, Okonofua EC, Tayade AS, Riley LJ. Epidemiology of de novo acute renal failure in hospitalized African Americans: comparing community-acquired vs hospital-acquired disease. Arch Intern Med. 2000;160(9):1309–13. doi: 10.1001/archinte.160.9.1309. [DOI] [PubMed] [Google Scholar]
- 114.Prescott GJ, Metcalfe W, Baharani J, Khan IH, Simpson K, Smith WC, et al. A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm264. [DOI] [PubMed] [Google Scholar]
- 115.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292–8. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 116.Anochie IC, Eke FU. Acute renal failure in Nigerian children: Port Harcourt experience. Pediatr Nephrol. 2005;20(11):1610–4. doi: 10.1007/s00467-005-1984-8. [DOI] [PubMed] [Google Scholar]
- 117.Vukusich A, Alvear F, Villanueva P, Gonzalez C, Francisco O, Alvarado N, et al. Epidemiology of severe acute renal failure in Metropolitan Santiago. Rev Med Chil. 2004;132(11):1355–61. [PubMed] [Google Scholar]
- 118.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012 Sep;82(5):516–24. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 119.Mehta RL. Timed and targeted therapy for acute kidney injury: a glimpse of the future. Kidney Int. 2010 Jun;77(11):947–9. doi: 10.1038/ki.2010.79. [DOI] [PubMed] [Google Scholar]