Abstract
Human pluripotent stem cells (hPSCs), including both embryonic stem cells and induced pluripotent stem cells, offer a potential cell source for research, drug screening, and regenerative medicine applications due to their unique ability to self-renew or differentiate to any somatic cell type. Before the full potential of hPSCs can be realized, robust protocols must be developed to direct their fate. Cell fate decisions are based on components of the surrounding microenvironment, including soluble factors, substrate or extracellular matrix, cell-cell interactions, mechanical forces, and 2D or 3D architecture. Depending on their spatio-temporal context, these components can signal hPSCs to either self-renew or differentiate to cell types of the ectoderm, mesoderm, or endoderm. Researchers working at the interface of engineering and biology have identified various factors which can affect hPSC fate, often based on lessons from embryonic development, and they have utilized this information to design in vitro niches which can reproducibly direct hPSC fate. This review highlights culture systems that have been engineered to promote self-renewal or differentiation of hPSCs, with a focus on studies that have elucidated the contributions of specific microenvironmental cues in the context of those culture systems. We propose the use of microsystems technologies for high-throughput screening of spatial-temporal presentation of cues, as this has been demonstrated to be a powerful approach for differentiating hPSCs to desired cell types.
Keywords: Embryonic stem cells, induced pluripotent stem cells, self-renewal, differentiation, ectoderm, mesoderm, endoderm, niche, microenvironment, cell culture engineering
1. Introduction
Human pluripotent stem cells (hPSCs), including both human embryonic stem cells (hESCs) (Thomson et al., 1998b) and human induced pluripotent stem cells (hiPSCs) (Takahashi et al., 2007, Yu et al., 2007) have tremendous potential in providing a cell source for tissue engineering applications including, but not limited to, regenerative therapies and in vitro model systems for studying development, disease, and pharmaceutical screening. The promise of hPSCs to be used for such applications is based on the unique properties of these cells, including unlimited self-renewal ability and the potential to form any somatic cell type from each of the three embryonic germ layers: the ectoderm, mesoderm, and endoderm. Realizing the true potential of hPSCs is predicated on the ability to develop tools and protocols to successfully convert hPSCs to cell types and tissues of interest.
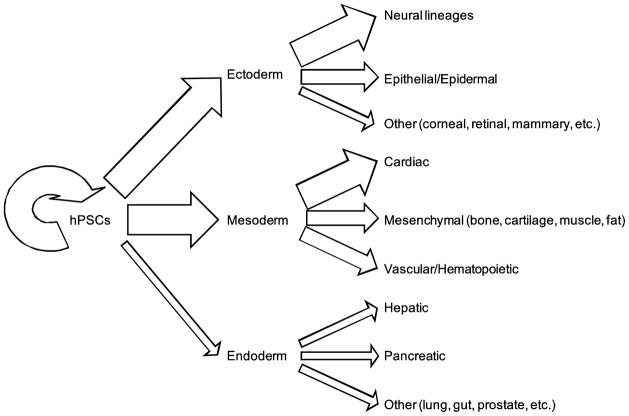
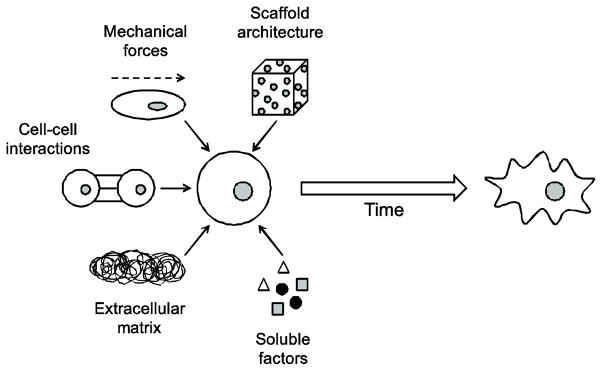
Cell fate decisions are based on a complex set of input signals from the surrounding cellular microenvironment. These microenvironmental cues can be presented to the cell in the form of soluble factors, the substrate or extracellular matrix (ECM) proteins onto which the cell is adhered, direct cell-cell interactions, mechanical forces or shear, the spatial organization (i.e. two-dimensional (2D) or three-dimensional (3D) architecture) of the microenvironment, or other various stimuli present in the cell’s immediate surroundings (Metallo et al., 2007). The introduction of a specific microenvironmental stimulus into the cellular milieu or “niche” can instruct hPSCs to self-renew and maintain their pluripotency or, alternatively, differentiate to any cell type, as depicted in Figure 1. In the case of hPSC differentiation, the spatio-temporal context of many microenvironmental cues is often as important as the cue itself.
Figure 1.
Schematic illustrating the traditional paradigm for engineering methods to manipulate PSC fate decisions by investigating the roles of individual microenvironmental cues and how the incorporation of these cues can instruct a cell to differentiate towards a particular lineage.
Researchers have engineered numerous strategies for either maintaining pluripotency of hPSCs or generating various cell types from an initial hPSC population in vitro. Figure 2 illustrates the relative progress to date on which lineages have been generated from hPSCs and thus how much of an understanding we have about which microenvironmental elements are critical in these differentiation strategies. In many cases, researchers have isolated or quantified the effects of specific microenvironmental factors on such hPSC fate decisions. In this review, we will highlight culture systems that have been engineered to promote self-renewal or differentiation of hPSCs to various cell types and specifically focus on studies that have isolated the contributions of specific microenvironmental elements in guiding hPSCs toward different cell fates in the context of those culture systems. In addition, we will provide examples of what has been found in mouse pluripotent stem cell systems in cases where an hPSC-based analog has not yet been demonstrated or examples that show homology in both mouse and human systems.
Figure 2.
Relative progress on engineering microenvironments to achieve various hPSC cell fates. The arrow thickness is proportional to the amount of research that has been conducted in directing hPSCs toward that specific cell fate.
2. Maintaining hPSC pluripotency
Prior to engineering microenvironments suitable for converting hPSCs to different somatic cell types, culture systems had to be designed to maintain the self-renewal and pluripotency, or “stemness”, of hPSCs. Upon the first derivation and isolation of hESC clones, researchers identified various microenvironmental elements as necessary for hESC self-renewal. Since then, new methods have been designed demonstrating that other microenvironmental factors are necessary, or at least conducive, for maintaining hPSC pluripotency and self-renewal in vitro. We highlight these microenvironmental cues below that have been identified in an effort to design an in vitro hPSC niche.
2.1. Role of soluble factors
The first isolation and culture of hESCs involved plating hESC colonies on a feeder layer of mouse embryonic fibroblasts (MEFs), which was found to maintain hESC self-renewal and pluripotency in culture (Amit et al., 2000, Thomson et al., 1998a). Either direct co-culture or culture in MEF-conditioned medium were demonstrated to successfully promote hPSC undifferentiated growth (Xu et al., 2001). This indicates that MEFs produce and secrete soluble factors that provide a suitable microenvironment for growth of hESCs.
While the exact combination of soluble factors secreted by MEFs to maintain hPSC pluripotency remains unknown, researchers have identified specific soluble factors that support hPSC self-renewal in vitro. For example, the addition of basic fibroblast growth factor (bFGF) to unconditioned medium can maintain undifferentiated hESCs in vitro at concentrations above 80 ng/mL, as indicated by the high percentage (>90%) of cells expressing pluripotency markers Oct4, SSEA4, and Tra1-60 (Levenstein et al., 2006). In addition, hESCs were found to maintain expression of the same pluripotency genes after several (>20) passages cultured in the presence of 100 ng/mL bFGF. The critical role of bFGF in promoting hPSC undifferentiated growth is widely recognized and well documented and thus bFGF has become an essential component of hPSC media in almost all feeder-free culture systems for hPSCs (Chen et al., 2011, Ludwig et al., 2006a, Ludwig et al., 2006b).
While the addition of MEFs or the use of MEF-conditioned medium can maintain hPSC self-renewal, some studies have sought to replace the use of MEFs by elucidating what factors MEFs provide that stimulate continual hPSC undifferentiated growth. One elegant study, for example, identified insulin-like growth factor-1 receptor (IGF1R) and ERBB2 receptor signaling as necessary for hESC self-renewal (Wang et al., 2007). To activate signaling of these two receptors, soluble factors such as heregulin-1B, activin A, insulin-like growth factor-1 (IGF-1), and bFGF were added to a defined medium in which hESCs were cultured. It was found that the hESC colonies cultured in this medium sustained pluripotency marker expression for several passages and the cells could form embryoid bodies and give rise to cell types derived from all three embryonic germ layers.
Other soluble factors, in the form of small molecules, have been shown to maintain pluripotency-specific gene expression in hPSCs. For example, the treatment of hESCs with a glycogen synthase kinase 3 (GSK-3) inhibitor, 6-bromoindirubin-3′-oxime (BIO), resulted in not only an undifferentiated hESC morphology, but also a comparable level of Oct4 expression compared to cells cultured in conditioned medium (Sato et al., 2004). In addition, these BIO-treated hESCs generated cell types from all three embryonic germ layers indicating that treatment with 2 μM BIO resulted in a maintained pluripotent state of hESCs. Another small molecule, erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA), has also been demonstrated to have a positive effect on maintaining pluripotency of hESCs in vitro without the addition of bFGF (Burton et al., 2010). While the addition of EHNA sustained Oct4 and Nanog expression in hESCs, the mechanism by which this small molecule promotes hESC self-renewal has yet to be elucidated.
Some studies have investigated the roles of multiple signaling pathways in maintaining pluripotency and how elements of the hPSC niche either activate or suppress these signaling pathways (Peerani et al., 2007). Such thorough studies are critical for designing new or improving current culture systems for engineering cellular microenvironments to either maintain hPSC self-renewal or to guide hPSC populations toward specific cell lineages.
2.2. Role of synthetic substrates
While cues in the form of soluble factors have been identified and utilized to maintain self-renewal of hPSCs, substrates on which hPSC colonies are plated have also shown substantial effects on the maintenance of hPSC pluripotency. While a majority of feeder-free cultures of hPSCs are performed on Matrigel, which has become the gold standard substrate for hPSC expansion, other substrates have been just as or more effective. For example, one study used an electrospun polycaprolactone (PCL)/collagen and a PCL/gelatin substrate to culture hESCs in the presence of MEFs (Gauthaman et al., 2009). The authors of this study found that culture of hESCs on either of these two nanofibrous substrates, compared to a conventional MEF co-culture system, resulted in similar hPSC cell morphology and pluripotency marker expression, but also demonstrated a 1.5 to 2-fold increase in the percentage of proliferative cells in culture.
Another study identified a synthetic surface functionalized with a heparin-binding peptide derived from vitronectin, GKKQRFRHRNRKG, that supports hPSC adhesion, self-renewal, and ability to form cell types derived from all three germ layers (Klim et al., 2010). In fact, hPSCs grown on this substrate demonstrated a similar growth rate compared to cells grown on Matrigel and actually showed a higher percentage of cells expressing SSEA4, SSEA3, TRA1-60, and TRA1-81 compared to the conventional Matrigel substrate after 17 passages. Together, these results indicate that this substrate functionalized with a synthetic peptide provides hPSCs with a microenvironment that may be more conducive for long-term undifferentiated hPSC growth compared to Matrigel.
Other substrates have been developed to maintain hPSC self-renewal and provide a non-xenogeneic alternative to Matrigel for hPSC expansion in culture. For example, a human recombinant laminin-511 substrate has been shown to maintain hPSC undifferentiated growth in vitro (Rodin et al., 2010). In fact, similar to the substrate investigated by Klim et al. (2010), this laminin substrate was shown to support similar growth rates of hPSCs compared to a Matrigel substrate, but could maintain hPSC populations with higher expression of pluripotency markers compared to that of Matrigel after 11 passages.
2.3. Culture architecture
The substrate on which hPSCs are cultured can have a profound effect on maintaining hPSC self-renewal and pluripotency, but the architecture of the culture itself can also promote hPSC growth. For example, there have been a number of studies investigating the use of 3D microwell culture systems for hESC culture as opposed to conventional 2D monolayer culture systems. For example, one study found that a fibronectin-coated microwell substrate with a MEF feeder layer demonstrated high viability of hESCs and a comparable expression of alkaline phosphatase (ALP), a marker for hPSCs, to hESCs grown in a conventional monolayer culture (Khademhosseini et al., 2006a). The microwell system provides a means of generating a homogenous distribution of aggregates in terms of size compared to monolayer culture, which results in a variable hESC aggregate distribution. A study from our lab exhibited an advantage in long-term culture of hESCs on a Matrigel-coated microwell substrate compared to a conventional 2D Matrigel substrate by demonstrating a higher percentage of Oct4 expressing cells in the microwell system after 18 days of hESC culture (Mohr et al., 2006). Utilization of these microwell substrates demonstrates an alternative means of culturing hPSCs by controlling microenvironment in an architectural context.
2.4. Other microenvironmental stimuli
Soluble factors and cell substrate are two elements of the microenvironment that have been thoroughly investigated in terms regulating hPSC pluripotency in vitro. However, other elements in engineering an hPSC niche have been investigated as well. For example, mechanical strain has been shown to inhibit hESC differentiation and promote self-renewal. A study in our laboratory demonstrated that 70% of hESCs exposed to a 10% average membrane expressed SSEA4 compared to 30% of hESCs that were not exposed to strain in a conditioned medium (Saha et al., 2006). A similar trend was observed with Oct4 expression where 90% of cells exposed to strain expressed Oct4 compared to 35% of cells that expressed Oct4 in the hESC population not exposed to strain. In a later study, it was determined that the inhibition of spontaneous hESC differentiation in hESCs exposed to cyclic biaxial mechanical strain is modulated through the transforming growth factor β (TGF-β)/Activin/Nodal pathway (Saha et al., 2008). The results in these studies demonstrated that mechanical strain in the context of a culture system with a Matrigel substrate in conditioned medium has a profound effect on hESC cell fate decisions.
Another element of the hPSC niche to consider is the concentration of oxygen in the media. One study clearly demonstrated a reduction in spontaneous differentiation in hypoxic (1–5% O2) conditions compared to normoxic (20% O2) conditions as measured by pluripotency marker expression and area of morphologically distinct undifferentiated cells (Ezashi et al., 2005). Furthermore, repeated passaging of hESCs in normoxic conditions resulted in amplification of differentiated cells and a corresponding reduction in self-renewing hESCs compared to repeated passaging in hypoxic conditions.
The extensive research on which microenvironmental elements are key to establishing an hPSC niche in vitro to promote self-renewal and pluripotency has resulted in the design of chemically defined and feeder-free hPSC culture systems. These new systems can be used to maintain hPSC self-renewal for numerous passages without any reduction in pluripotency marker expression or a loss in the ability to form cell types derived from all three embryonic germ layers. While the knowledge base for engineering a microenvironment to support undifferentiated hPSC growth is extensive and has allowed the development of simple, refined culture methods, the field of engineering microenvironments to efficiently differentiate hPSCs to various somatic cell types is, in general, still in its infancy.
3. Ectoderm lineages
The ectoderm germ layer in a developing embryo gives rise to several epithelial tissues including the epidermis, cornea, and mammary gland as well as the various types of neural lineages that comprise the central and peripheral nervous systems. We will summarize below the advancements in engineering microenvironments conducive for hPSC differentiation into clinically relevant ectodermal cell types.
3.1. Epidermal keratinocytes
Epidermal cells derived from hPSCs can be applied not only in constructing skin grafts for clinical use, but patient-specific hiPSC-derived epidermal keratinocytes can also be utilized in 3D tissue cultures in vitro to recapitulate diseased phenotypes and to monitor progression of various skin diseases. Numerous studies have identified key microenvironmental cues to initiate ectodermal, or at least an epithelial, commitment of hPSCs. In addition, researchers have also found cues to promote further differentiation of ectodermal cells toward an epidermal fate.
3.1.1. Role of soluble factors
3.1.1.1. Commitment to epithelial cells
Epidermal keratinocytes arise from an external ectodermal layer that envelops the developing embryo (Blanpain and Fuchs, 2009). The first differentiation event of hPSCs toward an epidermal keratinocyte lineage typically involves an initial commitment toward an epithelial cell fate. Many research groups have induced this epithelial commitment of hPSCs with the use of soluble factors. For example, a study by Metallo et al. (2008) demonstrated that retinoic acid (RA) can increase expression of the simple epithelial marker, cytokeratin 18 (K18), by 3-fold in EBs derived from hESCs. These primitive epithelial cells can be differentiated further into more mature epithelial cells as indicated by expression of a marker present in the basal layer of stratified squamous epithelial cells, cytokeratin 14 (K14). In fact, RA was shown to positively affect K14 expression in hESCs in a dose and time-dependent manner. Others have also used RA to induce epithelial differentiation of both mouse embryonic stem cells (mESCs) and hPSCs (Bain et al., 1995, Itoh et al., 2011, Kawamorita et al., 2002, Maye et al., 2004, Metallo et al., 2010, Sagha et al., 2009, Xia et al., 2007). Ascorbic acid has also been used to promote epithelial differentiation of hPSCs in strategies where RA is not incorporated (Aberdam et al., 2008, Guenou et al., 2009).
While RA can induce ectodermal commitment of hPSCs, it can also further differentiate cells toward neural fates. Researchers primarily interested in deriving ectodermal epithelial cells or more mature epidermal keratinocytes from hPSCs have found that bone morphogenetic protein 4 (BMP-4) efficiently inhibits neural morphogenesis in vitro, and thus will incorporate exogenous BMP-4 into hPSC culture (Aberdam et al., 2008, Hewitt et al., 2009, Itoh et al., 2011, Metallo et al., 2010, Metallo et al., 2008).
BMP-4 in synergy with RA can increase K18 expression 1.5-fold compared to cells treated with RA alone and that the treatment of hESCs in monolayer culture with BMP-4 and RA can result in a homogenous (>80%) population of K14+ cells (Metallo et al., 2010, Metallo et al., 2008). In a calcium-rich medium, K14+ cells were shown to subsequently stratify and express terminal-differentiation markers of the epidermis such as cytokeratin 10 (K10), involucrin, and filaggrin, demonstrating the epidermal potential of the cells.
3.1.1.2. Epithelial maturation
Subsequent to inducing ectodermal epithelial commitment of hPSCs, many research groups introduce a cocktail of exogenous soluble factors or culture cells in media containing soluble factors known to promote keratinocyte survival to induce keratinocyte differentiation. Such factors that are believed to be essential for a keratinocyte microenvironment and are thus incorporated in keratinocyte differentiation strategies include insulin, cholera toxin, epidermal growth factor (EGF), adenine, and hydrocortisone (Hewitt et al., 2009, Itoh et al., 2011, Metallo et al., 2010). Some studies culture hPSCs in commercially available media containing some or all of these soluble factors to promote epithelial commitment or maturation while other studies involve adding these exogenous soluble factors separately. To our knowledge, no comprehensive study exists that exhibits the individual influence of these factors on epithelial or epidermal differentiation of hPSCs, but one study demonstrated that the commercially available keratinocyte defined serum-free medium (K-DSFM, Invitrogen) formulation outperformed other media cocktails tested by at least 2-fold when measuring epidermal differentiation efficiency of hiPSCs (Itoh et al., 2011).
3.1.2. Culture architecture
3.1.2.1. EB vs. monolayer culture
To achieve successful differentiation of hPSCs into various cell types, researchers will often form embryoid bodies (EBs) from undifferentiated hPSCs and attempt to bias the spontaneous differentiation toward a particular cell type by adding soluble factors or by plating the EBs on different substrates. These methods of differentiation are typically inefficient but often necessary given our lack of knowledge on which microenvironmental cues promote differentiation to certain lineages. The architecture of the EB itself provides a microenvironment that can specifically guide differentiation of pockets of hPSCs toward certain cell types. Given the knowledge on epidermal keratinocyte culture and the natural architecture of skin, an in vitro hPSC directed differentiation method using monolayer culture is feasible. In fact, a directed differentiation strategy using monolayer culture compared to an EB method has been shown to improve differentiation efficiency of hPSCs into K14+ epithelial cells from 15% to 87% (Metallo et al., 2008). This indicates that a monolayer culture provides a suitable microenvironment for epithelial differentiation, which can result in higher purities and yields of differentiated cells compared to an EB-based differentiation protocol.
3.1.2.2. Organotypic culture
K14+ epithelial cells have shown epidermal potential not only in a 2D culture system, but also in a 3D organotypic culture system. When these cells are plated on a collagen matrix and raised to the air-liquid interface (ALI), stratification and terminal differentiation of epidermal keratinocytes can occur as indicated by K10, involucrin, and filaggrin expression (Itoh et al., 2011, Metallo et al., 2009). Other groups have demonstrated stratification of epithelial cells derived from hPSCs, but not all expressed terminal differentiation markers of the epidermis or displayed a distinct cornified envelope (Hewitt et al., 2009, Itoh et al., 2011). This indicates that subtle differences in microenvironmental cues present during differentiation may result in generating epithelial cells with different differentiation potentials.
While researchers have demonstrated successful methods for generating homogenous populations of epithelial progenitor or epidermal cells from hPSCs based on marker expression and by successful stratification and cornification in 3D culture, others demonstrate that these hPSC-derived cells have limited expansion potential and do not exhibit appropriate keratinocyte behavior in culture (Dabelsteen et al., 2009). It is conceivable that current culture strategies to induce epithelial/epidermal differentiation establish microenvironments for hPSCs that may be exclusively suitable for differentiation, but do not include factors essential for proper keratinocyte function and propagation. If this is the case, these additional cues required to recapitulate “appropriate” keratinocyte behavior in vitro must be elucidated in further studies.
3.2. Neural lineages
There is considerable interest in deriving various neural cell types from hPSCs for applications in regenerative therapeutics as well as in vitro disease modeling for neurodegenerative diseases including Parkinson’s disease and spinal muscular atrophy (Ebert et al., 2009, Hwang et al., 2009). Research efforts within the past decade have demonstrated substantial progress in engineering culture methods for generating neural progenitors and more specialized neural cells, such as neurons or glial cells from hPSCs. Furthermore, researchers have identified microenvironmental cues, summarized in Table 1, in the form of growth factors, ECM proteins, synthetic substrates, and scaffolds to guide hPSC toward various neural cell lineages for eventual incorporation into tissue engineering constructs.
3.2.1. Role of soluble factors
3.2.1.1. Neuroepithelial commitment
Most efforts in generating neural cells from hPSCs in vitro involve an initial commitment of hPSCs toward a neuroectodermal or neuroepithelial cell fate, similar to how neurogenesis is initiated during embryogenesis. These morphologically distinct neural precursor cells form rosette structures in culture resembling early neural tube formation observed in vivo (Dhara and Stice, 2008, Hu and Zhang, 2009, Li et al., 2005, Pankratz et al., 2007, Wang et al., 2011a, Zhang et al., 2001). The earliest studies to generate these neural precursors utilized an EB-based protocol where 94% of EBs plated in the presence of bFGF gave rise to these rosette structures while the absence of bFGF produced no colonies with organized rosettes (Zhang et al., 2001). Survival and proliferation of this neural precursor population was also found to be highly dependent on the presence of bFGF, and thus has been incorporated into more recent protocols to generate neural progenitor populations from hPSCs (Dhara and Stice, 2008, Ebert et al., 2009, Itsykson et al., 2005). However, other labs have developed EB-based culture systems that do not require exogenous addition of bFGF but still successfully induce neural differentiation of hPSCs (Belinsky et al., 2011, Hu and Zhang, 2009, Kang et al., 2007a, Ogawa et al., 2011, Suter et al., 2009, Wang et al., 2011a). These contrasting reports on effects of bFGF in neural differentiation indicate that either bFGF addition is dependent on the microenvironmental context or endogenous bFGF secreted by differentiating cells is sufficient for neural differentiation (Hu and Zhang, 2009).
Researchers have modified established culture systems for inducing neural commitment of hPSCs by incorporating other soluble factors in culture. For example, loss of BMP activity is known to induce neuroectoderm commitment in a developing embryo (Dhara and Stice, 2008). An EB-based culture system developed by Itsykson et al. (2005) involves the addition of noggin, a BMP-antagonist, to the culture medium to initiate neural commitment. Using this EB-based protocol, a significant increase in cells expressing neural precursor markers A2B5, PSA-NCAM, Musashi, and NCAM was observed when cultured in the presence of noggin (95.6%, 96.2%, 91.3%, and 73.4%, respectively) compared to cells cultured without noggin (69.6%, 51.3%, 66.4%, and 31.5%, respectively). In addition, no change was observed in the amount of PSA-NCAM, Musashi, or nestin-expressing cells when cultured with or without bFGF, indicating that the contribution of bFGF to neural differentiation is insignificant in the context of culture with noggin. Noggin addition is also employed in various 2D culture systems to initiate neural commitment of hPSCs (Baharvand et al., 2007, Gerrard et al., 2005, Pera et al., 2004). Other small molecule inhibitors of BMP, such as dorsomorphin, have also demonstrated to induce neural and neuronal differentiation of hSPCs (Birenboim et al., 2013, Morizane et al., 2011).
RA has been shown to play an important role in regulating neural cell fate decisions in both a mouse and human system (Dhara and Stice, 2008, Pankratz et al., 2007). A study conducted by the Langer laboratory clearly exhibited the positive effect of RA on the neural commitment of hESCs when cells were cultured in a poly(lactic-co-glycolic acid)/poly(L-lactic acid)-based scaffold with either a Matrigel or fibronectin coating (Levenberg et al., 2010). Incorporation of RA into culture systems has been shown to be useful for facilitating both early and late neural differentiation.
3.2.1.2. Neuronal and glial commitment
Neuroepithelial cells can be further differentiated to neuronal and glial lineages. Both RA and sonic hedgehog (SHH) have been implicated in guiding hPSC-derived neural progenitor cells to motoneurons. In an elegant study by Li et al. (2005), hESC-derived neuroectoderm precursor cells were cultured in the presence of RA, SHH, or both and the presence of both RA and SHH resulted in the greatest amount of motoneurons, as indicated by the percentage of cells expressing HB9, a motoneuron-specific transcription factor.
Another study identified phenazopyridine as a soluble factor that would not only improve neuronal differentiation of hESCs compared to other conventional methods, but would also generate neuronal cells that maintained the potential to form all three neuronal lineages: glutameric, GABAmeric, and tyrosine hydroxylase (TH1)-expressing neurons (Suter et al., 2009). Phenazopyridine-treated hESCs produced a homogenous population of nestin+/Tuj1low neuronal precursor cells after 4 weeks of differentiation and yielded a 1.5-fold increase in neural tube outgrowths in three-dimensional culture after 6 weeks of differentiation relative to hESCs not exposed to phenazopyridine.
Other soluble factors such as glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, platelet-derived growth factor, cyclic adenosine monophosphate, ascorbic acid, and epidermal growth factor and commercially available defined media supplements such as B27 and N2 have been incorporated into neural differentiation protocols to establish microenvironments that are either more conducive for initial neural commitment of hPSCs or for further differentiating neuroepithelial cells to more specialized neurons or glial cells (Belinsky et al., 2011, Ebert et al., 2009, Kang et al., 2007a, Li et al., 2005, Ogawa et al., 2011, Preynat-Seauve et al., 2009, Suter et al., 2009, Wang et al., 2011a).
3.2.2. Role of substrate or ECM
To induce neuronal differentiation from neural progenitors, several laboratories plate hPSC-derived neural precursor cells on a laminin-coated poly(L-ornithine) (PLO) substrate (Baharvand et al., 2007, Ebert et al., 2009, Li et al., 2005, Suter et al., 2009, Zhang et al., 2001). Others have found alternative substrates to enhance neuronal differentiation. For example, one study demonstrated that carbon nanotubes grafted with poly(acrylic acid) (PAA) and coated with laminin serves as a better substrate for hESC neuronal differentiation when compared to a laminin-coated flat PAA control or a conventional PLO substrate (Chao et al., 2009). A subsequent study from the same laboratory demonstrated that a poly(methacrylic acid) (PMAA)-grafted carbon nanotube substrate yielded even better neuronal differentiation of hESCs resulting in a 220% improvement in adhesion efficiency and a 2.5-fold higher population expression of Tuj1 compared to the conventional PLO substrate (Chao et al., 2010).
Several laboratories have focused efforts on achieving neural differentiation of hPSCs using synthetic systems with minimal biological agents, as they offer cheaper and potentially more robust alternatives to systems that require the addition of various growth factors. For example, one research group developed a polyurethane acrylate-based nanoscale ridge/groove pattern array to facilitate differentiation of hESCs into neuronal cells without the use of RA or a laminin substrate (Lee et al., 2010). This substrate pattern, compared to a flat surface control, clearly favored neurectoderm and, specifically, neuronal differentiation, indicating a spatial or mechanical dependence of neuronal differentiation. Neuronal cell fate of hESCs was also favored in another study that utilized an electospun polyurethane substrate, which provided a relatively pure population (80–100%) of Tuj1+/TH1+ cells within the hESC-derived cell population (Carlberg et al., 2009).
3.2.3. Culture architecture: 2D vs. 3D microenvironments
Most laboratories that have engineered systems to direct hPSCs toward neural cell fates have utilized EB-based methods requiring a manual selection step involving colony picking or careful enzymatic separation. However, monolayer (2D) culture systems have been developed as well to induce neural differentiation of hESCs as well using either co-culture systems or completely defined systems (Baharvand et al., 2007, Shin et al., 2006). One study by Baharvand et al. (2007) used a directed differentiation system where hESCs in monolayer culture on Matrigel are induced to undergo neural differentiation in the presence of bFGF and noggin, followed by plating on a laminin-coated PLO substrate resulting in > 90% cells expressing Tuj1, synaptophysin, and neurofilament proteins. Like most EB-based protocols, this differentiation scheme also used mechanical isolation of neural tube-like structures for further differentiation. However, to our knowledge, no direct comparison of EB vs. monolayer culture with respect to neural differentiation efficiency of hPSCs has been conducted.
More novel culture methods have been established to improve efficiency and reproducibility of neural differentiation of hPSCs. For example, multiple hESC lines cultured at ALI compared to two-dimensional cultures resulted in a greater frequency of rosette formations, a more morphologically homogeneous mature (Tuj1+) cell subpopulation, segregation between neural progenitor niches and more advanced neuronal cell populations, and a more distinct organization of cells in three dimensions (Preynat-Seauve et al., 2009). Such a system exhibits how culture at ALI may better recapitulate 3D architectures observed in vivo compared to submerged 2D cultures.
3.2.4. Temporal dependence of cues
In engineering culture systems conducive for differentiation, time can be overlooked as an essential component of the microenvironment. The developing embryo is an agglomeration of countless microenvironments, each defined by a unique spatio-temporally regulated milieu of soluble factors, extracellular matrix proteins, and cell-cell contact. Regarding neural differentiation, there are factors that are implicated in directing cells to different cell fates depending on when these cues are initiated. For example, the effects of RA addition to differentiating neural cells are highly time-dependent. An elegant paper by Pankratz et al. (2007) demonstrated that culture of plated EBs without the presence of RA resulted in an anterior neural phenotype, but if RA was added to the medium 0–2 days after EBs are plated no neural rosettes formed. When RA was added to the medium 4 days after EB plating, neural tube-like rosettes formed and continued exposure to RA resulted in a posterior neural phenotype. These experiments clearly demonstrate that there is a specific time window for RA to induce a posterior phenotype in cells already committed to a neural fate. Temporal regulation of growth factors such as SHH, FGFs, and BMPs that can operate synergistically with RA have also been implicated in hierarchical neural cell fate decisions in vivo and need to be considered when attempting to recapitulate these events in vitro using hPSCs (Dhara and Stice, 2008, Zhang, 2006).
The interest in generating neural cells for tissue engineering applications has garnered some fascinating developments in the field of hPSC engineering over the past decade. However, there remains a need to optimize current differentiation strategies to generate neural cells from hPSCs and improve overall efficiency to ultimately translate these laboratory-scale differentiation systems to manufacturing or clinical scales.
3.3. Retinal and corneal cells
Most studies investigating the commitment of hPSCs toward ectodermal lineages focus on either epidermal or neural differentiation. However, there is an interest in deriving other ectodermal cells from hPSCs including retinal and corneal cells for applications in studying blindness and cataracts. One study, aimed at recapitulating the limbal stem cell niche of the cornea in vitro, used a culture system consisting of a medium conditioned by limbal fibroblasts in combination with various ECM substrates to generate corneal epithelial cells from hESCs. It was found that a collagen IV substrate, in comparison to a laminin or fibronectin substrate, resulted in the highest percentage of p63+ cells and the highest limbal colony forming efficiency (Ahmad et al., 2007). This was expected by the authors because collagen IV is prominent in the basement membrane in the limbus of the cornea. The resulting cells expressed corneal epithelial markers cytokeratin 3 and 12.
A study by Buchholz et al. clearly demonstrated the differentiation and selection of retinal pigmented epithelial cells from hiPSCs by removing bFGF from the medium and subsequently plating pigmented cells onto a gelatin substrate (Buchholz et al., 2009). This study did not isolate any microenvironmental parameters to study the effect on retinal pigmented epithelium differentiation efficiency, but did recognize that singularization of cells resulted in loss of epithelial morphology and pigment indicating a requirement of cell-cell contact to successfully differentiate and propagate a culture of functional retinal pigmented epithelia. Another study investigated the effects of oxygen concentration on the generation of retinal progenitor cells from hPSCs. Hypoxia (2% O2) enhanced this differentiation compared to atmospheric concentrations resulting in a 10-fold higher concentration of Pax6+/Chx10+ cells (Bae et al., 2011). In fact, oxygen concentration is often overlooked as a key parameter of the stem cell microenvironment or niche but has been demonstrated to play a significant role in regulating differentiation of neural cells and cells from the mesoderm germ layer including mesenchymal and hematopoietic cells in addition to retinal progenitor cells (Mohyeldin et al., 2010).
4. Mesoderm lineages
Mesoderm, the “middle” germ layer of an organism, includes such tissues as muscle, blood, and bone. Mesoderm formation originates during the gastrulation stage of embryonic development, beginning with formation of the primitive streak, commonly identified by the transcription factor brachyury (T). In the embryo, epiblast cells migrate through the primitive streak and undergo an epithelial to mesenchymal transition (EMT) (Gadue et al., 2005), hallmarks of which include loss of polarity, downregulation of E-cadherin, and acquisition of a migratory phenotype (Xu et al., 2009). In vitro, early mesodermal differentiation can be achieved in hESCs cultured on substrates functionalized with peptide ligands for α5β1 and α6β1 integrins (Liu et al., 2011).
4.1. Cardiomyocytes
Cardiomyocytes are the muscle cells responsible for rhythmic contractions of the heart. These cells have a limited proliferative capacity at the adult stage (Soonpaa and Field, 1998), so loss of cardiomyocytes due to myocardial infarction or disease can lead to permanent loss of heart function. Heart failure is the leading cause of death in the United States (Lloyd-Jones, 2010), and the best current treatment option is organ transplant, limited by the number of available donor hearts. A great clinical need exists for human cardiomyocytes which could be used in drug screening or to repopulate damaged regions of patient hearts. Pluripotent stem cells have the potential to produce large numbers of cardiomyocytes for such applications, and their differentiation to cardiomyocytes has been extensively studied and modulated using engineering strategies, summarized in Table 2. Cardiomyocytes are identified in culture by the presence of spontaneously contracting cells, or on a molecular level, by expression of the cardiac transcription factors Nkx2.5 and GATA4 and definitive cardiac markers such as MLC2a, MLC2v, α-MHC, MF20, α-actinin, cTnT, and cTnI.
Table 2.
Summary of microenvironmental factors that have been demonstrated to influence directing hPSCs toward a cardiac cell fate.
| Category | Factor | Effect | References |
|---|---|---|---|
| Soluble factors | BMP4 added to EBs in suspension | 4-fold increase in beating frequency | Takei et al. 2009 |
| Soluble factors | Sequential addition of Activin A and BMP4 to monolayer cultures | Cardiogenesis achieved in H7 hESCs | Laflamme et al. 2007 |
| Soluble factors | BMP4, bFGF, Activin A, VEGF, and DKK1 added at varying concentrations/times | Cardiogenesis achieved in multiple cell lines with protocol modifications | Yang et al. 2008, Kattman et al. 2011 |
| Soluble factors | >45 variables optimized, including polyvinyl alcohol, BMP4, FGF2, and insulin concentrations/times | Universal protocol for cardiogenesis in 11 cell lines | Burridge et al. 2011 |
| Soluble factors | Activin A/BMP4 protocol plus Matrigel sandwich | Induced EMT and efficient cardiogenesis | Zhang et al. 2012 |
| Soluble factors | Activin A/BMP4 protocol plus BIO pre-treatment | Efficient cardiogenesis | Hazeltine et al. 2012, Lian et al. 2012, Lian et al. 2013 |
| Soluble factors | Sequential addition of CHIR99021, IWP4 to modulate Wnt pathway | Efficient cardiogenesis in fully defined system | Lian et al. 2012, Lian et al. 2013 |
| Soluble factors | Isoflurane addition to hESCs | Improved survival of cardiac progenitors | Kim et al. 2011 |
| Soluble factors | Ghrelin addition to hESCs | Enhanced cardiogenesis | Yang et al. 2011 |
| Co-culture | hESC-derived cardiomyocytes isolated or cultured with non- myocytes | Non-myocytes required for electrophysiological maturation | Kim et al. 2010 |
| 3D environment | Cuboidal microwells with 100– 500 μm side lengths | All sizes enhance cardiogenesis relative to TCPS; 300 μm is optimal size | Mohr et al. 2010, Azarin et al. 2012 |
| 3D environment | Micropatterned features of 200– 800 μm diameters | Large endoderm-biased and small neural-biased EBs enhanced cardiogenesis | Bauwens et al. 2008 |
| 3D environment | 3D microwells seeded with 100–4000 hESCs | Highest cardiogenesis in 1000 cell aggregates | Bauwens et al. 2011 |
| 3D environment | Concave PDMS microwells with 200–1000 μm diameters | Highest cardiogenesis in 1000 μm size | Choi et al. 2010 |
| 3D environment | Micropatterned features of 100–400 μm diameters | Highest cardiogenesis in 200 μm size | Sasaki et al. 2009 |
| 3D environment | Cardiac progenitors cultured in 3D electrospun scaffold | Increased proliferation | Schenke-Layland et al. 2011 |
| Mechanical forces | Laminar shear stress applied to mESCs | Histone modifications and upregulation of cardiac markers | Illi et al. 2005 |
| Mechanical forces | Size-controlled EBs cultured in hypoxic conditions in bioreactor | Increased beating percentages | Niebruegge et al. 2009 |
| Mechanical forces | Rotary suspension culture of EBs | Increased cardiogenesis | Sargent et al. 2009 |
| Mechanical forces | Encapsulated ESCs cultured in spinner flasks | Increased cardiac gene expression | Jing et al. 2010 |
| Electrical stimulation | Electric fields applied to mESC-EBs | Increasing field strength increased cardiogenesis | Sauer et al. 1999 |
| Electrical stimulation | Point-source electrical stimulation with controlled current | Stimulating day 7 EBs for 4 days at 30 μA was best for cardiogenesis | Chen et al. 2009a |
4.1.1. Embryoid body/aggregate size control
The earliest reports of hESC and hiPSC differentiation to cardiomyocytes employed the use of EBs (Kehat et al., 2001, Zhang et al., 2009), which are spherical aggregates of cells cultured in suspension for several days in serum-containing medium. EBs are then plated and exhibit spontaneously contracting regions as cardiomyocytes are generated. The name for these 3D bodies reflects that they mimic the microenvironment of the developing embryo; however, like an embryo, EBs produce a heterogeneous mixture of cell types with low yield of cardiomyocytes. Efforts have been made to engineer embryoid bodies to increase the percentage of cardiomyocytes they can generate.
Pluripotent stem cells are typically grown in colonies of various sizes, and this size heterogeneity is propagated when colonies are made into embryoid bodies. Two strategies have commonly been employed to control the size of hPSC colonies and thus, to control embryoid body or aggregate size: microwells and micropatterning. Our lab used a cuboidal polyurethane microwell system with lateral dimensions ranging from 100–500 μm to demonstrate that all sizes of 3D microwell culture enhanced cardiogenesis relative to 2D tissue culture polystyrene (TCPS) culture (Mohr et al., 2010). A starting colony size of 300 μm was optimal for cardiogenesis in hESCs, although cardiogenic 100 μm aggregates contained more cardiomyocytes per EB. This effect was linked to upregulation of Wnt/β-catenin signaling in EB derivatives of hESCs cultured in microwells (Azarin et al., 2012). The Zandstra lab used micropatterned features of 200–800 μm in diameter to control hESC colony size prior to making EBs and demonstrated that cardiogenesis depended both on EB size and the ratio of endoderm-biased (high Gata6/Pax6 ratio) to neural-biased (low Gata6/Pax6 ratio) cells in the EB (Bauwens et al., 2008). Large endoderm-biased EBs and small neural-biased EBs exhibited high levels of cardiogenesis. When differing numbers of hESCs were seeded into 3D microwells, intermediate-sized aggregates of 1000 cells were found to have the highest cardiogenesis, and this effect was linked to signals from neighboring extraembryonic endoderm cells (Bauwens et al., 2011). A concave PDMS microwell system has been used to demonstrate that, when comparing 200–1000 μm diameter aggregate sizes, larger aggregates of mESCs exhibited higher α-actinin expression and beating frequency (Choi et al., 2010). However, when mESCs were seeded and differentiated on micropatterned islands of 100–400 μm in diameter, 200 μm aggregates exhibited the highest cardiogenesis, based on flow cytometry data from an α-MHC EGFP reporter line (Sasaki et al., 2009). These examples, with their seemingly conflicting findings, illustrate the importance of context when interpreting the effects of a single cue on lineage commitment.
4.1.2. Co-culture with non-cardiomyocytes
Another technique used to induce cardiomyocyte differentiation is co-culture with non-cardiomyocytes. One early example involved co-culture of hESCs with mouse visceral endoderm-like cells to induce hESC differentiation to functional cardiomyocytes (Mummery et al., 2003). Another study demonstrated the role of non-cardiomyocytes in facilitating functional maturation of hESC-derived cardiomyocytes (Kim et al., 2010). When hESC-derived cardiomyocytes were cultured in isolation from non-myocytes, they did not mature, but maturity could be rescued by adding non-myocytes back into culture. Although co-culture can produce a desired outcome, its mechanism of action remains unclear.
4.1.3. Role of soluble factors
A more defined strategy to engineer cardiac differentiation involves addition of growth factors or cytokines to medium to mimic the signaling environment of cardiac specification. Activin/Nodal and BMP signaling are important in cardiomyocyte differentiation. In one report, BMP-4 was added to the medium of EBs in suspension and resulted in up to 97.7% of contracting EBs, 4-fold higher than control samples without BMP-4 (Takei et al., 2009). In a monolayer system, sequential addition of Activin A and BMP-4 was sufficient to drive cardiogenesis in H7 hESCs (Laflamme et al., 2007). The Keller lab has developed protocols utilizing BMP-4, bFGF, Activin A, vascular endothelial growth factor (VEGF) and dickkopf-1 (DKK-1) for efficient cardiogenesis in mouse and human PSC aggregates, but these protocols require optimization for each cell line of interest, which could be problematic in the advent of patient-specific hiPSC-lines (Kattman et al., 2011, Yang et al., 2008). A universal protocol was recently arrived upon by optimizing 4 different stages of differentiation: hPSC growth, EB formation/mesoderm induction, cardiac specification, and cardiomyocyte development (Burridge et al., 2011). This approach highlights the dynamic nature of the niche, and effective protocols must offer temporal changes that mimic different developmental stages.
In the past year, major advances have been made in monolayer-based directed differentiation to cardiomyocytes. Building on the Murry lab’s Activin A/BMP-4 protocol, the Kamp lab led efforts to develop a Matrigel “matrix sandwich” method, which induced hPSCs to undergo an epithelial-to-mesenchymal transition and efficiently differentiate to cardiomyocytes (Zhang et al., 2012). Our lab modified the Activin A/BMP-4 protocol to include a pre-treatment step with the Wnt activator BIO resulting in > 90% purity of cardiomyocytes (Hazeltine et al., 2012, Lian et al., 2012, Lian et al., 2013). We next developed a fully defined, growth-factor free protocol for cardiomyocyte generation from hPSCs (Lian et al., 2012, Lian et al., 2013). This protocol relies on temporal modulation of Wnt signaling through sequential application of CHIR99021 and IWP4 small molecules.
4.1.4. Fluid flow and bioreactor culture
In vivo, forces generated by proximal blood flow play a role in organogenesis. Culture methods which incorporate a dynamic flow element have been shown to improve cardiogenesis when compared to traditional static culture methods. For example, mESCs exposed to laminar shear stress showed several histone modifications, which paralleled upregulation of early cardiac markers (Illi et al., 2005).
Bioreactors offer a 3D, dynamic fluid environment ideal for embryoid body culture. Bioreactor culture was shown to increase beating percentage in size-controlled hESC-derived EBs, with additional benefits shown in hypoxic conditions representative of the embryonic environment (Niebruegge et al., 2009). Rotary suspension culture has been demonstrated to increase beating percentage as well as cardiac gene and protein expression in mESC-derived EBs (Sargent et al., 2009). A bioreactor study in the Tzanakakis lab demonstrated increased cardiac gene expression in mESCs and hESCs encapsulated in liquid core poly-L-lysine-coated alginate capsules cultured in spinner flasks (Jing et al., 2010). In addition to improving cardiogenesis, bioreactors provide a scalable system that may prove useful for producing large numbers of cardiomyocytes for therapeutic applications.
4.1.5. Electrical stimulation
Electrical fields in the developing embryo also play a role in cardiogenesis, and incorporating electrical stimulation into culture systems can increase yield of cardiomyocytes. When electrical fields were applied to mESC-derived EBs, increasing electrical field strength was found to increase both the number and size of beating regions (Sauer et al., 1999). Addition of 1 nM H2O2 had a similar positive effect, implying that electrical fields were acting through the generation of reactive oxygen species (ROS). hESCs have also been shown to generate ROS upon electrical stimulation; however, ROS were not shown to improve cardiac differentiation in this study (Serena et al., 2009). Another study utilized point-source electrical stimulation with a controlled current, which better mimics the electrical microenvironment of the heart (Chen et al., 2009a). After investigating various mESC-derived EB differentiation stages and stimulation amplitudes, stimulating day 7 EBs for 4 days at 30 μA was found most beneficial for cardiogenesis, evidenced by increased cardiac gene expression.
4.1.6. Engineering expansion and sorting to increase yield and purity
One approach to improve the yield of cardiomyocytes from hPSCs is to enhance cell survival and expansion throughout the differentiation process. Incorporation of pro-survival factors can be beneficial to this end. For example, the anaesthetic isoflurane has been shown to improve survival of Nkx2.5+ cells derived from hESCs by offering protection from oxidative stress (Kim et al., 2011b). Ghrelin, a peptide with cardioprotective activity, has recently been demonstrated to increase cardiogenesis in hESC-derived EBs through an undefined mechanism (Yang et al., 2011). We can also attempt to mimic the niche of cardiac progenitor cells (CPCs) which are intermediates in the journey from hPSCs to cardiomyocytes. One elegant study examined the in vivo CPC niche and sought to reconstruct it in vitro (Schenke-Layland et al., 2011). A 3D gelatin/polycaprolactone electrospun scaffold, collagen IV coating, and IQ1, a small molecule to specifically inhibit p300-dependant β-catenin signaling, increased CPC proliferation.
In order to improve cardiomyocyte purity it is possible to genetically engineer hPSC lines to facilitate sorting and selection. This has been demonstrated with an hESC-derived α-MHC GFP reporter line, where cardiomyocytes were isolated at early stages of differentiation (Ritner et al., 2011). Cardiomyocytes may also be selected for using antibiotics, such as an example utilizing hESCs with α-MHC-driven puromycin resistance (Kim et al., 2010). The exciting recent identification of a cardiomyocyte surface marker, SIRPA, facilitated their sorting without the need for genetic modification (Dubois et al., 2011). Ultimately cardiomyocytes may need to be mixed back with other cell types present in the heart, such as endothelial cells and smooth muscle cells.
4.2. Mesenchymal stem cells and their derivatives
Mesenchymal stem cells or stromal cells (MSCs) are multipotent adult stem cells capable of becoming specialized cells including osteoblasts (bone), chondrocytes (cartilage), or adipocytes (fat). They are commonly harvested from bone marrow; however, this is an invasive procedure, and MSCs from older adults have reduced expansion and differentiation potential (Roobrouck et al., 2008). Therefore, there is interest in using hPSCs as an alternative source of MSCs, and in understanding both how to keep the cells multipotent for scale-up and how to induce lineage-specific differentiation. Osteoblasts, commonly identified by expression of collagen type I and staining for mineralization with Alizarin Red, have applications in regenerative medicine, as do chondrocytes, identifiable by collagen type II expression and proteoglycan staining with Alcian blue. Adipocytes are of interest because they offer a model system for studying obesity and related diseases, and they can be identified in culture by production and secretion of hormones.
4.2.1. Maintaining multipotency
Several studies have demonstrated the derivation of MSCs from hPSCs and their sustained maintenance of multipotency. In the Thomson and Slukvin labs, hESCs and hiPSCs were differentiated to MSC lines through sequential co-culture with OP9 mouse bone marrow stromal cells, culture in semisolid medium, and plating onto a fibronectin/collagen matrix in MSC medium (Vodyanik et al., 2010). The MSC lines were characterized by their ability for long-term maintenance and osteo-, chondro-, and adipogenic differentiation. The MSCs were found to share a common precursor with endothelial cells, which was dubbed the mesenchymoangioblast. In mouse and human ESCs, sustained activation of the Wnt pathway through use of a GSK-3β inhibitor led to generation of mesendodermal progenitor clones which maintained multipotency for over a year and could become endothelial cells as well as various mesodermal lineages (Bakre et al., 2007). In a third study, hiPSCs were differentiated to MSCs via a clinically compliant monolayer protocol (Lian et al., 2010). When transplanted into mice with hind-limb ischemia, the hiPSC-MSCs promoted vascular and muscle regeneration in a superior fashion to adult bone marrow-derived MSCs. This is an excellent demonstration of the utility of hPSC-MSCs and motivates further research into their derivation and maintenance. Most efforts thus far have focused on the obtaining derivatives of MSCs, and engineering efforts have been utilized to improve these processes.
4.2.2. Role of soluble factors
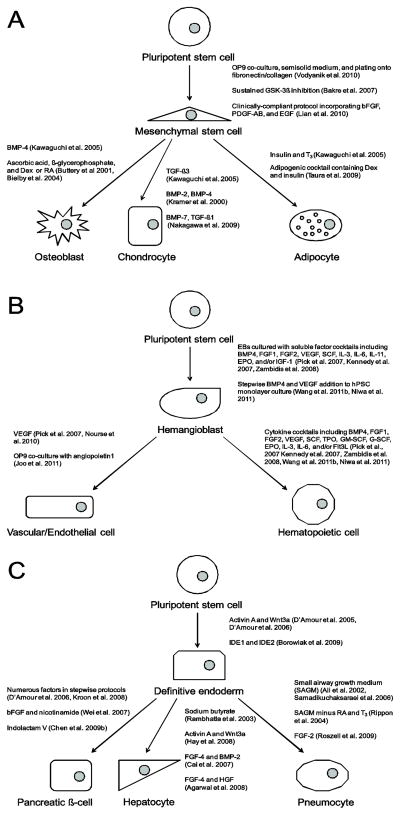
Many studies have identified soluble factors which can be used to obtain osteoblasts, chondrocytes, and adipocyctes, and some further demonstrate the tissue engineering potential of these cells. A summary of these findings is shown in Figure 3A. In one study, mESC-derived EBs were cultured in the presence of RA, then plated and exposed to a growth factor cocktail (Kawaguchi et al., 2005). Addition of insulin and triiodothyronine (T3) was found to specifically induce adipogenesis, BMP-4 to induce osteogenesis, and TGF-β3 to induce chondrogenesis. Focusing on osteogenesis alone, another study identified that supplementing the medium of dissociated mESC-EBs with a combination of ascorbic acid, β-glycerophosphate, and dexamethasone (Dex) or RA led to osteogenesis and formation of Alizarin Red-positive bone nodules in vitro (Buttery et al., 2001). The same protocol was later shown to translate directly to hESCs, and hESC-derived osteoblasts were demonstrated to form mineralized tissue in vivo (Bielby et al., 2004). For chondrogenesis, BMP-2 and BMP-4 were found to have a positive effect on mESC-derived EBs while TGF-β1 had a negative effect, characterized by Alcian blue staining and chonodrocyte gene and protein expression (Kramer et al., 2000). Using an EB-free protocol, hESCs were cultured in the presence of BMP-7, TGF-β1, or a combination (Nakagawa et al., 2009). The combination of both factors resulted in the largest yield of chondrocytes, while the highest expression of chondrocyte genes occurred in the BMP-7 only condition. For adipogenesis, hESCs and hiPSCs have been differentiated with similar efficiencies by culturing EBs in the presence of RA, then seeding onto a PLO/fibronectin matrix and adding an adipogenic cocktail containing Dex and insulin among other factors (Taura et al., 2009). In all cases, soluble factor combination, concentration, and timing of addition regulate differentiation efficiency, which could likely be improved by further optimization.
Figure 3.
Outline of strategies for deriving (a) MSCs and their derivatives, (b) hematopoietic and vascular cells, and (c) DE and its derivatives from hPSCs using soluble factors.
4.2.3. Modulating aggregation level for differentiation
Various culture methods have been employed to demonstrate that cell aggregation can influence differentiation to mesenchymal lineages. These include monolayer, EB, and hybrid approaches. To demonstrate the 3D vs. 2D extremes, hESCs underwent osteogenesis in either an EB or monolayer system (Karner et al., 2007). While cells differentiated via both methods produced mineralized matrix, the monolayer cells had greater osteogenic potential. In another example of osteogenesis, cells in mESC-derived EBs were crosslinked by exploiting avidin-biotin binding (Gothard et al., 2010). The crosslinked EBs exhibited increased mesoderm differentiation propensity and improved osteogenic differentiation in both osteoinductive and control media. In a study of chondrogenesis, mESC-derived EBs were plated intact or dissociated and plated as a monolayer, high-density monolayer, or a pelleted mass (Tanaka et al., 2004). The high-density monolayer and pelleted mass exhibited greater chondrogenesis, evidenced by increased Sox9 and collagen type II expression.
4.2.4. Culture architecture: artificial scaffolds for differentiation
Several studies have exploited dimensionality and scaffold architecture to influence differentiation to mesenchymal lineages. In one example, hESC-derived EBs were cultured with growth factors to induce osteogenesis, then dissociated and seeded onto 2D and 3D architectures of nanofibrous poly(L-lactic acid) (Smith et al., 2010). In both 2D and 3D environments, the nanofibers enhanced osteogenic differentiation, measured via mRNA expression and mineralization, when compared to traditional solid film or solid-walled methods. Another study demonstrated that osteogenesis occured in the absence of a scaffold (Handschel et al., 2011). mESCs were aggregated to microspheres in a bioreactor and cultured in the presence of ascorbic acid, β-glycerophosphate, and Dex, and the cells produced mineralized matrix. In an adipogenesis study, mESCs were seeded onto 3D polycaprolactone electrospun scaffolds designed to mimic the architecture of adipose tissue or onto 2D disk controls (Kang et al., 2007b). The 3D environment led to formation of uniform cell aggregates, a key input for efficient differentiation. The adipocytes formed in 3D scaffolds exhibited proper insulin response and morphology. In another study of osteogenesis, mouse induced pluripotent stem cells (miPSCs) were generated from mouse fibroblasts and then differentiated to osteoblasts via an EB method (Bilousova et al., 2011). miPSC-derived osteoblasts were seeded into Gelfoam sponge scaffolds and implanted into mice. Mineralization and vascularization occurred within the scaffolds, and the osteoblast phenotype persisted for 12 weeks in vivo. This is one of many examples illustrating that 3D scaffolds are useful not only to influence differentiation trajectories, but also to enhance functionality and reparative capacity of differentiated cells.
4.3. Hematopoietic and vascular lineages
Both hematopoietic and vascular cell types arise from within the mesoderm germ layer in a developing embryo via a common progenitor, often referred to as a hemangioblast. Researchers currently seek methods for generating vascular (vascular smooth muscle and endothelial) cells from hPSCs for in vitro disease modeling and for tissue engineering constructs since most tissues require some degree of vasculature for efficient nutrient supply and waste disposal. Generating hematopoietic cells from hPSCs is of great interest to researchers because of the variety of clinical applications for which hematopoietic cells can be used including treatments for cancer and various immune disorders. To date, there has been progress made on both of these fronts in differentiating hPSCs to vascular and hematopoietic cells often via a hemangioblast intermediate. Figure 3B highlights some of these strategies for deriving hemangioblasts and subsequently vascular and hematopoietic cells from hPSCs.
4.3.1. Hematopoietic lineages
Because hematopoietic cells are nonadherent cells, there are very few studies that investigate effects of ECM proteins or synthetic substrates as microenvironmental cues that may enhance hematopoietic differentiation of hPSCs. Most methods for guiding hPSCs toward hematopoietic cell fates involve co-culturing hPSCs with an OP9 mouse stromal cell line (Choi et al., 2009) or using EB-based differentiation and the addition of soluble factors. While these EB-based strategies are largely inefficient, some researchers have identified soluble factor cues that can improve initial hematopoietic commitment of hPSCs using EBs. For example, one elegant study investigated the roles of BMP-4, FGF2, VEGF, and stem cell factor (SCF) on the hematopoietic differentiation of hESCs (Pick et al., 2007). Addition of BMP-4 to EBs in suspension induced almost 100-fold higher expression of MIXL1, a primitive streak marker, and induced expression of other primitive streak and hematopoietic mesoderm genes, such as brachyury (T), Goosecoid, KDR, and SCL, in a dose-dependent manner regardless of the presence of the other growth factors. Others have confirmed the requirement of BMP-4 to form hemangioblast colonies from EBs (Kennedy et al., 2007). FGF2 has been shown to improve hemangioblast viability and, correspondingly, yield by at least 2-fold compared to cultures that did not contain FGF2, and the combination of all four factors resulted in the highest hematopoietic colony forming efficiency of about 150 per 100,000 cells as indicated by hematopoietic markers CD34, CD45, and CD33 (Pick et al., 2007). Such findings regarding the importance of FGF2, VEGF, and BMP-4 in hematopoietic differentiation of hPSCs have been corroborated in other studies (Kennedy et al., 2007, Wang et al., 2011b).
Additional studies have also utilized similar sets of growth factors in generating hematopoietic cell types. For example, a protocol by Yang et al. that utilized an EB-based differentiation method involving EB exposure to BMP-4, FGF2, Activin A, VEGF, and DKK-1 in a specific temporal manner was modified in another study to induce erythromyeloid and, more specifically, B-cell lymphopoiesis of hiPSCs following hematopoietic commitment (Carpenter et al., 2011, Yang et al., 2008). In one recent study, using knowledge of which soluble factors promote hematopoietic differentiation of hPSCs in EB-based differentiation strategies, researchers developed a serum-free monolayer differentiation scheme to derive various hematopoietic cells from hPSCs (Niwa et al., 2011). Similar to the EB-based study by Pick et al., BMP-4 was found to induce primitive streak formation in hPSC colonies, as demonstrated by 4-fold higher expression of mesoderm markers, brachyury (T) and MIXL1, and a correspondingly lower expression of Sox1, a neurectoderm marker, and Nanog (Niwa et al., 2011, Pick et al., 2007). The removal of BMP-4 and the addition of VEGF and SCF to this monolayer culture resulted in hematopoietic mesodermal progenitors expressing KDR, CD117, CXCR4, and CD34 (Niwa et al., 2011).
While BMP-4, FGF2, and VEGF have been found to play key roles in directing hPSC differentiation to hemangioblasts, other soluble factors have also been implicated in guiding these hPSC-derived hemangioblasts towards hematopoietic cell fates. TGF-β inhibition enhanced hematopoietic conversion of CD31+ progenitors to CD43+ cells by about 2.5-fold and increased the number of CD43+/CD45+ hematopoietic progenitor cells from 20% to 50% after 18 days of differentiation (Wang et al., 2011b). This recent study elegantly outlined which growth factors synergistically or antagonistically regulate the multistep differentiation of hPSCs to hematopoietic cells, eliminating the need for a manual sorting step, which has been used by many researchers to isolate hematopoietic progenitors from the heterogeneous populations yielded by EB differentiation (Carpenter et al., 2011, Chadwick et al., 2003, Choi et al., 2009, Wang et al., 2004, Zambidis et al., 2008).
Further differentiation of hematopoietic progenitor cells derived from hPSCs can be induced with various cytokine cocktails containing different combinations of soluble factors widely used in hematopoietic differentiation, including SCF, thrombopoietin (TPO), interleukin 3 (IL3), interleukin 6 (IL6), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin (EPO) to generate either myeloid- or erythropoietic differentiation of hPSCs (Chadwick et al., 2003, Choi et al., 2009, Niwa et al., 2011, Wang et al., 2004, Zambidis et al., 2008, Zambidis et al., 2005). Some of these cytokines have also been implicated in earlier stages of hematopoietic differentiation of hPSCs in combination with BMP-4 to improve yield of CD34+/CD45+ hematopoietic progenitor cells over systems that utilized BMP-4 alone (Chadwick et al., 2003, Zambidis et al., 2008, Zambidis et al., 2005). While BMP-4 was responsible for promoting self-renewal of the hematopoietic progenitors, the continued exposure to the cocktail of other cytokines promoted the differentiation and maturation of the hPSC-derived hematopoietic cells. There is a wealth of knowledge as to which combinations of soluble factors induce specialization of hematopoietic progenitors toward specific hematopoietic cell types given the extent of research to date on hematopoietic stem cell (HSC) differentiation, providing an explanation as to why such extensive cytokine cocktails are known and thus incorporated into current hPSC differentiation strategies.
4.3.2. Vascular cells
Researchers have engineered microenvironments for successfully guiding hPSCs toward hematopoietic cell types, as described above, by first achieving hemangioblast differentiation. Similar differentiation strategies are also used to derive endothelial cells from hPSCs given the bipotent nature of hemangioblast cells in both mouse and human systems (Bai and Wang, 2008, Kane et al., 2011, Noghero et al., 2010). However, there are only a limited number of studies that investigate specific contributions from soluble factors or substrates on hPSC differentiation to vascular cells.
VEGF is arguably considered the best inducer or cue for endothelial differentiation. In a thorough study, VEGF added to EBs was found to induce endothelial differentiation in a dose-dependent manner, in a specific temporal context, and outperformed standard EB culture and OP9 co-culture differentiation strategies in terms of VE-cadherin, von Willebrand Factor (vWF), and CD31 expression (Nourse et al., 2010). The cell populations analyzed in this study also did not express hematopoietic markers such as CD45, CD14, and CD115 and formed vascular networks in vivo. Numerous studies have introduced VEGF to hPSC culture medium to induce endothelial differentiation of hPSCs and have found success in doing so in a number of different contexts. For example, VEGF incorporation into a hyaluronic acid hydrogel induced endothelial differentiation and sprouting of seeded hESCs which was not observed in the absence of VEGF (Gerecht et al., 2007). Other studies also demonstrated the requirement of VEGF to attain endothelial differentiation of hPSCs (Ferreira et al., 2007, Tatsumi et al., 2011).
Another soluble factor found to promote endothelial differentiation of hPSCs is angiopoietin-1. In the context of the OP9 co-culture method for inducing hemagioblastic differentiation of hPSCs, angiopoietin-1 has been shown to have the greatest effect on endothelial colony expansion, even greater than VEGF (Joo et al., 2011). In addition, angiopoietin-1 addition results in a 2-fold greater amount of CD31+/CD144+ endothelial cells and a 2-fold reduction in CD45+ hematopoietic cells by promoting endothelial conversion of hemangioblast precursors over hematopoietic commitment. It should be noted that the effects of angiopoietin-1 on endothelial differentiation and survival were found to be dose-dependent.
The presence of BIO, a GSK-3 inhibitor, in the cellular microenvironment can also have a positive influence on endothelial differentiation of hPSCs. In a feeder-free system where hESCs were plated on a collagen I substrate, BIO addition generated cells that co-expressed VE-cadherin and VEGFR-2 (Tatsumi et al., 2011). It should be noted that in this particular case, the requirement for BIO was highly context dependent, as very few current endothelial differentiation strategies implement BIO or other GSK-3 inhibitors. However, its indisputable role in promoting endothelial differentiation in a feeder-free system might prove useful as differentiation strategies continue to evolve toward efficient, defined, and non-xenogeneic systems.
As opposed to microenvironments engineered for hematopoietic differentiation where a cell-matrix interaction is arguably irrelevant, it has been found that endothelial cell differentiation can be enhanced with the use of an RGD peptide. Combined with angiopoietin-1, the addition of an RGD peptide to the microenvironment resulted in a 2-fold increase in CD31+/CD144+ cells (Joo et al., 2011). Another study from the Langer laboratory incorporated an RGD peptide into a dextran-acrylate-based hydrogel, resulting in a higher percentage of CD34+ cells, but not KDR+ cells (Ferreira et al., 2007). In a detailed study, monolayer culture of IMR90-4 hiPSCs on a Matrigel substrate was shown to yield ~66% PECAM-1+/GLUT-1+ blood-brain barrier (BBB) endothelial cells, a population which could be purified and further matured on a collagen-fibronectin matrix (Lippmann et al., 2012). Future studies should comprehensively investigate different ECM or synthetic substrates to improve current endothelial differentiation methods.
For the most part, current strategies to promote vascular and hematopoietic differentiation of hPSCs still involve the formation of EBs or the co-culture with xenogeneic cell lines. However, future studies should continue to focus on more defined, efficient culture methods that use stepwise microenvironmental changes to help navigate hPSC through the multiple progenitor stages that precede terminal endothelial and hematopoietic differentiation. The challenge for researchers in the future will be to identify key microenvironmental cues in the context of defined culture systems that act as “switches” to promote differentiation of an entire cell population towards one cell fate while inhibiting differentiation towards another cell lineage.
5. Endoderm lineages
Endoderm lineages comprise the internal organs, such as the pancreas, liver, lungs, and intestines. Similar to mesoderm, endoderm formation in vivo also originates with primitive streak formation. Migrating epiblast cells encounter signaling pathway gradients (BMP, Nodal, Wnt, and FGF) in the primitive streak which aid in specification to definitive endoderm (Gadue et al., 2005). Achieving endoderm lineages in vitro is desirable for regenerative medicine, as these cells could be utilized instead of organ transplant as a treatment for diabetes (pancreatic β-islets), liver diseases (hepatocytes), and respiratory diseases (pneumocytes). Endoderm is the final germ layer to form and its specification pattern is complex, so attempts to generate endoderm lineages from PSCs in vitro have not yet reached the level of progression of ectoderm and mesoderm lineages. Most work to date has focused on mimicking the signaling environment with addition of soluble factors, but some studies have incorporated additional parameters which will be discussed in the following sections.
5.1. Definitive endoderm
The first step in differentiation to endodermal cell types is specification to definitive endoderm (DE), identified by expression of SOX17, FOXA2, GSC, and CXCR4 (D’Amour et al., 2005). Several studies have focused on obtaining this precursor cell type with high efficiency, primarily through addition of soluble factors and controlling aggregate size.
5.1.1. Role of soluble factors
In 2005 a landmark protocol was reported for differentiation of hESCs to DE via treating hESC monolayers with Activin A in a low serum environment (D’Amour, Agulnick, 2005). The resulting population was up to 80% positive for SOX17, GSC, and FOXA2, and was further enriched by sorting for cells expressing the surface marker CXCR4. The same group later demonstrated enhanced DE specification through addition of Wnt3a on the first day of Activin A treatment (D’Amour et al., 2006). Phosphatidylinositol-3-kinase signaling and insulin/IGF were later found to antagonize the effects of Activin A on DE specification in hESCs, which can be rescued through addition of LY 294002 inhibitor (McLean et al., 2007). These studies, along with soluble factor approaches to induce further differentiation, are outlined in Figure 3C.
5.1.2. Controlling aggregate size
Although DE specification is effective in monolayer culture, culturing cell aggregates in a bioreactor is a more desirable scenario for scale-up. The Tzanakakis lab cultured hESCs on microcarriers in stirred-suspension bioreactors, and achieved DE specification efficiencies of 80% upon treatment with Activin A and Wnt3a in low serum (Lock and Tzanakakis, 2009). hESCs encapsulated in calcium alginate microcapsules, forcing aggregate homogeneity, have also been effectively differentiated to DE upon treatment with Activin A and Wnt3a (Chayosumrit et al., 2010). In a third report employing aggregate size control, hESCs were seeded onto Matrigel islands of 200–1200 μm diameter (Lee et al., 2009b). When treated with Activin A and BMP-2, smaller colonies expressed genes associated with endoderm (Sox17, GSC, and Cer1) while larger colonies tended towards mesoderm.
5.2. Pancreatic β-cells
Pancreatic β-cells are responsible for secreting insulin in response to rising blood glucose levels. Type I diabetes is caused by autoimmune destruction of β-cells while type II diabetes is caused by insulin resistance. Over 20 million Americans have some form of diabetes, and this number is expected to more than double by 2050 (Lloyd-Jones, 2010). PSC-derived β-cells offer the promise of a novel treatment option for diabetes or a model system to study its onset and progression. During differentiation pancreatic progenitors are commonly identified by expression of Pdx1, while more mature pancreatic cells are identified by secretion of insulin and its by-product c-peptide, to distinguish de novo insulin from that present in culture medium, in response to glucose. Most efforts thus far have employed soluble factors to obtain cells sharing some characteristics of β-cells, commonly referred to as hormone- or insulin-producing cells.
5.2.1. Role of soluble factors
The most successful protocols to obtain hormone-producing cells from PSCs involve addition of soluble factors in a stepwise fashion reminiscent of in vivo differentiation. One such protocol was developed by optimizing morphogen and inhibitor concentrations as well as timing and length of addition for 5 differentiation stages: DE, primitive gut tube, posterior foregut, pancreatic endoderm/endocrine precursor, and hormone-expressing endocrine cell (D’Amour et al., 2006). The resulting cells secreted various hormones, although the response to glucose was minimal. A later study employed a similar 4-step protocol to obtain pancreatic endoderm cells (Kroon et al., 2008). These cells were engrafted into mice, and upon glucose stimulation they secreted insulin and c-peptide at similar levels as engrafted human islets Another group developed a serum-free protocol in which Activin A and RA were sequentially employed for differentiation of hESCs, and bFGF and nicotinamide were employed for maturation of the resulting pancreatic cells (Wei et al., 2007). These cells were able to secrete insulin in response to glucose and restored normal blood glucose levels upon transplantation into diabetic mice.
Recent efforts have focused on identifying small molecules which can direct PSC differentiation to pancreatic cells. A screen of over 4000 compounds identified two, IDE1 and IDE2, which enhanced mESC and hESC differentiation to DE (Borowiak et al., 2009). Upon treatment with another small molecule, Indolactam V (Chen et al., 2009b), DE cells became pancreatic progenitor cells, with more than 50% expressing Pdx1 (Borowiak et al., 2009). Future efforts are likely to focus on identifying small molecules to drive specification to insulin-producing cells.
5.2.2. Culture architecture: incorporating a 3D environment
Additional efforts have combined soluble factors with 3D architecture to induce superior structural and functional maturation of pancreatic cells. In one example, mESCs differentiated to pancreatic cells via a 5-step protocol in a 3D collagen scaffold assembled into an islet-like tissue structure (Wang and Ye, 2009). These cells exhibited superior functional maturity to cells differentiated in 2D, evidenced by fivefold greater insulin release by the 3D cells upon exposure to high glucose medium, as opposed to twofold increase in insulin release by the 2D cells. In another study, hESCs were treated with Activin A, BMP-4, and bFGF (Xu et al., 2011). EBs formed from these cells were embedded into Matrigel in the presence of additional growth factors for further differentiation. 89% of the clusters were Pdx1+, some containing branch-like structures, and upon further differentiation, many cells in the clusters stained positive for insulin and c-peptide.
5.3. Hepatocytes
Hepatocytes are the main cell type found in the liver, which plays a key role in metabolism and detoxification. Although the liver has some regenerative capacity, this is impaired in end-stage liver disease. Liver transplant and bio-artificial liver devices are the only current treatment options, and transplantation of hPSC-derived hepatocytes offers an attractive alternative. Hepatocytes can be identified in culture by production of alpha-fetoprotein (AFP), albumin, and urea. While initial efforts focused on soluble factors, a variety of engineering approaches have since been employed to improve hepatocyte differentiation from hPSCs.
5.3.1. Role of soluble factors
In the first report of hepatocyte differentiation, hESCs were treated with sodium butyrate (Rambhatla et al., 2003). This resulted in massive cell death, but the remaining cells comprised a homogeneous hepatic population. A later study attempted hepatic differentiation of hESCs with Activin A/Wnt3a and Activin A/sodium butyrate treatments (Hay et al., 2008). Activin A/Wnt3a resulted in highly efficient differentiation, consistent with the authors’ observations of high Wnt3a expression in immunohistochemistry of developing human livers. Other studies utilized Activin A treatment followed by FGF4 and BMP-2 or hepatocyte growth factor (HGF) to obtain hepatocytes from hESCs (Agarwal et al., 2008, Cai et al., 2007). The hESC-derived hepatocytes in both studies were implanted into mice livers and displayed integration into native tissue.
5.3.2. Other microenvironmental stimuli
Extracellular matrix has been used in conjunction with soluble factors to drive hepatic differentiation of hESCs. In one study, various combinations of ECM (collagen I, laminin, and Matrigel) and growth factors (HGF, BMP-4, FGF4, RA, and Activin A) were applied to hESCs (Ishii et al., 2008). Sequential Activin A and HGF treatment on Matrigel was the best condition for hepatic differentiation, evidenced by highest activity of an AFP-GFP reporter cell line.
Co-culture is often desirable to induce differentiation, and microarray systems have been employed to bring a greater level of control into this potentially complex and undefined microenvironment. One group micropatterned alternating 300 μm spots of collagen I (for hepatic cell attachment) or fibronectin (for mESC attachment) (Lee et al., 2009a). Upon culture in hepatic differentiation medium, the co-cultured mESCs expressed endoderm and liver genes at higher levels compared to mESCs cultured alone. Another group immobilized HGF, bFGF, and BMP-4 into a combination of collagen I/fibronectin and micropatterned 500 μm spots of the mixture (Tuleuova et al., 2010). Spots containing HGF led to transient expression of Sox17 and expression of AFP and albumin in mESCs, which was further enhanced by co-culture with hepatic stellate cells.
A 3D environment has been demonstrated to benefit hepatic differentiation from hESCs. In one study, EBs were cultured on 2D or within 3D collagen and growth factors were added to induce hepatocyte differentiation (Baharvand et al., 2006). Both 2D and 3D environments yielded cells expressing hepatocyte markers, but in 3D, albumin and glucose-6-phosphotase (G6P) were detected earlier, and higher levels of AFP and urea were produced. In another study, hESCs were cultured in a hollow fiber-based 3D perfusion bioreactor, and hepatic differentiation was induced with stepwise growth factor addition (Miki et al., 2011). The 3D culture led to upregulation in hepatic gene expression and increased functional maturation.
5.4. Pneumocytes
The lung is an organ with complex structure and is comprised of a variety of different cell types, presenting a challenge for the development of stem cell therapies. Many efforts have focused on the generation of alveolar tissue of the gas-exchange unit, specifically type II pneumocytes, identifiable in culture by surfactant protein C (SPC) expression. Type II pneumocytes have been called stem cells of the pulmonary epithelium for their ability to proliferate and differentiate into type I pneumocytes after injury (Bishop, 2004). Despite the lung’s maintenance of some regenerative capacity, lung transplant remains the best option in end-stage lung disease. To advance efforts for tissue engineering of the lung, soluble factor and ECM approaches have been employed to obtain type II pneumocytes from PSCs.
5.4.1. Role of soluble factors
Soluble factors present in small airway growth medium (SAGM) are sufficient to obtain type II pneumocytes from mESCs and hESCs (Ali et al., 2002, Samadikuchaksaraei et al., 2006). When mESC- and hESC-derived EBs were cultured in SAGM, type II pneumocytes were present by day 30, based on SPC expression and morphology. During later attempts to improve yield of type II pneumocytes from mESCs, removing RA and T3 from SAGM resulted in threefold higher SPC expression (Rippon et al., 2004). In a monolayer-based protocol, mESCs were directed to DE and treated with FGF2 to induce SPC expression (Roszell et al., 2009). The mESC-derived type II pneumocytes were injected into preterm mice and found to distribute around the developing lung.
5.4.2. Role of ECM
One study employed various ECM coatings on both TCPS and poly-DL-lactic acid (PDLLA) for differentiation of mESCs to pneumocytes (Lin et al., 2010). Matrigel and laminin-332 coatings on TCPS and PDLLA led to enhanced SPC expression, identifying a polymer scaffold that may be useful for lung tissue engineering applications. Another study compared the efficiency of acellular lung, Gelfoam, Matrigel, and collagen I on formation of complex lung tissue from mESCs (Cortiella et al., 2010). The acellular lung led to better cell retention and more complex 3D organization of the resulting tissue suggesting that synthetic scaffolds must still be improved to approach the properties of native ECM.
6. Challenges and Directions
The stem cell niche is comprised of many factors, including soluble factors, the substrate and extracellular matrix, cell-cell interactions, mechanical forces, and scaffold architecture. Numerous culture systems have been designed to present these factors to PSCs in a controlled fashion and achieve a desired outcome, either self-renewal or differentiation to a specific cell type. While significant progress has been made to improve efficiency of PSC self-renewal and differentiation to certain clinically desirable cell types such as neurons and cardiomyocytes, less progress has been made towards other cell types such as endoderm lineages, potentially due to the complexity of their specification process (Gadue et al., 2005).
To accelerate progress on differentiation to challenging lineages, we encourage adoption of protocols that go beyond the typical single-factor approaches (depicted in Figure 1) to better mimic the complexity of a developing embryo, which is the in vivo niche of a differentiating pluripotent stem cell. Embryogenesis takes place in a 3D environment, but the majority of cell culture still takes place on 2D surfaces. Many approaches described here incorporate 3D architecture, either through EB formation to initiate differentiation, or incorporation of a scaffold to achieve greater yields or functional maturity. Another key consideration is that the developing embryo is tremendously dynamic with respect to time, the fourth dimension. Some of the most successful protocols described in this review employ a stepwise approach, changing media composition at each developmental stage to reflect the changing needs and trajectory of the cell. Finally, we must consider the influence of each component of the niche with respect to its context. For example, one soluble factor by itself could produce multiple outcomes, depending on its concentration and timing of introduction, as well as the identity of other components present in the niche. All components work together to send a collective net signal to the cell, which drives its fate decision. As the cell differentiates, it exerts influence on the niche, perhaps by secreting soluble factors or ECM components, which influence the cell and its neighbors. This combinatorial paradigm is outlined in Figure 4.
Figure 4.
Schematic illustrating how a new paradigm for designing culture systems to guide PSC fate decisions should take into account the combinatorial effect of microenvironmental cues as opposed to the individual contributions of each cue on a cell’s fate. In addition, the dynamic nature of a cell undergoing differentiation should be taken into account since the effects of these microenvironmental factors are contextually dependent on the state of the cell. A final consideration is the ability of the cell to continually modify its microenvironment, which can change the combinatorial net signal on itself or neighboring cells.
How does one begin to design and optimize such a complicated culture system? Many combinations of factors must be tested, and the number of plates and cells required can quickly become unmanageable with traditional techniques. Emerging microsystems technologies allow for increased throughput when testing the combinatorial influence of multiple factors. For example, microfabricated scaffolds, combinatorial microarrays, and microfluidics devices have been employed to make surprising discoveries about cell-environment interactions (Khademhosseini et al., 2006b, Yang et al., 2009). Once sufficient experimental data have been obtained, four-dimensional modeling is a useful technology to predict the effect of perturbing a single factor of the niche on cell fate, as it accounts for complexity in both spatial organization and time (Setty et al., 2008).
Although they offer an excellent screening tool, we need to use caution when interpreting conclusions from microscale systems. Microscale systems rely on small cell populations, even down to the single-cell level, while most clinical applications will require cell populations on the order of billions. All factors act in conjunction with other components of the niche, and drastically increasing the number of neighboring cells will alter the context of a single factor. After discoveries have been made with microscale systems, we need to do pilot experiments on the macroscale level to determine if modifications are required for scale-up.
A final factor to consider when designing culture systems for directing pluripotent stem cell fate is the altered differentiation propensity between various hESC and hiPSC lines. For example, hESC lines exhibit varied tendencies to differentiate towards pancreatic and cardiac lineages (Osafune et al., 2008), and hiPSC lines have been reported to exhibit reduced efficiency and increased variability relative to hESC lines in their differentiation propensity to neural lineages (Hu et al., 2010). Microarray data can be used to identify the predisposition of an hPSC line to differentiate down a lineage of choice, such as the negative correlation of miR-371-3 expression in undifferentiated hPSCs to Pax6 expression after neural differentiation (Kim et al., 2011a). More robust signals may need to be employed to differentiate hPSCs lacking a given predisposition, such as adding two inhibitors of SMAD signaling for efficient neural differentiation (Chambers et al., 2009).
In conclusion, significant progress has been made in reconstructing the niche in vitro to direct PSC fate decisions. Researchers working at the interface of engineering and biology have identified the spatial and temporal effects of various microenvironmental factors. New technologies are being adopted to accelerate discovery and implementation of protocols to obtain desirable cell types. Continued pursuit of this work will help PSCs to reach their full potential as a tool in regenerative medicine, in vitro modeling, and drug discovery.
Table 1.
Summary of microenvironmental factors that have been demonstrated to influence directing hPSCs toward neural cell fates.
| Category | Factor | Effect | References |
|---|---|---|---|
| Soluble factors | Addition of bFGF to EB culture | 94% of EBs gave rise to neural rosette structures compared to 0% without bFGF | Zhang et al. 2001 |
| Soluble factors | Addition of noggin to EB culture | Increases in neural precursor markers A2B5, PSA-NCAM, Musashi, AND NCAM of hESCs | Itsykson et al. 2005 |
| Soluble factors | Addition of RA and SHH | Guides hESC-derived neural progenitors to HB9-expressing motoneurons | Li et al. 2005a |
| Soluble factors | Addition of phenazopyridine | Improves neuronal commitment of hESCs and can give rise to all three neuronal cell types | Suter et al. 2009 |
| Soluble factors | Addition of RA (in the appropriate temporal context) | hESCs commit to posterior neural cell fate | Pankratz et al. 2007 |
| ECM/Substrate | PMAA- or PAA-coated carbon nanotubes | Increase in Tuj1 expression compared to PLO control |
Chao et al. 2009 Chao et al. 2010 |
| ECM/Substrate | Polyurethane acrylate-based ridge/groove pattern array | Promoted nestin and Tuj1, and later HuC/D and MAP2 expression cultured without RA | Lee et al. 2010 |
| ECM/Substrate | Electrospun polyurethane substrate | Promoted high purity populations of Tuj1+/TH1+ cells | Carlberg et al. 2009 |
| 3D Environment | Culture at ALI | Greater frequency of rosettes and more Tuj1+ cells compared to submerged monolayer culture | Preynat-Seauve et al. 2009 |
Acknowledgments
We apologize to our colleagues whose work we could not include due to space constraints. This work was supported by NIH grant R01 EB007534 and NSF grant EFRI 0735903.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laurie B. Hazeltine, Email: hazeltine@wisc.edu.
Joshua A. Selekman, Email: jselekman@wisc.edu.
References
- Aberdam E, Barak E, Rouleau M, de LaForest S, Berrih-Aknin S, Suter D, et al. A pure population of ectodermal cells derived from human embryonic stem cells. Stem Cells. 2008;26:440–4. doi: 10.1634/stemcells.2007-0588. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Holton K, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–27. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Stewart R, Yung S, Kolli S, Armstrong L, Stojkovic M, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145–55. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- Ali N, Edgar A, Samadikuchaksaraei A, Timson C, Romanska H, Polak J, et al. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Engineering. 2002;8:541–50. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–8. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33:2041–9. doi: 10.1016/j.biomaterials.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae D, Mondragon-Teran P, Hernandez D, Ruban L, Mason C, Bhattacharya SS, et al. Hypoxia Enhances the Generation of Retinal Progenitor Cells from Human Induced Pluripotent and Embryonic Stem Cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0225. [DOI] [PubMed] [Google Scholar]
- Baharvand H, Hashemi S, Ashtian S, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. International Journal of Developmental Biology. 2006;50:645–52. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- Baharvand H, Mehrjardi NZ, Hatami M, Kiani S, Rao M, Haghighi MM. Neural differentiation from human embryonic stem cells in a defined adherent culture condition. Int J Dev Biol. 2007;51:371–8. doi: 10.1387/ijdb.72280hb. [DOI] [PubMed] [Google Scholar]
- Bai H, Wang ZZ. Directing human embryonic stem cells to generate vascular progenitor cells. Gene Ther. 2008;15:89–95. doi: 10.1038/sj.gt.3303005. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–57. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bakre M, Hoi A, Mong J, Koh Y, Wong K, Stanton L. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. Journal of Biological Chemistry. 2007;282:31703–12. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- Bauwens C, Peerani R, Niebruegge S, Woodhouse K, Kumacheva E, Husain M, et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–10. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- Bauwens C, Song H, Thavandiran N, Ungrin M, Masse S, Nanthakumar K, et al. Geometric Control of Cardiomyogenic Induction in Human Pluripotent Stem Cells. Tissue Engineering Part a. 2011;17:1901–9. doi: 10.1089/ten.TEA.2010.0563. [DOI] [PubMed] [Google Scholar]
- Belinsky GS, Moore AR, Short SM, Rich MT, Antic SD. Physiological properties of neurons derived from human embryonic stem cells using a dibutyryl cyclic AMP-based protocol. Stem Cells Dev. 2011;20:1733–46. doi: 10.1089/scd.2010.0501. [DOI] [PubMed] [Google Scholar]
- Bielby R, Boccaccini A, Polak J, Buttery L. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Engineering. 2004;10:1518–25. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- Bilousova G, Jun D, King K, De Langhe S, Chick W, Torchia E, et al. Osteoblasts Derived from Induced Pluripotent Stem Cells Form Calcified Structures in Scaffolds Both in Vitro and in Vivo. Stem Cells. 2011;29:206–16. doi: 10.1002/stem.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birenboim R, Markus A, Goldstein RS. Simple generation of neurons from human embryonic stem cells using agarose multiwell dishes. J Neurosci Methods. 2013 doi: 10.1016/j.jneumeth.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Bishop A. Pulmonary epithelial stem cells. Cell Proliferation. 2004;37:89–96. doi: 10.1111/j.1365-2184.2004.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen A, Tang W, Fox J, et al. Small Molecules Efficiently Direct Endodermal Differentiation of Mouse and Human Embryonic Stem Cells. Cell Stem Cell. 2009;4:348–58. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–34. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- Burridge P, Thompson S, Millrod M, Weinberg S, Yuan X, Peters A, et al. A Universal System for Highly Efficient Cardiac Differentiation of Human Induced Pluripotent Stem Cells That Eliminates Interline Variability. Plos One. 2011;6 doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P, Adams DR, Abraham A, Allcock RW, Jiang Z, McCahill A, et al. Identification and characterization of small-molecule ligands that maintain pluripotency of human embryonic stem cells. Biochem Soc Trans. 2010;38:1058–61. doi: 10.1042/BST0381058. [DOI] [PubMed] [Google Scholar]
- Buttery L, Bourne S, Xynos J, Wood H, Hughes F, Hughes S, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Engineering. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Carlberg B, Axell MZ, Nannmark U, Liu J, Kuhn HG. Electrospun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells. Biomed Mater. 2009;4:045004. doi: 10.1088/1748-6041/4/4/045004. [DOI] [PubMed] [Google Scholar]
- Carpenter L, Malladi R, Yang CT, French A, Pilkington KJ, Forsey RW, et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117:4008–11. doi: 10.1182/blood-2010-08-299941. [DOI] [PubMed] [Google Scholar]
- Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–15. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- Chambers S, Fasano C, Papapetrou E, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnology. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao TI, Xiang S, Chen CS, Chin WC, Nelson AJ, Wang C, et al. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2009;384:426–30. doi: 10.1016/j.bbrc.2009.04.157. [DOI] [PubMed] [Google Scholar]
- Chao TI, Xiang S, Lipstate JF, Wang C, Lu J. Poly(methacrylic acid)-grafted carbon nanotube scaffolds enhance differentiation of hESCs into neuronal cells. Adv Mater. 2010;22:3542–7. doi: 10.1002/adma.201000262. [DOI] [PubMed] [Google Scholar]
- Chayosumrit M, Tuch B, Sidhu K. Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials. 2010;31:505–14. doi: 10.1016/j.biomaterials.2009.09.071. [DOI] [PubMed] [Google Scholar]
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Xie X, Wilson K, Sun N, Wu J, Giovangrandi L, et al. Current-Controlled Electrical Point-Source Stimulation of Embryonic Stem Cells. Cellular and Molecular Bioengineering. 2009a;2:625–35. doi: 10.1007/s12195-009-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox J, Maehr R, Osafune K, Davidow L, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nature Chemical Biology. 2009b;5:258–65. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–67. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Chung B, Lee D, Khademhosseini A, Kim J, Lee S. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010;31:4296–303. doi: 10.1016/j.biomaterials.2010.01.115. [DOI] [PubMed] [Google Scholar]
- Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of Acellular Natural Lung Matrix on Murine Embryonic Stem Cell Differentiation and Tissue Formation. Tissue Engineering Part a. 2010;16:2565–80. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- D’Amour K, Agulnick A, Eliazer S, Kelly O, Kroon E, Baetge E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnology. 2005;23:1534–41. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour K, Bang A, Eliazer S, Kelly O, Agulnick A, Smart N, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotechnology. 2006;24:1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Dabelsteen S, Hercule P, Barron P, Rice M, Dorsainville G, Rheinwald J. Epithelial cells derived from human embryonic stem cells display p16INK4A senescence, hypermotility, and differentiation properties shared by many P63+ somatic cell types. Stem Cells. 2009;27:1388–99. doi: 10.1002/stem.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J Cell Biochem. 2008;105:633–40. doi: 10.1002/jcb.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois N, Craft A, Sharma P, Elliott D, Stanley E, Elefanty A, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nature Biotechnology. 2011;29:1011–U82. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–8. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28:2706–17. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Huber T, Nostro M, Kattman S, Keller G. Germ layer induction from embryonic stem cells. Experimental Hematology. 2005;33:955–64. doi: 10.1016/j.exphem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gauthaman K, Venugopal JR, Yee FC, Peh GS, Ramakrishna S, Bongso A. Nanofibrous substrates support colony formation and maintain stemness of human embryonic stem cells. J Cell Mol Med. 2009;13:3475–84. doi: 10.1111/j.1582-4934.2009.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23:1234–41. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- Gothard D, Roberts S, Shakesheff K, Buttery L. Engineering Embryonic Stem-Cell Aggregation Allows an Enhanced Osteogenic Differentiation In Vitro. Tissue Engineering Part C-Methods. 2010;16:583–95. doi: 10.1089/ten.TEC.2009.0462. [DOI] [PubMed] [Google Scholar]
- Guenou H, Nissan X, Larcher F, Feteira J, Lemaitre G, Saidani M, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet. 2009;374:1745–53. doi: 10.1016/S0140-6736(09)61496-3. [DOI] [PubMed] [Google Scholar]
- Handschel J, Naujoks C, Depprich R, Lammers L, Kübler N, Meyer U, et al. Embryonic stem cells in scaffold-free three-dimensional cell culture: osteogenic differentiation and bone generation. Head Face Med. 2011;7:12. doi: 10.1186/1746-160X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D, Fletcher J, Payne C, Terrace J, Gallagher R, Snoeys J, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12301–6. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt KJ, Shamis Y, Carlson MW, Aberdam E, Aberdam D, Garlick JA. Three-dimensional epithelial tissues generated from human embryonic stem cells. Tissue Eng Part A. 2009;15:3417–26. doi: 10.1089/ten.tea.2009.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Weick J, Yu J, Ma L, Zhang X, Thomson J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4335–40. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295–304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D, Kim D, Kim D. Human ES and iPS cells as cell sources for the treatment of Parkinson’s disease: Current state and problems. J Cell Biochem. 2009 doi: 10.1002/jcb.22411. [DOI] [PubMed] [Google Scholar]
- Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, et al. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circulation Research. 2005;96:501–8. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- Ishii T, Fukumitsu K, Yasuchika K, Adachi K, Kawase E, Suemori H, et al. Effects of extracellular matrixes and growth factors on the hepatic differentiation of human embryonic stem cells. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;295:G313–G21. doi: 10.1152/ajpgi.00072.2008. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kiuru M, Cairo MS, Christiano AM. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2011;108:8797–802. doi: 10.1073/pnas.1100332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsykson P, Ilouz N, Turetsky T, Goldstein RS, Pera MF, Fishbein I, et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;30:24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Jing D, Parikh A, Tzanakakis E. Cardiac Cell Generation From Encapsulated Embryonic Stem Cells in Static and Scalable Culture Systems. Cell Transplantation. 2010;19:1397–412. doi: 10.3727/096368910X513955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HJ, Kim H, Park SW, Cho HJ, Kim HS, Lim DS, et al. Angiopoietin-1 promotes endothelial differentiation from embryonic stem cells and induced pluripotent stem cells. Blood. 2011;118:2094–104. doi: 10.1182/blood-2010-12-323907. [DOI] [PubMed] [Google Scholar]
- Kane NM, Xiao Q, Baker AH, Luo Z, Xu Q, Emanueli C. Pluripotent stem cell differentiation into vascular cells: a novel technology with promises for vascular re(generation) Pharmacol Ther. 2011;129:29–49. doi: 10.1016/j.pharmthera.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007a;25:419–24. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- Kang X, Xie Y, Powell H, Lee L, Belury M, Lannutti J, et al. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials. 2007b;28:450–8. doi: 10.1016/j.biomaterials.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Karner E, Unger C, Sloan A, Ahrlund-Richter L, Sugars R, Wendel M. Bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Stem Cells and Development. 2007;16:39–52. doi: 10.1089/scd.2006.0010. [DOI] [PubMed] [Google Scholar]
- Kattman S, Witty A, Gagliardi M, Dubois N, Niapour M, Hotta A, et al. Stage-Specific Optimization of Activin/Nodal and BMP Signaling Promotes Cardiac Differentiation of Mouse and Human Pluripotent Stem Cell Lines. Cell Stem Cell. 2011;8:228–40. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kawaguchi J, Mee P, Smith A. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–69. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kawamorita M, Suzuki C, Saito G, Sato T, Sato K. In vitro differentiation of mouse embryonic stem cells after activation by retinoic acid. Hum Cell. 2002;15:178–82. doi: 10.1111/j.1749-0774.2002.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. Journal of Clinical Investigation. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–87. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademhosseini A, Ferreira L, Blumling J, Yeh J, Karp JM, Fukuda J, et al. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006a;27:5968–77. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006b;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Majdi M, Xia P, Wei K, Talantova M, Spiering S, et al. Non-Cardiomyocytes Influence the Electrophysiological Maturation of Human Embryonic Stem Cell-Derived Cardiomyocytes During Differentiation. Stem Cells and Development. 2010;19:783–95. doi: 10.1089/scd.2009.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee G, Ganat Y, Papapetrou E, Lipchina I, Socci N, et al. miR-371-3 Expression Predicts Neural Differentiation Propensity in Human Pluripotent Stem Cells. Cell Stem Cell. 2011a;8:695–706. doi: 10.1016/j.stem.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Kim J, Oh A, Choi Y, Ku S, Kim Y, Lee N, et al. Isoflurane decreases death of human embryonic stem cell-derived, transcriptional marker Nkx2.5(+) cardiac progenitor cells. Acta Anaesthesiologica Scandinavica. 2011b;55:1124–31. doi: 10.1111/j.1399-6576.2011.02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7:989–94. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J, Hegert C, Guan K, Wobus A, Muller P, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mechanisms of Development. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson L, Kadoya K, Bang A, Kelly O, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnology. 2008;26:443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Laflamme M, Chen K, Naumova A, Muskheli V, Fugate J, Dupras S, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Lee J, Tuleuova N, Jones C, Ramanculov E, Zern M, Revzin A. Directing hepatic differentiation of embryonic stem cells with protein microarray-based co-cultures. Integrative Biology. 2009a;1:460–8. doi: 10.1039/b905757a. [DOI] [PubMed] [Google Scholar]
- Lee L, Peerani R, Ungrin M, Joshi C, Kumacheva E, Zandstra P. Micropatterning of human embryonic stem cells dissects the mesoderm and endoderm lineages. Stem Cell Research. 2009b;2:155–62. doi: 10.1016/j.scr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, et al. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–6. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Ferreira LS, Chen-Konak L, Kraehenbuehl TP, Langer R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat Protoc. 2010;5:1115–26. doi: 10.1038/nprot.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–74. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–21. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Lian Q, Zhang Y, Zhang J, Zhang H, Wu X, Zhang Y, et al. Functional Mesenchymal Stem Cells Derived From Human Induced Pluripotent Stem Cells Attenuate Limb Ischemia in Mice. Circulation. 2010;121:1113–U91. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine L, Azarin S, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1848–E57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protocols. 2013;8:162–75. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang A, Rippon H, Bismarck A, Bishop A. Tissue Engineering of Lung: The Effect of Extracellular Matrix on the Differentiation of Embryonic Stem Cells to Pneumocytes. Tissue Engineering Part a. 2010;16:1515–26. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
- Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–91. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Kaufman D, Shen W. A synthetic substrate to support early mesodermal differentiation of human embryonic stem cells. Biomaterials. 2011;32:8058–66. doi: 10.1016/j.biomaterials.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones Heart Disease and Stroke Statistics-2010 Update: A Report From the American Heart Association (vol 121, pg e46, 2010) Circulation. 2010;121:E260-E. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lock L, Tzanakakis E. Expansion and Differentiation of Human Embryonic Stem Cells to Endoderm Progeny in a Microcarrier Stirred-Suspension Culture. Tissue Engineering Part a. 2009;15:2051–63. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Levenstein M, Jones J, Berggren W, Mitchen E, Frane J, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006a;24:185–7. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006b;3:637–46. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Maye P, Becker S, Siemen H, Thorne J, Byrd N, Carpentino J, et al. Hedgehog signaling is required for the differentiation of ES cells into neurectoderm. Dev Biol. 2004;265:276–90. doi: 10.1016/j.ydbio.2003.09.027. [DOI] [PubMed] [Google Scholar]
- McLean A, D’Amour K, Jones K, Krishnamoorthy M, Kulik M, Reynolds D, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- Metallo C, Azarin S, Moses L, Ji L, de Pablo J, Palecek S. Human embryonic stem cell-derived keratinocytes exhibit an epidermal transcription program and undergo epithelial morphogenesis in engineered tissue constructs. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo C, Ji L, de Pablo J, Palecek S. Directed differentiation of human embryonic stem cells to epidermal progenitors. Methods Mol Biol. 2010;585:83–92. doi: 10.1007/978-1-60761-380-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Ji L, de Pablo JJ, Palecek SP. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells. 2008;26:372–80. doi: 10.1634/stemcells.2007-0501. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Mohr JC, Detzel CJ, de Pablo JJ, Van Wie BJ, Palecek SP. Engineering the stem cell microenvironment. Biotechnol Prog. 2007;23:18–23. doi: 10.1021/bp060350a. [DOI] [PubMed] [Google Scholar]
- Miki T, Ring A, Gerlach J. Hepatic Differentiation of Human Embryonic Stem Cells Is Promoted by Three-Dimensional Dynamic Perfusion Culture Conditions. Tissue Engineering Part C-Methods. 2011;17:557–68. doi: 10.1089/ten.TEC.2010.0437. [DOI] [PubMed] [Google Scholar]
- Mohr J, Zhang J, Azarin S, Soerens A, de Pablo J, Thomson J, et al. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–93. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–42. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89:117–26. doi: 10.1002/jnr.22547. [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes - Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lee S, Reddi A. Induction of Chondrogenesis From Human Embryonic Stem Cells Without Embryoid Body Formation by Bone Morphogenetic Protein 7 and Transforming Growth Factor beta 1. Arthritis and Rheumatism. 2009;60:3686–92. doi: 10.1002/art.27229. [DOI] [PubMed] [Google Scholar]
- Niebruegge S, Bauwens C, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, et al. Generation of Human Embryonic Stem Cell-Derived Mesoderm and Cardiac Cells Using Size-Specified Aggregates in an Oxygen-Controlled Bioreactor. Biotechnology and Bioengineering. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- Niwa A, Heike T, Umeda K, Oshima K, Kato I, Sakai H, et al. A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors. PLoS One. 2011;6:e22261. doi: 10.1371/journal.pone.0022261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noghero A, Bussolino F, Gualandris A. Role of the microenvironment in the specification of endothelial progenitors derived from embryonic stem cells. Microvasc Res. 2010;79:178–83. doi: 10.1016/j.mvr.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol. 2010;30:80–9. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tokumoto Y, Miyake J, Nagamune T. Induction of oligodendrocyte differentiation from adult human fibroblast-derived induced pluripotent stem cells. In Vitro Cell Dev Biol Anim. 2011;47:464–9. doi: 10.1007/s11626-011-9435-2. [DOI] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez R, Fitz-Gerald C, Sato Y, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nature Biotechnology. 2008;26:313–5. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–20. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–55. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–80. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–14. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- Preynat-Seauve O, Suter DM, Tirefort D, Turchi L, Virolle T, Chneiweiss H, et al. Development of human nervous tissue upon differentiation of embryonic stem cells in three-dimensional culture. Stem Cells. 2009;27:509–20. doi: 10.1634/stemcells.2008-0600. [DOI] [PubMed] [Google Scholar]
- Rambhatla L, Chiu C, Kundu P, Peng Y, Carpenter M. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplantation. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- Rippon H, Ali N, Polak J, Bishop A. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning and Stem Cells. 2004;6:49–56. doi: 10.1089/1536230041372328. [DOI] [PubMed] [Google Scholar]
- Ritner C, Wong S, King F, Mihardja S, Liszewski W, Erle D, et al. An Engineered Cardiac Reporter Cell Line Identifies Human Embryonic Stem Cell-Derived Myocardial Precursors. Plos One. 2011;6 doi: 10.1371/journal.pone.0016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–5. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- Roobrouck V, Ulloa-Montoya F, Verfaillie C. Self-renewal and differentiation capacity of young and aged stem cells. Experimental Cell Research. 2008;314:1937–44. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Roszell B, Mondrinos M, Seaton A, Simons D, Koutzaki S, Fong G, et al. Efficient Derivation of Alveolar Type II Cells from Embryonic Stem Cells for In Vivo Application. Tissue Engineering Part a. 2009;15:3351–65. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagha M, Karbalaie K, Tanhaee S, Esfandiari E, Salehi H, Sadeghi-Aliabadi H, et al. Neural induction in mouse embryonic stem cells by co-culturing with chicken somites. Stem Cells Dev. 2009;18:1351–60. doi: 10.1089/scd.2008.0341. [DOI] [PubMed] [Google Scholar]
- Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–37. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–33. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon H, Polak J, Bielby R, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Engineering. 2006;12:867–75. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- Sargent C, Berguig G, McDevitt T. Cardiomyogenic Differentiation of Embryoid Bodies Is Promoted by Rotary Orbital Suspension Culture. Tissue Engineering Part a. 2009;15:331–42. doi: 10.1089/ten.tea.2008.0145. [DOI] [PubMed] [Google Scholar]
- Sasaki D, Shimizu T, Masuda S, Kobayashi J, Itoga K, Tsuda Y, et al. Mass preparation of size-controlled mouse embryonic stem cell aggregates and induction of cardiac differentiation by cell patterning method. Biomaterials. 2009;30:4384–9. doi: 10.1016/j.biomaterials.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Sauer H, Rahimi G, Hescheler J, Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. Journal of Cellular Biochemistry. 1999;75:710–23. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Nsair A, Van Handel B, Angelis E, Gluck J, Votteler M, et al. Recapitulation of the embryonic cardiovascular progenitor cell niche. Biomaterials. 2011;32:2748–56. doi: 10.1016/j.biomaterials.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, et al. Electrical stimulation of human embryonic stem cells: Cardiac differentiation and the generation of reactive oxygen species. Experimental Cell Research. 2009;315:3611–9. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty Y, Cohen I, Dor Y, Harel D. Four-dimensional realistic modeling of pancreatic organogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20374–9. doi: 10.1073/pnas.0808725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, et al. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006;24:125–38. doi: 10.1634/stemcells.2004-0150. [DOI] [PubMed] [Google Scholar]
- Smith L, Liu X, Hu J, Ma P. The Enhancement of human embryonic stem cell osteogenic differentiation with nano-fibrous scaffolding. Biomaterials. 2010;31:5526–35. doi: 10.1016/j.biomaterials.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soonpaa M, Field L. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circulation Research. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- Suter DM, Preynat-Seauve O, Tirefort D, Feki A, Krause KH. Phenazopyridine induces and synchronizes neuronal differentiation of embryonic stem cells. J Cell Mol Med. 2009;13:3517–27. doi: 10.1111/j.1582-4934.2009.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takei S, Ichikawa H, Johkura K, Mogi A, No H, Yoshie S, et al. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. American Journal of Physiology-Heart and Circulatory Physiology. 2009;296:H1793–H803. doi: 10.1152/ajpheart.01288.2008. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Murphy C, Murphy C, Kimura M, Kawai S, Polak J. Chondrogenic differentiation of murine embryonic stem cells: Effects of culture conditions and dexamethasone. Journal of Cellular Biochemistry. 2004;93:454–62. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Suzuki Y, Sumi T, Sone M, Suemori H, Nakatsuji N. Simple and highly efficient method for production of endothelial cells from human embryonic stem cells. Cell Transplant. 2011;20:1423–30. doi: 10.3727/096368910X547444. [DOI] [PubMed] [Google Scholar]
- Taura D, Noguchi M, Sone M, Hosoda K, Mori E, Okada Y, et al. Adipogenic differentiation of human induced pluripotent stem cells: Comparison with that of human embryonic stem cells. Febs Letters. 2009;583:1029–33. doi: 10.1016/j.febslet.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998a;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998b;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tuleuova N, Lee J, Lee J, Ramanculov E, Zern M, Revzin A. Using growth factor arrays and micropatterned co-cultures to induce hepatic differentiation of embryonic stem cells. Biomaterials. 2010;31:9221–31. doi: 10.1016/j.biomaterials.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik M, Yu J, Zhang X, Tian S, Stewart R, Thomson J, et al. A Mesoderm-Derived Precursor for Mesenchymal Stem and Endothelial Cells. Cell Stem Cell. 2010;7:718–29. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Tang Z, Park IH, Zhu Y, Patel S, Daley GQ, et al. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011a;32:5023–32. doi: 10.1016/j.biomaterials.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Tang X, Sun X, Miao Z, Lv Y, Yang Y, et al. TGFβ inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Res. 2011b doi: 10.1038/cr.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–9. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye K. Three-Dimensional Differentiation of Embryonic Stem Cells into Islet-like Insulin-Producing Clusters. Tissue Engineering Part a. 2009;15:1941–52. doi: 10.1089/ten.tea.2008.0181. [DOI] [PubMed] [Google Scholar]
- Wei J, Shi Y, Zhao D, Chen S, Yong J, Zhang J, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Research. 2007;17:333–44. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- Xia C, Wang C, Zhang K, Qian C, Jing N. Induction of a high population of neural stem cells with anterior neuroectoderm characters from epiblast-like P19 embryonic carcinoma cells. Differentiation. 2007;75:912–27. doi: 10.1111/j.1432-0436.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Research. 2009;19:156–72. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Browning V, Odorico J. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mechanisms of Development. 2011;128:412–27. doi: 10.1016/j.mod.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Mei Y, Langer R, Anderson D. High Throughput Optimization of Stem Cell Microenvironments. Combinatorial Chemistry & High Throughput Screening. 2009;12:554–61. doi: 10.2174/138620709788681916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu G, Wei R, Hou W, Gao M, Zhu M, et al. Ghrelin promotes differentiation of human embryonic stem cells into cardiomyocytes. Acta Pharmacologica Sinica. 2011;32:1239–45. doi: 10.1038/aps.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Soonpaa M, Adler E, Roepke T, Kattman S, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR plus embryonic-stem-cell-derived population. Nature. 2008;453:524–U6. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik M, Smuga-Otto K, Antosiewicz-Bourget J, Frane J, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–14. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–70. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, et al. Extracellular Matrix Promotes Highly Efficient Cardiac Differentiation of Human Pluripotent Stem Cells: The Matrix Sandwich Method. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wilson G, Soerens A, Koonce C, Yu J, Palecek S, et al. Functional Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells. Circulation Research. 2009;104:E30–E41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006;16:132–42. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]