Abstract
The ubiquitin-proteasome system (UPS) plays a central role in maintaining protein homeostasis, emphasized by a myriad of diseases that are associated with altered UPS function such as cancer, muscle-wasting, and neurodegeneration. Protein ubiquitination plays a central role in both the promotion of proteasomal degradation as well as cellular signaling through regulation of the stability of transcription factors and other signaling molecules. Substrate specificity is a critical regulatory step of ubiquitination and is mediated by ubiquitin ligases. Recent studies implicate ubiquitin ligases in multiple models of cardiac diseases such as cardiac hypertrophy, atrophy, and ischemia/reperfusion injury, both in a cardioprotective and maladaptive role. Therefore, identifying physiological substrates of cardiac ubiquitin ligases provides both mechanistic insights into heart disease as well as possible therapeutic targets. Current methods identifying substrates for ubiquitin ligases rely heavily upon non-physiologic in vitro methods, impeding the unbiased discovery of physiological substrates in relevant model systems. Here we describe a novel method for identifying ubiquitin ligase substrates utilizing Tandem Ubiquitin Binding Entities (TUBE) technology, two-dimensional differential in gel electrophoresis (2-D DIGE), and mass spectrometry, validated by the identification of both known and novel physiological substrates of the ubiquitin ligase MuRF1 in primary cardiomyocytes. This method can be applied to any ubiquitin ligase, both in normal and disease model systems, in order to identify relevant physiological substrates under various biological conditions, opening the door to a clearer mechanistic understanding of ubiquitin ligase function and broadening their potential as therapeutic targets.
Keywords: ubiquitin, ubiquitin-proteasome system, E3, ubiquitin ligase, substrate screen, proteomics, tandem ubiquitin binding entities
Introduction
Protein ubiquitination and the ubiquitin-proteasome system (UPS) play a critical role in the regulation of protein homeostasis, emphasized by a myriad of diseases associated with aberrant UPS function. Ubiquitin is added to a substrate protein through a covalent linkage to a lysine residue on a substrate protein catalyzed by a class of enzymes called ubiquitin ligases; as an additional layer of regulation, this mechanism is counter-regulated by a class of enzymes called de-ubiquitination enzymes (DUBs) (1,2). The effect of protein ubiquitination depends upon which lysine residue within the protein the ubiquitin is attached to as well as the length and linkage type of the added ubiquitin. The UPS is most commonly thought of in terms of regulating the turnover of mis-folded and damaged proteins by the addition of canonical K48 polyubiquitin chains and subsequent proteasomal degradation. However, non-canonical linkages, for example K63 polyubiquitin chains, or single ubiquitin molecules (monoubiquitination) can also occur and mediates non-proteolytic mechanisms such as modulating protein localization, protein-protein interactions, activity or stability (3–5). The essential nature of protein ubiquitination is well illustrated in the heart where dysfunction, of both proteolytic and non-proteolytic mechanisms, has been associated with multiple disorders, including cardiac hypertrophy, heart failure, diabetes and ischemia-reperfusion injury (5).
Substrate specificity of the ubiquitination reaction occurs at the level of the ubiquitin ligase; as such, ubiquitin ligases are attractive therapeutic targets for diseases involving aberrant protein ubiquitination (4,6). Muscle-specific RING finger protein 1 (MuRF1, Trim63) is a striated muscle-specific ubiquitin ligase involved in protein quality control of the muscle sarcomere by targeting numerous proteins for polyubiquitin-dependent proteasomal degradation, including troponin I, muscle actin, β/slow myosin heavy chain and myosin binding protein-C (7–10). Ubiquitin ligases, including MuRF family proteins, function as distinct molecular regulators by which the heart controls not only sarcomeric structure, but also cellular signaling pathways implicated in multiple models of cardiac disease, both in maladaptive and cardioprotective roles (5,9,11–16). Targeting ubiquitin ligases in the heart may allow for more precise, single therapy manipulation of a smaller, specific subset of substrate proteins that contribute to disease-causing mechanisms while avoiding the negative cardiovascular effects observed with global proteasome inhibition (17,18). Yet the substrates targeted by MuRF1 and their DUB counterparts remain incompletely understood and more robust identification methods for identifying ubiquitin ligase substrates is required for development of successful therapies (19).
Traditional ubiquitin ligase substrate discovery utilizes protein-protein interaction based methods, such as yeast-two hybrid and co-immunoprecipitation paired with mass spectrometry. Although refined recently by several modifications to increase efficiency in substrate identification (9,20,21), interaction-based methods are hindered by the transient, weak nature of the ubiquitin ligase-substrate interaction. To circumvent the limitations of interaction-based methods, high-throughput in vitro approaches, such as in vitro ubiquitination biochemistry coupled with protein microarrays, have proven to be successful at identifying ubiquitin ligase substrates (22,23). The use of in vitro-based methods is limited, however, to the content printed on protein arrays, limiting the substrate candidate pool. Importantly, neither yeast-two hybrids nor in vitro methods for ubiquitin ligase substrate identification are performed in physiologically relevant conditions, thereby limiting and biasing substrate discovery (16,24).
Given the limitations of existing ubiquitin ligase substrate screening methods, our aim was to develop a methodology to better identify candidate ubiquitin ligase substrates under physiological conditions. Tandem Ubiquitin Binding Entities (TUBE) technology allows unbiased ubiquitome isolation through high affinity binding to polyubiquitinated proteins. TUBE also protects polyubiquitiated proteins from de-ubiquitination and degradation during processing, allowing for detection of even low abundant species (25). Furthermore, the use of different TUBE types that have higher affinities for specific polyubiquitin lysine linkages can be used to enrich for subsets of the ubiquitome, providing another potential level of specificity to the screen. We used a subtractive approach combining TUBE technology, 2-D DIGE, and mass spectrometry, to develop a method for ubiquitin ligase substrate identification that is translatable to physiologically relevant inputs, either from cells or tissues. We describe and validate this method demonstrated by the identification of both previously identified as well as novel physiological substrates of the ubiquitin ligase MuRF1 in primary cardiomyocytes.
Methods
Neonatal Rat Ventricular Myocyte Isolation and Culture
Neonatal rat ventricular myocytes (NRVM) were isolated from day old Sprague–Dawley pups utilizing the Worthington Neonatal Cardiomyocyte Isolation System as previously described and according to manufacturer’s instructions (26). Briefly, isolated neonatal hearts were sequentially digested at 4 °C overnight with trypsin and then at 37 °C for 2 h with collagenase type II. After preplating to minimize nonmyocyte contamination, cells were plated on tissue culture dishes precoated with laminin (Sigma). NRVM were cultured at 37 °C and 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen) with 10% horse serum, 5% fetal bovine serum and 100 µM 5-bromo-2'-deoxyuridine (BRDU) to inhibit non-myocyte growth for the first 24 h post isolation and subsequently in a 4:1 ratio of DMEM:Minimum Essential Medium with 100 µM BRDU. Freshly isolated NRVM were cultured for 72 h prior to adenoviral infection.
Adenoviral Constructs
Full-length mouse MuRF1 was cloned into the Myc-pCMV vector. Adenovirus plasmids Ad-GFP and Ad-MuRF1 (the later expressing GFP and Myc-tagged MuRF1 bicistronically) were constructed in pADTrack-CMV and used as previously described (9).
Adenoviral Infection and TUBE Enrichment for Ubiquitinated Proteins
Six independent 15 cm plates of cultured NRVM were transduced with Ad-GFP or Ad-MuRF1 at a multiplicity of infection (MOI) of 10 for 18 h. Lysates were prepared by scraping, trituration and brief sonication of cells in cell lysis buffer containing 50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 1 mM EDTA, 1% NP-40, 10% glycerol, 50 µM deubiquitinating enzyme inhibitor PR619 (LifeSensors) and 1X HALT protease/phosphatase inhibitor (Pierce) followed by clarification by centrifugation at 15,000 × g. Lysates from all plates transduced with Ad-GFP or Ad-MuRF1 were pooled and total protein concentration determined by BCA protein assay (Pierce). Lysate containing 1 mg of total protein was incubated for 18 h at 4 °C with 60 µl of prewashed Agarose TUBE 2 beads (LifeSensors) or Control Agarose beads (LifeSensors). Unbound supernatant was removed and frozen at −80 °C. Beads were thoroughly washed in 20 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 0.1% Tween-20 (TBST) and ubiquitinated proteins eluted in 0.2 M glycine, pH 2.5 and neutralized with 1 M Tris pH9.0. Elutions were stored at −80 °C prior to 2D-DIGE and proteomic analysis. Alternatively, ubiquitinated proteins were eluted by resuspending the beads in 20 µl SDS reducing sample buffer and boiling for 5 m followed by centrifugation at 13000 × g for 5 m. Beads were discarded and eluted samples analyzed by SDS-PAGE and immunoblotting for Hspd1 (Abcam, ab59457) or total ubiquitin (Lifesensors, VU-1).
Two-Dimensional Differential In Gel Electrophoresis (2D-DIGE)
To detect differential protein expression, samples were first cleaned by methanol/chloroform precipitation and dissolved in lysis buffer (8 M urea, 20 mM tris-HCl, pH 8.5, 4% CHAPS). Aliquots of the MuRF1 TUBE eluate, MuRF1 TUBE supernatant, MuRF1 agarose control supernatant, GFP TUBE eluate, GFP TUBE supernatant, and GFP agarose control supernatant were labeled with either Cy3 or Cy5 fluorescent dyes. An internal control (IC) was prepared by pooling equal amounts of protein (15 µg) from all samples, and then labeled with 200 pmol of Cy2 for every 15 µg of protein. The labeling reaction was carried out on ice for 30 min, protected from light. To quench the reaction, 1 µL of 10 mM lysine was added, and the reaction was then incubated for an additional 10 m on ice in the dark. After labeling, corresponding samples were combined. An equal volume of 2X sample buffer (8 M urea, 4% CHAPS, 20 mg/mL DTT, 2% (v/v) IPG buffer 4–7 (GE Healthcare)) was added and the mixture was placed on ice for 15 m. Rehydration buffer (8 M urea, 4% CHAPS, 2 mg/mL DTT, 1% (v/v) IPG buffer 4–7) was added to a final volume of 250 µL. For each gel IC, Cy3, and Cy5 labeled samples were mixed before applying to immobilized pH gradient (IPG) strips (24 cm, pI range 4–7, GE Healthcare). Isoelectric focusing and the subsequent SDS-PAGE (second dimension) were performed as previously described (27). Three independent gels were run: the first comparing MuRF1 TUBE eluate to GFP TUBE eluate, the second comparing MuRF1 TUBE supernatant to MuRF1 agarose control supernatant and the third comparing GFP TUBE supernatant to GFP agarose control supernatant. Gels were analyzed using DeCyder 7.0 software (GE Healthcare). A “spot number” of 4500 was used to generate spot maps using the differential in-gel analysis (DIA) component. Spot maps were filtered via the built-in algorithm using a Max slope of 1.0, and manually edited to remove dust particle signals. Expression changes were assessed using the Volume Ratio between samples within each gel.
Spot selection and MALDI-TOF
Protein spots displaying greater than 1.5 fold expression changes were marked as “picks” and the list of “picks” from all three gel comparisons were aligned to determine spots that were identified as “picks” by all three comparisons. Spots that were identified as “picks” across all multiple gels were selected for further analysis and removed from the 2D gels using an Ettan Spot Picker (GE Healthcare) and submitted to the Michael Hooker Proteomics Center (University of North Carolina) for protein identification by matrixassisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry. With the aid of a Progest Protein Digestion System (Digilab), proteins were digested with trypsin, and the resulting peptides were extracted. Peptides were mixed with matrix (α-Cyano-4-Hydroxycinnamic Acid) and analyzed using a MALDI-TOF/TOF mass spectrometer (Applied Biosystems 4800 Plus). MS spectra were obtained in reflector positive ion mode and peaks with signal-to-noise ratio above 10 were selected for MS/MS analysis (maximum of 45 MS/MS spectra per spot). All spectra were searched using GPS Explorer, Version 3.6 (AB Sciex) linked to the Mascot (Matrix Science, Inc.) search engine and compared to the IPI rat database downloaded from European Bioinformatics Institute.
Immunofluorescence
24 h post infection with Ad-GFP or Ad-MuRF1, cells were washed with PBS, fixed, and permeabilized in phosphate-buffered 2% paraformaldehyde/0.2% Triton X-100 for 30 minutes at 4 °C. Immunofluorescence labeling was carried out with a mouse anti-GFP (Sigma, G6795) followed by a FITC-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories Inc.) and DAPI (Sigma-Aldrich). Images were acquired by fluorescence-inverted microscopy.
Western blotting
24 h post infection with Ad-GFP or Ad-MuRF1, cells were washed with PBS and lysates prepared by scraping, trituration and brief sonication of cells in cell lysis buffer containing 50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 1 mM EDTA, 1% NP-40, 10% glycerol, 50 µM deubiquitinating enzyme inhibitor PR619 (LifeSensors), and 1X HALT protease/phosphatase inhibitor (Pierce) followed by clarification by centrifugation at 15,000 × g. Proteins were separated by SDS-PAGE, transferred to PVDF and detected by Western blot analysis with anti-myc (Sigma, C3956), anti-MuRF1 (R&D Systems, AF5366), or anti-GAPDH (Sigma, G8795).
In Vitro Ubiquitination Reactions
In vitro ubiquitination reactions were performed as previously described (28). In brief, 2 µM human MuRF1 (LifeSensors), 0.25 µM Ube1 (Boston Biochem), 2.5 µM UbcH5c and 10 mg/ml ubiquitin and 500 ng of recombinant Hspd1 (Enzo Life Sciences) or ATP5b (Abnova) were incubated in 20 µM HEPES, pH 7.4, 10 µM KCl, 5mM ATP, 5mM MgCl2, and 1X Energy Regeneration Solution (ERS, Boston Biochem) for 3 h at 30 °C. Samples were analyzed by SDS/PAGE and immunoblotting for ATP5b (Aviva Systems Biology, ARP48186) or Hspd1 (Abcam, ab59457).
Animals
The MuRF1 transgenic (MuRF1-Tg) mice used in this study were previously described (29). All animal protocols were reviewed and approved by the University of North Carolina Institutional Animal Care Advisory Committee and were in compliance with the rules governing animal use as published by the National Institutes of Health.
Immunoprecipitation from MuRF1 Transgenic Hearts
Whole heart ventricles were excised from anesthetized male wild-type and MuRF1-Tg mice and homogenized on ice in T-Per tissue protein extraction reagent (Pierce) containing 1X HALT protease/phosphatase inhibitor (Pierce), 50uM PR619 (Lifesensors), 50uM MG132 (Millipore) and 10mM N-ethylmaleimide (Sigma) first in a 2 ml glass tissue grinder homogenizer followed by brief homogenization by handheld tissue homogenizer. Homogenates were clarified by centrifugation at 10,000 × g. Protein G Dynabeads (Invitrogen) pre-conjugated to 2 µg of Hspd1 (Abcam, ab59457) or Tpm1 (Abcam, ab133292) antibody or appropriate IgG control were incubated for 1 h at 4 °C with clarified homogenates. Beads were washed five times with Tris-Buffered Saline with 0.02% Tween-20 and subsequently, proteins were eluted in SDS-sample buffer and analyzed by SDS-PAGE and western blotting.
Results
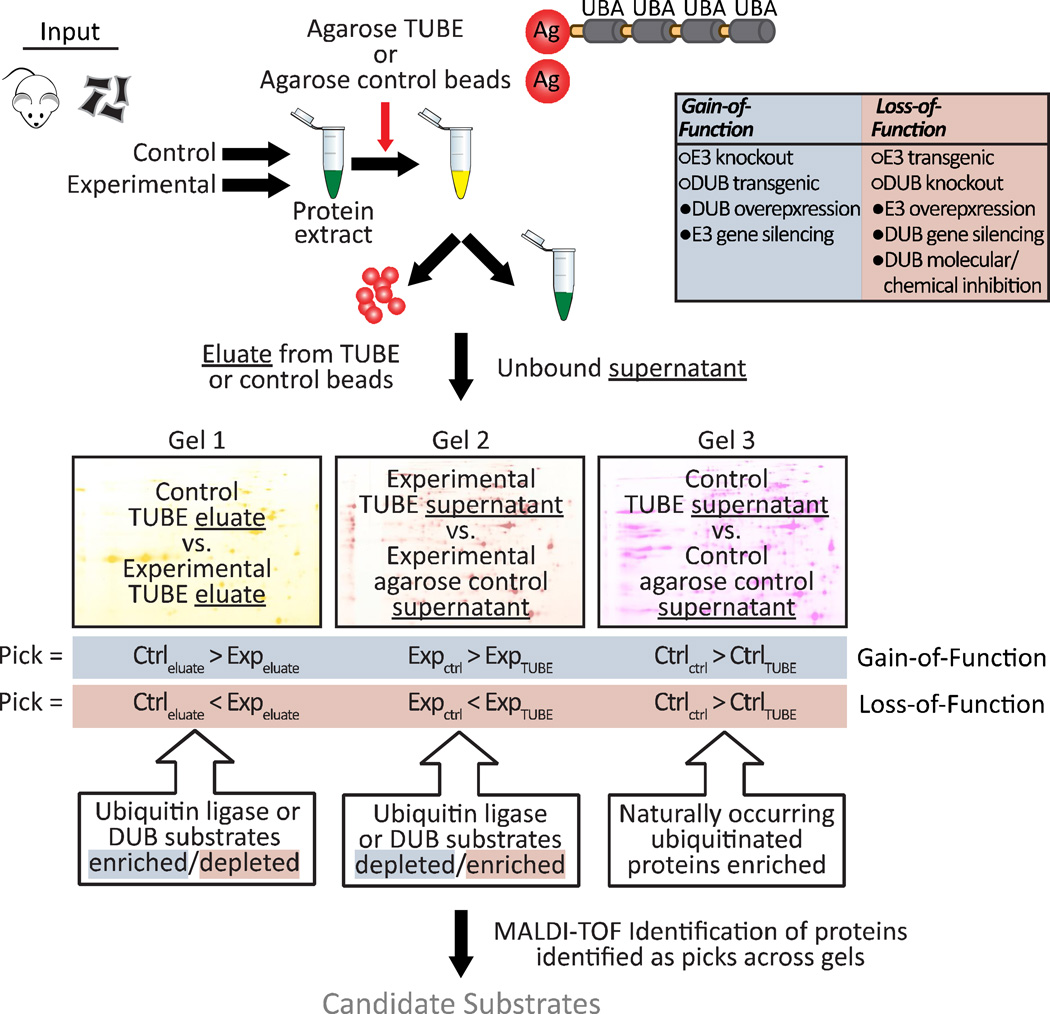
Outline of the methodology used to screen for ubiquitin ligase substrates
Identification of substrates for ubiquitin ligases have traditionally relied on either artificial systems, such as yeast two-hybrid screens, or inefficient candidate substrate screens. Our goal for a more robust and flexible substrate screen included the use of biologically relevant cell systems combined with a proteomic approach for identification. A key component of this method is the selection, as well as the lack of selection, of polyubiquitinated proteins immunoprecipitated by Tandem Ubiquitin Binding Entities (TUBE) conjugated to agarose beads. This allows us to compare the relative ratio of proteins that bind to TUBE (eluate) as well as compare proteins that did not bind (supernatant) across multiple conditions. We have provided a graphical overview of the method (Fig. 1) and discuss below the major components of this approach.
Figure 1. Schematic model representing the ubiquitin ligase/deubiquitinating enzyme screening platform.
Protein is isolated from control and experimental animal tissue or cell culture samples where the expression or activity of a ubiquitin ligase or deubiquitinating enzyme of interest is manipulated to increase or decrease, dubbed a gain-of-function or loss-of-function manipulation. Isolated protein is then quantitated and incubated overnight at 4 °C with Tandem Ubiquitin Binding Entities (TUBE) or agarose control beads. Both the bound (eluate) and unbound (supernatant) fractions are collected and subjected to 2D-DIGE. Three different 2D-DIGE gels are run, each also including a pooled internal standard sample. Gel 1 compares the control sample ubiquitin enrichment to the experimental sample ubiquitin enrichment, identifying proteins whose ubiquitination is dependent upon the ubiquitin ligase of interest. The second and third gels allow comparisons of the TUBE-selected ubiquitomes within the experimental condition (Gel 2) or control condition (Gel 3) by comparing the ubiquitin-depleted supernatants from the sample incubated with TUBE to the ubiquitin-rich sample incubated with agarose control beads. The comparison on Gel 2 identifies proteins whose ubiquitination is potentially dependent upon the ubiquitin ligase of interest. The Gel 3 comparison reveals naturally occurring ubiquitinated proteins, as here, the ubiquitin ligase of interest is unperturbed. Spots are identified as “picks” by DeCyder Analysis Software based upon the determination of relative changes in intensity between the two samples and picks are aligned across all three gel comparisons to select spots for subsequent MS/MS peptide sequencing and protein identification.
1) Protein source
Our method relies on a subtractive approach (i.e. comparing one state to another); therefore the starting material must include two conditions wherein the expression or activity of the ubiquitin ligase is manipulated. We classify systems where ubiquitin ligase activity is either increased or decreased as gain-of-function or loss-of-function, respectively (see Fig. 1 for examples). The starting material can range from protein isolated from animal tissues, primary cells, or stable cell culture models in which either the ubiquitin ligase or a counter-regulatory de-ubiquitination enzyme is manipulated.
2) Ubiquitome isolation
Protein extracts are isolated and quantified from each condition and an equal amount of protein is subsequently added to either agarose control beads or beads conjugated with TUBE. We used TUBE that bind equally to K48- and K63-ubiquitin linkages, however, there are other TUBE varieties that preferentially bind certain lysine linkages that can be used depending on the type of substrates desired (e.g. canonical versus non-canonical). From each condition we collect both the eluate from the TUBE, containing the selected ubiquitome, as well as the unbound supernatants from both the control agarose and TUBE, which provide an additional measure of the ubiquitome from each condition.
3) 2D-DIGE and pick selection
In order to reduce the number of false positives, we established a three spot comparison to identify substrate picks. Within each 2D-DIGE gel, relative differences between the two conditions are quantified. We established three independent comparisons that are analyzed in parallel to increase the likelihood of positive substrate identification. The first comparison of the pick selection contrasts the eluted ubiquitome from the experimental and control condition. In the case of gain-of-function studies, a spot pick would be increased in the experimental condition compared to the control condition (converse for loss-of-function models). The second and third gels allow comparisons of the TUBE-selected ubiquitomes within the experimental condition (Gel 2) or control condition (Gel 3). In these later comparisons, enriched ubiquitinated proteins are identified by comparing the unbound fraction from the control agarose beads (ubiquitin-enriched) versus the unbound fraction from the TUBE agarose (ubiquitin-depleted). Naturally occurring ubiquitinated proteins in the control condition are identified in Gel 3 whereas Gel 2 identifies the enriched (or depleted in the case of loss-of-function) pool of proteins in the experimental conditions.
4) Spot pick identification
All of the spots that meet the pick criteria for each Gel are tabulated and subsequently used to identify picks that are common to all multiple comparisons (see Figure 1 for the differences in pick criteria in gain-of-function versus loss-of-function models). These picks are then subjected to MALDI-TOF for protein identification and classified as potential ubiquitin ligase substrates.
MuRF1 expression in primary cardiomyocytes
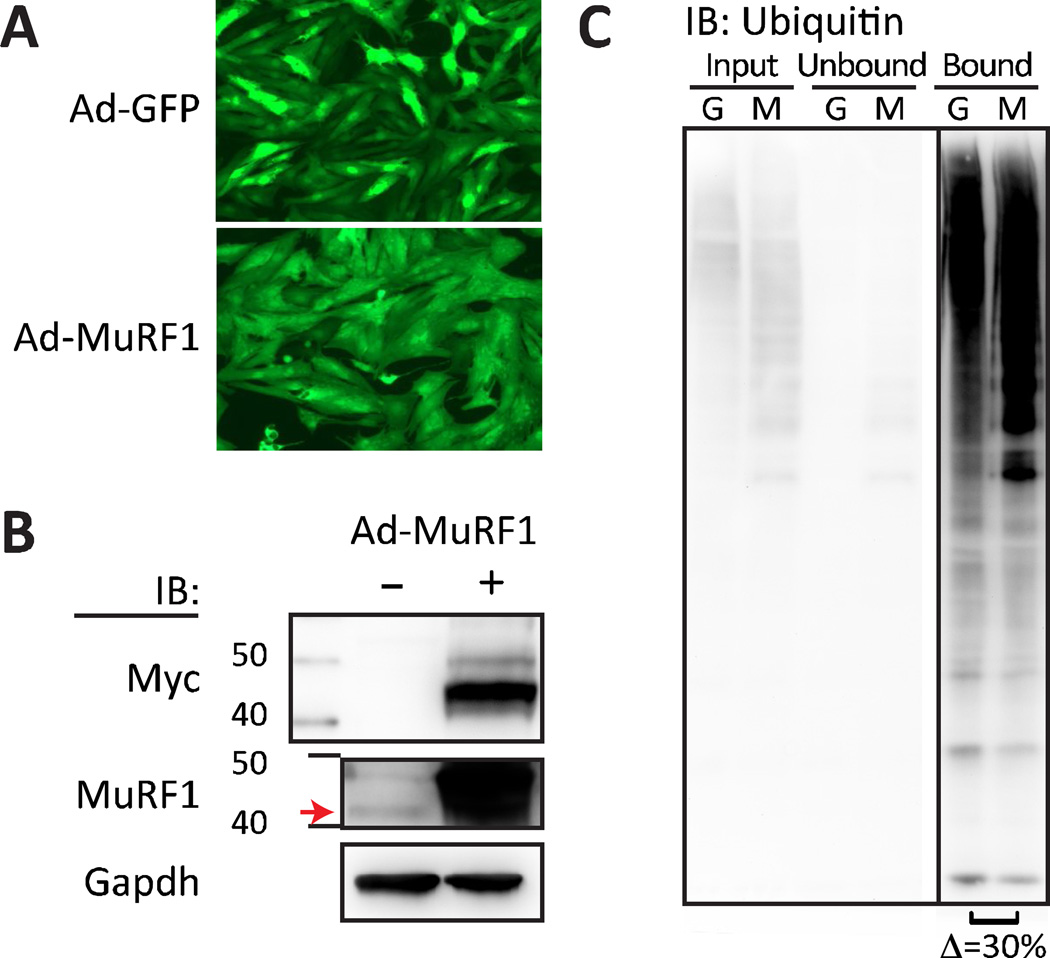
Using traditional substrate identification approaches, our laboratory as well as and other groups identified substrates for MuRF1, a striated muscle-specific ubiquitin ligase. As a proof of principle for our method, we screened for MuRF1 substrates in primary rat cardiomyocytes and hypothesized we would identify both previously established substrates as well as potential novel substrates. We utilized a transient gain-of-function model in primary cardiomyocytes as previously described through the use of adenoviral-mediated expression of either the reporter green fluorescent protein (GFP) alone or in combination with MuRF1 (mouse) at a multiplicity of infection of 10 MOI per cell (Fig. 2A). Confirmation of the expression of the MuRF1 transgene was performed via immunoblot detection of the myc epitope tag (Fig. 2B, top) that corresponded to an approximate 25-fold increase in MuRF1 transgene expression relative to the endogenous MuRF1 expression in control cells, measured by densitometry, after 18 h of adenoviral transduction. These data confirm the successful expression of the MuRF1 transgene in primary cardiomyocytes.
Figure 2. MuRF1 ectopic expression and TUBE-mediated ubiquitin enrichment.
Fluorescent imaging and immunoblot verified MuRF1 ectopic expression and ubiquitin enrichment prior to 2D-DIGE a Representative fluorescence micrographs of primary cardiomyocytes after 24 h of transduction with adenovirus expressing green fluorescent reporter protein alone (Ad-GFP) or in combination with Myc-tagged MuRF1 (Ad-MuRF1) at MOI of 10. b Representative immunoblots (IB) of Myc, MuRF1, and GAPDH protein levels in extracts isolated from primary cardiomyocytes transduced with Ad-GFP (−) or Ad-MuRF1 (+) adenovirus for 24 h. The red arrow indicates endogenous MuRF1, with ectopically-expressed myc-tagged MuRF1 migrating at a slightly higher molecular weight. c Representative immunoblot of total ubiquitin from TUBE enrichment in extracts isolated from primary cardiomyocytes transduced with Ad-GFP (G) or Ad-MuRF1 (M) for 24 h as performed in the 2D-DIGE MuRF1 substrate screen. Lanes 1 and 2: input samples; lanes 3 and 4: unbound TUBE supernatant collected; lanes 5 and 6: ubiquitinated protein enrichment eluted from TUBE. From 3 independent experiments we observed an average of 30 ± 14.7% increase in total ubiquitinated protein with MuRF1 ectopic expression as measured by densitometry.
MuRF1 ubiquitome isolation
Prior to 2D-DIGE analyses, we first confirmed that we could successfully isolate the TUBE-selected ubiquitome. Lysates were prepared, quantified, and incubated for 18 h in the presence of either control agarose or TUBE beads. Both the unbound (supernatant) and bound (eluate) fractions were collected and separated via SDS-PAGE. The bound fractions from the TUBE beads in both the Ad-GFP and Ad-MuRF1 conditions contained highly enriched levels of ubiquitinated proteins compared to the unbound fraction determined via immunoblot analysis (Fig. 2C). We also consistently noticed a 30% increase in the total densitometry of ubiquitin immunoreactivity in cells transduced with Ad-MuRF1 (Fig. 2C, right) suggesting an overall increase in total protein ubiquitination in the presence of increased MuRF1 expression. Given the successful selection of ubiquitinated proteins using the TUBE isolation procedure, we moved forward to the differential gel analysis to attempt to identify specific proteins that are more readily ubiquitinated in the presence of increased MuRF1 expression.
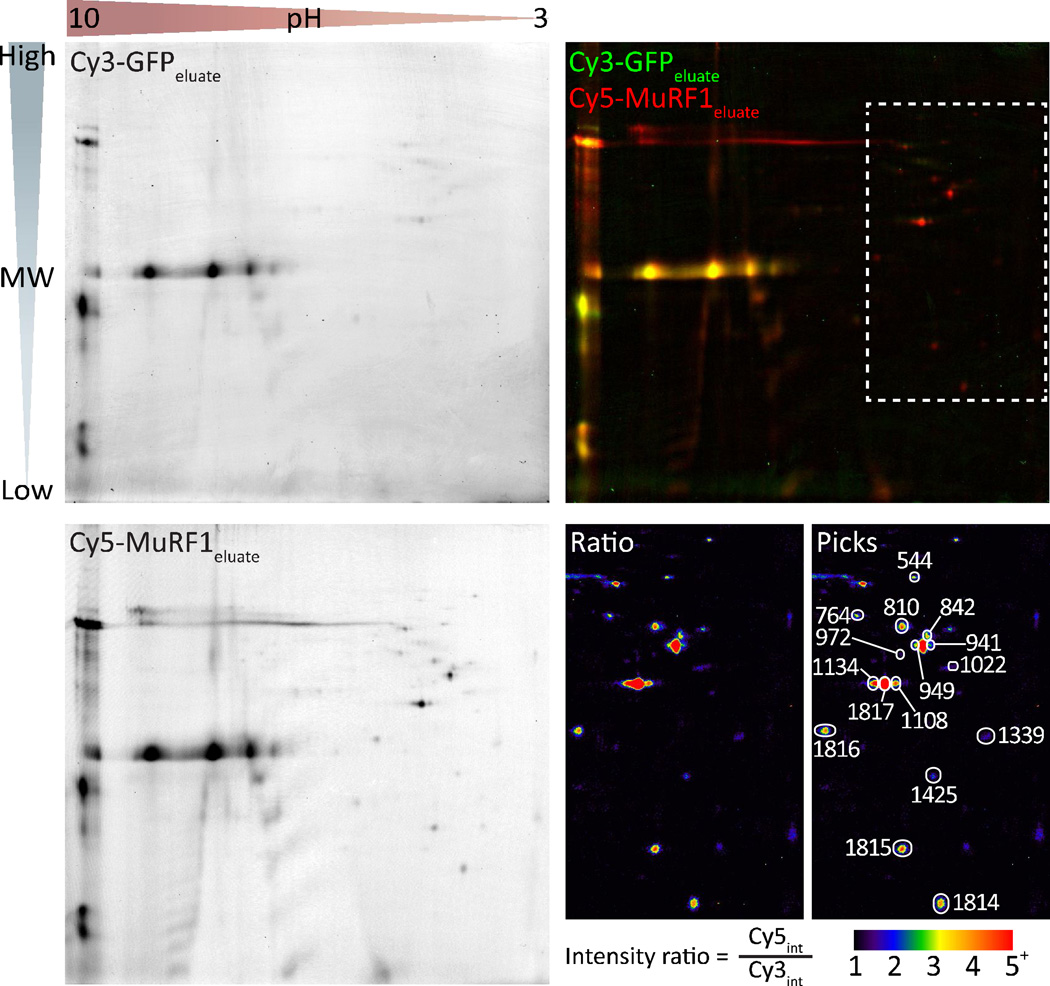
2D-DIGE and selection of picks corresponding to increased MuRF1 expression
Using the three gel conditions outlined above (Fig. 1) we used 2D-DIGE to identify candidate picks from each of the three comparisons. The primary comparison was between the elution profiles of the GFP versus MuRF1 ubiquitome (Gel 1). As shown in Figure 3, 2D-DIGE resolved differentially fluorescent-labeled pools of proteins in the same gel separated by both molecular weight (MW, vertical) and isoelectric point (pH, horizontal). A relative ratio of protein species was determined by the ratio of fluorescent intensity at identified spots in the gel. The image of the Cy3-labeled GFP eluate (green) and Cy5-labeled MuRF1 eluate (Fig. 3 top left and bottom left, respectively) were overlaid to locate differentially expressed spots (Fig. 3 top right). In the eluate comparison, we were interested in spots that were ≥1.5-fold more red than green (yellow spots represent similar protein amounts) indicating potential protein species that were more abundant in the MuRF1 eluate sample. Differential spots in the other two gels that met both quality standards and were changed in the expected direction were also identified as “picks” (for additional gel image data, see Supplementary Fig. 1).
Figure 3. 2D-DIGE gel of the TUBE-isolated ubiquitome.
2D-DIGE image analysis of ubiquitin-enriched samples eluted from TUBE identified spots for mass spectrometry protein identification. Proteins eluted from TUBE incubated with protein extract from Ad-GFP or Ad-MuRF1 transduced cardiomyocytes were labeled with Cy3 and Cy5, respectively, and separated by molecular weight and isoelectric point (Cy3-GFPeluate and Cy5-MuRF1eluate, top left and bottom left, respectively). Relative changes in protein spots were calculated using the ratio of fluorescence intensity of each fluorescent channel visualized by coloring and overlaying the Cy3-GFPeluate (green) and Cy5-MuRF1eluate images (top right). The region containing the 16 spots selected for mass spectrometry identification (top right, hashed white box) was magnified and used to generate a ratio image (Cy5/Cy3) to highlight the fold-enrichement and identification of each picked spot (bottom right).
Picks that pass all three selection criteria and subsequent protein identification by MALDI-TOF identify candidate MuRF1 substrates
We tabulated all of the individual spots that passed the selection criteria from each gel comparison; for a list of all the spots “picked” in each comparison, see Supplementary Table 1. In total, there were nine spots that satisfied all three gel comparison criteria (highest confidence, Supplementary Table 1), and an additional seven spots that satisfied criteria in Gel 1 and Gel 2 (high confidence, Supplementary Table 1) for gain-of-function substrate identification (Fig. 1). These 16 spots all fell within the intermediate to low pH range, spanning a molecular weight range of 20–75 × 103 Daltons (Fig. 3 upper right, boxed region). As a reference, we included a ratio image of this region to highlight the fold enrichment of these samples in the MuRF1 TUBE elution relative to the GFP TUBE elution as well as an annotated ratio image to highlight and label the 16 spots (Fig. 3, bottom right). The 16 spots were picked from an independently run “pick” gel and submitted for protein identification using MALDI-TOF. Peptide sequencing identified a total 20 polypeptides identified from the 16 spots (Table 1, Supplementary Table 2 and Supplementary data file). Consistent with our hypothesis and validation of our proof-of-principal, three of the 20 proteins were previously identified in our yeast two-hybrid screen (9) or a published yeast two-hybrid screen (30) and all but one of the proteins (Coq9) have been published as an identified polyubiquitinated protein (30–33). Additionally, the functional classification of the proteins identified included both structural (sarcomeric) protein components and mitochondrial substrates, two known locations within the cardiomyocyte where MuRF1 is known to function (30,34,35), suggesting this approach may, in fact, identify bona-fide MuRF1 substrates.
Table 1. Identities and characterization of MuRF1 substrates identified by MS/MS analysis of selected 2D-DIGE spots.
Symbols, descriptions and PANTHER protein classifications for each of the MuRF1 substrates identified by mass spectroscopy analysis are listed. Also indicated are those proteins previously identified as MuRF1 substrates by yeast two-hybrid screening (Y2H (9,30) and Cam Patterson – data not shown) and those proteins previously reported as MuRF1 substrates (or interacting proteins) in skeletal muscle.
| Symbol | Protein description | Reported substrate |
Identified by Y2H |
PANTHER Protein Class |
|---|---|---|---|---|
| ACTB | Actin, cytoplasmic 1 | actin and actin related protein | ||
| ACTC1 | Actin, alpha cardiac muscle 1 | skeletal (40) |
yes (9) | actin and actin related protein |
| ATP5B | ATP synthase subunit beta, mitochondrial | yes (30) | ATP synthase/ion channel/hydrolase |
|
| COQ9 | Ubiquinone biosynthesis protein COQ9, mitochondrial |
cofactor biosynthesis* | ||
| HNRNPF | Heterogeneous nuclear ribonucleoprotein F | ribosomal protein | ||
| HSPA5 | 78 kDa glucose-regulated protein | Hsp70 family chaperone | ||
| HSPD1 | 60 kDa heat shock protein, mitochondrial | chaperonin | ||
| LDHB | L-lactate dehydrogenase B chain | dehydrogenase | ||
| MDH1 | Malate dehydrogenase, cytoplasmic | dehydrogenase | ||
| MURF1 | E3 ubiquitin-protein ligase TRIM63 (MuRF1) | auto Ub | ubiquitin-protein ligase | |
| MYL2 | Myosin regulatory light chain 2, ventricular/cardiac muscle isoform |
skeletal (41) |
yes (9) | cytoskeletal protein |
| MYL3 | Myosin light chain 3 | skeletal (41) |
yes (9) | actin family cytoskeletal protein/calmodulin |
| PDIA6 | Protein disulfide-isomerase A6 | isomerase | ||
| PPP1R7 | Protein phosphatase 1 regulatory subunit 7 | phosphatase modulator/microtubule family cytoskeletal protein |
||
| TOLLIP | Toll-interacting protein | adapter protein* | ||
| TPM1 | Isoform 1 of Tropomyosin alpha-1 chain | actin binding motor protein | ||
| TUBA1A | Tubulin alpha-1A chain | tubulin | ||
| TUBB2C | Tubulin beta-2C chain | tubulin | ||
| TUBB5 | Isoform 1 of Tubulin beta-5 chain | tubulin | ||
| VIM | Vimentin | structural protein/intermediate filament |
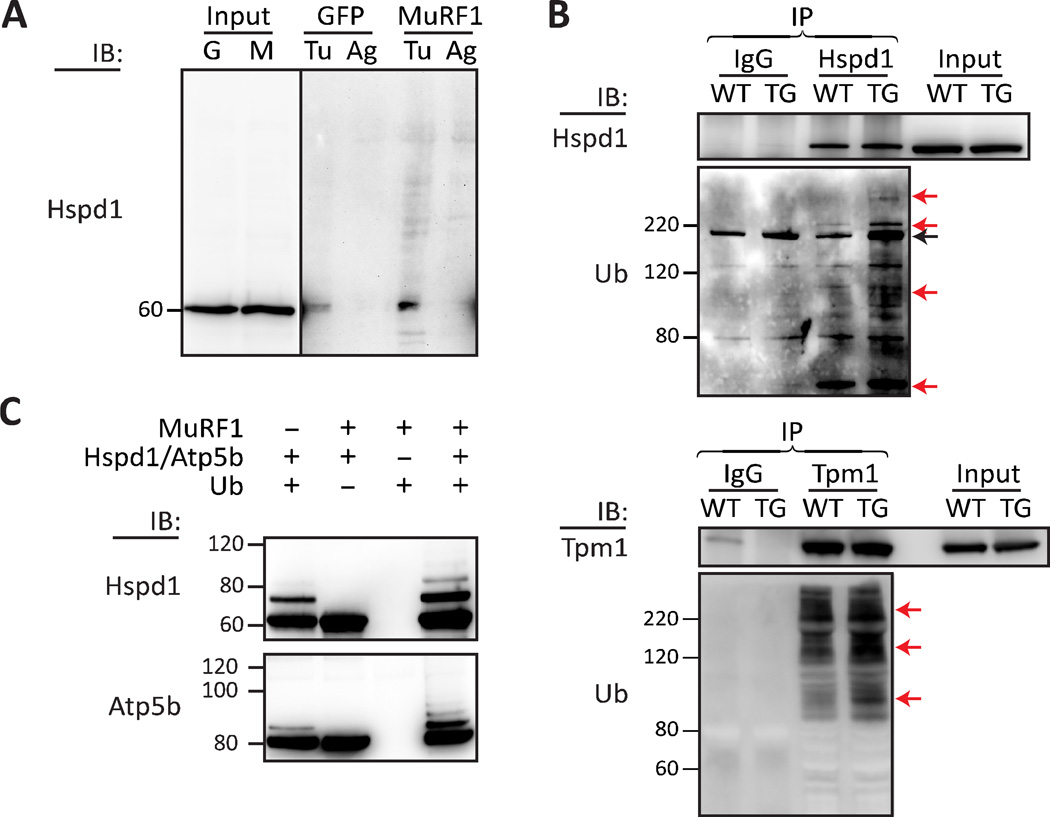
Validation of Hspd1, Tpm1, and Atp5b as substrates of MuRF1
To determine if the candidate proteins identified in our 2D-DIGE method were substrates of MuRF1, we took advantage of available antibodies and purified recombinant proteins to validate selected candidates. First, we measured the pattern of Hspd1 (Fig. 3, spot #764) modification in primary cardiomyocytes transduced with either Ad-GFP or Ad-MuRF1 via SDS-PAGE/immunoblot analysis from independent experiments prepared with TUBE or control agarose identically as described in the 2D-DIGE screen. As expected, in cells overexpressing MuRF1, we detected several higher molecular weight proteins bound to the TUBE beads that were reactive to the Hspd1 antibody (Fig. 4A), consistent with a MuRF1-dependent increase in Hspd1 polyubiquitination. To determine if our primary cell system of MuRF1 overexpression accurately reflected the action of increased MuRF1 activity in vivo, we immunoprecipitated Hspd1 as well as another MuRF1 substrate candidate Tpm1 (Fig. 3, spot #1339) from heart tissue isolated from either wild-type or MuRF1-transgenic mice (Fig. 4B). Consistent with the pattern observed in primary cardiomyocytes (Fig. 4A), after successful immunoprecipitation of either candidate substrate protein, using immunoblot analysis we observed increases in higher molecular weight proteins reactive to an antibody that recognizes ubiquitin (Fig 4B) in MuRF1-trangenic hearts compared to wild-type hearts. These data suggest that in vivo, both Hspd1 and Tpm1 are cardiac substrates of MuRF1; furthermore, the translatability of using this ubiquitin ligase screen in our primary cardiomyocyte culture model to identify in vivo cardiac substrates demonstrates the utility of developing methods that are performed in a biologically relevant context. Finally, we used a purified recombinant protein system to test if the candidate substrates Hspd1 and Atp5b (Fig. 3, spot #941, #949, #972) could be polyubiquitinated by MuRF1 in a cell-free in vitro system. Interestingly, in the absence of MuRF1 we observed E2-mediated monoubiquitination (an emerging property of E2 enzymes; 36, 37) of both Hspd1 and Atp5b,. As expected, we found both Hspd1 and Atp5b to be polyubiquitinated in the presence of MuRF1, providing further validation that these proteins are direct substrates of MuRF1. Through the use of multiple approaches we demonstrated that all three candidate substrates identified by our 2D-DIGE method were validated as MuRF1 substrates.
Figure 4. Validation of screen-identified proteins Hspd1, Tpm1, and Atp5b as substrates of MuRF1.
In vitro and in vivo data demonstrate that the screen-identified proteins Hspd1, Tpm1 and Atp5b, are MuRF1 substrates. a Representative immunoblot (IB) of Hspd1 protein levels in extracts isolated from primary cardiomyocytes transduced with Ad-GFP (G) or Ad-MuRF1 (M) adenovirus for 24 h. Lane 1 and 2: input samples (light exposure, see Supplementary Fig. 2); lane 3 and 4: Ad-GFP samples eluted from either TUBE (Tu) or agarose control beads (Ag); Lane 5 and 6: Ad-MuRF1 samples eluted from TUBE (Tu) or agarose control beads (Ag). b Immunoprecipitations (IP) of Hspd1 and Tpm1 in extracts isolated from wild-type (WT) or MuRF1 transgenic (TG) mouse hearts, subsequently immunoblotted (IB) for Hspd1 or Tpm1 and ubiquitin (Ub). Lane 1 and 2: IgG control IP; lane 3 and 4: Hspd1 (top) or Tpm1 (bottom) IP; Lane 5 and 6: 10% input of extract. Red arrows indicate ubiquitin-reactive Hspd1 or Tpm1 species in MuRF1 Tg hearts (lane 4) that are not present or are of lower relative abundance in wild-type hearts (lane 3). The black arrow indicates a non-specific band also present in the IgG control IP. c In vitro ubiquitination assays for MuRF1 ubiquitination of Hspd1 and Atp5b performed in presence or absence of purified ubiquitin or MuRF1 as indicated and detected by immunoblot analysis (IB) for Hspd1 (top) or Atp5b (bottom).
Discussion
Ubiquitin ligases and their counter-regulatory de-ubiquitinating enzymes (DUBs) play unique roles in protein ubiquitination and the UPS as the regulators of substrate specificity; therefore, substrate identification is critical to the mechanistic understanding of the UPS as well as our ability to modify protein ubiquitination in order to modulate disease. Despite this, the available methods to identify ubiquitin ligase and DUB substrates do not provide robust or unbiased means to identify physiological substrates, often relying upon non-physiological in vitro approaches. Given the current limitations in ubiquitin substrate screens, we developed a novel method for the identification of physiological ubiquitin ligase/DUB substrates (Fig. 1). As a proof-of-principle for our method, we successfully utilized a transient gain-of-function model to screen for MuRF1 substrates in primary cardiomyocytes (Fig. 2) and identified both previously identified MuRF1 substrates as well as novel substrate proteins (Table 1). Not surprisingly, nearly all of the candidate substrates were previously identified as polyubiquitinated proteins (30–33) ; additionally, the novel candidate substrates were functionally classified in cellular locations where MuRF1 is known to function including the sarcomere and mitochondria (30,34,35), reflective of the physiological approach utilized by our method. Furthermore, we validated several novel MuRF1 substrates both in vitro (Hspd1, Atp5b) and in vivo (Hspd1, Tpm1). In fact, the increased in polyubiquitination of Hspd1 and Tpm1 in hearts of MuRF1 transgenic animals not only validates the ability of our screen to identify novel substrates but also demonstrates the unique translatability of this screening method performed in a biologically relevant context to an independent in vivo model.
Our method circumvents major pitfalls of traditional substrate screens by: 1) using a physiological setting that is relevant to the ubiquitin ligase/DUB or disease model; 2) utilizing TUBE technology to isolate and protect the ubiquitome (25), independent of the strength of the physical interaction between the ubiquitin ligase/DUB and substrate; and 3) employing 2DDIGE with multiple subtractive comparisons to reduce the number of false positives. For our proof-of-principle we used a biologically relevant primary cell culture system; however, TUBE-based ubiquitome isolation can be used with a myriad of other protein sources including stable cell culture models, isolated preparations of subcellular compartments (for example, mitochondria, Rubel and Patterson – data not shown), and animal tissues. The flexibility in starting material allows the study substrates of a ubiquitin ligase, or the action of an opposing DUB (Fig. 1), under specific physiological or pathophysiological conditions. We performed our screen using TUBE technology that has an equal affinity to K63 and K48 ubiquitin linkages; however, there are other TUBE variants that bind specific lysine linkages allowing the screen to be biased towards non-canonical ubiquitinated substrates if desired. Moreover, we used semi-quantitative proteomics using 2D-DIGE, allowing high resolution separation and reduction of sample complexity prior to mass spectrometric analysis, and enhancing accuracy of protein identification, while avoiding issues of gel-to-gel variability associated with traditional 1D or 2D gel-based proteomics (38). To minimize false positives, we coupled our 2D-DIGE strategy with a multi-sample cross-comparison to achieve higher confidence in spot-picking prior to MALDI-TOF peptide identification, the stringency of which could be increased or decreased based on the user’s preference for confidence (Supplementary Table 1).
We realize that there are other modifications to this protocol that could be used to refine substrate identification. Although we did not include proteasome inhibition to our protocol, an approach that is often used in interaction-based methods, the inclusion of a proteasome inhibitor such as MG132 for cell-based protein sources prior to protein extraction would likely increase the pool of K48-linked polyubiquitinated proteins. In addition, there are other protein identification methods that could be used in place of 2D-DIGE/MALDI-TOF as there are limitations to a gel-based approach including difficulty detecting very hydrophobic proteins, proteins with extreme molecular weights and pI values, as well as the potential limited availability of 2D-DIGE facilities and personnel with the required expertise. With minimal modification this method could be used with gel-free quantitative proteomics strategies such Tandem Mass Tags (TMT) or Isobaric Tags for Relative and Absolute Quantification (iTRAQ) to identify and quantitate proteins in each of the collected eluate and supernatant samples (39). It should be noted that, even with the use of a gel-free based approach, the same subtractive approach could be used to reduce false positives. With the robust, flexible nature of the protocol described here, we are hopeful that this method will be broadly applied to the study of both ubiquitin ligases and DUBs and through the identification of their substrates, aid in the understanding of these unique and important regulatory proteins.
Supplementary Material
Acknowledgements
We would like to thank the Michael Hooker Proteomics Center at UNC for protein identification services and Andrea Portbury for her critical review of this manuscript.
References
- 1.Colland F. The therapeutic potential of deubiquitinating enzyme inhibitors. Biochemical Society transactions. 2010 Feb;38(Pt 1):137–143. doi: 10.1042/BST0380137. [DOI] [PubMed] [Google Scholar]
- 2.Lim K-H, Baek K-H. Deubiquitinating enzymes as Therapeutic Targets in Cancer. Current pharmaceutical design. 2012 Nov 23; doi: 10.2174/1381612811319220013. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals “Protein Modifications: Beyond the Usual Suspects” review series. EMBO reports. 2008 Jun;9(6):536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelmann MJ, Nicholson B, Kessler BM. Pharmacological targets in the ubiquitin system offer new ways of treating cancer, neurodegenerative disorders and infectious diseases. Expert reviews in molecular medicine. 2011 Jan;13:e35. doi: 10.1017/S1462399411002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zolk O, Schenke C, Sarikas A. The ubiquitin-proteasome system: focus on the heart. Cardiovascular research. 2006 Jun;70(3):410–421. doi: 10.1016/j.cardiores.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Wilkie Neil, Davies S. Drug Discovery World, Drug Discovery and Development News. [Google Scholar]
- 7.Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, et al. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. Journal of molecular biology. 2001 Mar;306(4):717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 8.Spencer JA, Eliazer S, Ilaria RL, Richardson JA, Olson EN. Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. The Journal of cell biology. 2000 Aug;150(4):771–784. doi: 10.1083/jcb.150.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedar V, McDonough H, Arya R, Li H-H, Rockman HA, Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proceedings of the National Academy of Sciences of the United States of America. 2004 Dec 28;101(52):18135–18140. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polge C, Heng A-E, Jarzaguet M, Ventadour S, Claustre A, Combaret L, et al. Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011 Nov;25(11):3790–3802. doi: 10.1096/fj.11-180968. [DOI] [PubMed] [Google Scholar]
- 11.Willis MS, Zungu M, Patterson C. Cardiac muscle ring finger-1--friend or foe? Trends in cardiovascular medicine. 2010 Jan;20(1):12–16. doi: 10.1016/j.tcm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mearini G, Schlossarek S, Willis MS, Carrier L. The ubiquitin-proteasome system in cardiac dysfunction. Biochimica et biophysica acta. 2008 Dec;1782(12):749–763. doi: 10.1016/j.bbadis.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Powell SR, Herrmann J, Lerman A, Patterson C, Wang X. The ubiquitin-proteasome system and cardiovascular disease. Progress in molecular biology and translational science. 2012 Jan;109:295–346. doi: 10.1016/B978-0-12-397863-9.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell SR. The ubiquitin-proteasome system in cardiac physiology and pathology. American journal of physiology. Heart and circulatory physiology. 2006 Jul;291(1):H1–H19. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zeng Y, Wang M, Tian C, Ma X, Chen H, et al. Cardiac-specific overexpression of E3 ligase Nrdp1 increases ischemia and reperfusion-induced cardiac injury. Basic research in cardiology. 2011 May;106(3):371–383. doi: 10.1007/s00395-011-0157-0. [DOI] [PubMed] [Google Scholar]
- 16.Li H-H, Du J, Fan Y-N, Zhang M-L, Liu D-P, Li L, et al. The ubiquitin ligase MuRF1 protects against cardiac ischemia/reperfusion injury by its proteasome-dependent degradation of phospho-c-Jun. The American journal of pathology. 2011 Mar;178(3):1043–1058. doi: 10.1016/j.ajpath.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X, Kem DC. Proteasome inhibition during myocardial infarction. Cardiovascular research. 2010 Jan;85(2):312–320. doi: 10.1093/cvr/cvp309. [DOI] [PubMed] [Google Scholar]
- 18.Nowis D, Maczewski M, Mackiewicz U, Kujawa M, Ratajska A, Wieckowski MR, et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. The American journal of pathology. 2010 Jun;176(6):2658–2668. doi: 10.2353/ajpath.2010.090690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshaies RJ, Joazeiro CAP. RING domain E3 ubiquitin ligases. Annual review of biochemistry. 2009 Jan;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Chen Y, Yang T, Gao Q, Yuan M, Ma L. Targeted Ubiquitination and Degradation of G-Protein-Coupled Receptor Kinase 5 by the DDB1-CUL4 Ubiquitin Ligase Complex. In: Hess S, editor. PloS one. 8. Vol. 7. Public Library of Science; 2012. Jan, p. e43997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peschiaroli A, Dorrello NV, Guardavaccaro D, Venere M, Halazonetis T, Sherman NE, et al. SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Molecular cell. 2006 Aug;23(3):319–329. doi: 10.1016/j.molcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Kus B, Gajadhar A, Stanger K, Cho R, Sun W, Rouleau N, et al. A high throughput screen to identify substrates for the ubiquitin ligase Rsp5. The Journal of biological chemistry. 2005 Aug;280(33):29470–29478. doi: 10.1074/jbc.M502197200. [DOI] [PubMed] [Google Scholar]
- 23.Loch CM, Eddins MJ, Strickler JE. Protein microarrays for the identification of praja1 e3 ubiquitin ligase substrates. Cell biochemistry and biophysics. 2011 Jun;60(1–2):127–135. doi: 10.1007/s12013-011-9180-x. [DOI] [PubMed] [Google Scholar]
- 24.Qian S-B, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006 Mar;440(7083):551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, Rodriguez MS. Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO reports. 2009 Nov;10(11):1250–1258. doi: 10.1038/embor.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toraason M, Luken ME, Breitenstein M, Krueger JA, Biagini RE. Comparative toxicity of allylamine and acrolein in cultured myocytes and fibroblasts from neonatal rat heart. Toxicology. 1989 May;56(1):107–117. doi: 10.1016/0300-483x(89)90216-3. [DOI] [PubMed] [Google Scholar]
- 27.Osorio C, Sullivan PM, He DN, Mace BE, Ervin JF, Strittmatter WJ, et al. Mortalin is regulated by APOE in hippocampus of AD patients and by human APOE in TR mice. Neurobiology of Aging. 2007 Dec;28(12):1853–1862. doi: 10.1016/j.neurobiolaging.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Höhfeld J, et al. CHIP is a U-boxdependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. The Journal of biological chemistry. 2001 Nov;276(46):42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 29.Willis MS, Schisler JC, Li L, Rodríguez JE, Hilliard EG, Charles PC, et al. Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circulation research. 2009 Jul 2;105(1):80–88. doi: 10.1161/CIRCRESAHA.109.194928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. Journal of molecular biology. 2005 Jul;350(4):713–722. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Molecular cell. 2011 Oct;44(2):325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. The Journal of cell biology. 2009 Jun;185(6):1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Molecular & cellular proteomics : MCP. 2011 Oct;10(10):M111.013284. doi: 10.1074/mcp.M111.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portbury AL, Willis MS, Patterson C. Tearin’ up my heart: proteolysis in the cardiac sarcomere. The Journal of biological chemistry. 2011 Mar;286(12):9929–9934. doi: 10.1074/jbc.R110.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis MS, Schisler JC, Li L, Rodríguez JE, Hilliard EG, Charles PC, et al. Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circulation research. 2009 Jul 2;105(1):80–88. doi: 10.1161/CIRCRESAHA.109.194928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David Y, Ternette N, Edelmann MJ, Ziv T, Gayer B, Sertchook R, et al. E3 ligases determine ubiquitination site and conjugate type by enforcing specificity on E2 enzymes. The Journal of biological chemistry. 2011 Dec 23;286(51):44104–44115. doi: 10.1074/jbc.M111.234559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoeller D, Hecker C-M, Wagner S, Rogov V, Dötsch V, Dikic I. E3-independent monoubiquitination of ubiquitin-binding proteins. Molecular cell. 2007 Jun 22;26(6):891–898. doi: 10.1016/j.molcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Friedman DB, Hoving S, Westermeier R. Isoelectric focusing and two-dimensional gel electrophoresis. Methods in enzymology. 2009 Jan;463:515–540. doi: 10.1016/S0076-6879(09)63030-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhu W, Smith JW, Huang C-M. Mass spectrometry-based label-free quantitative proteomics. Journal of biomedicine & biotechnology. 2010 Jan;2010:840518. doi: 10.1155/2010/840518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polge C, Heng A-E, Jarzaguet M, Ventadour S, Claustre A, Combaret L, et al. Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011 Nov;25(11):3790–3802. doi: 10.1096/fj.11-180968. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. The Journal of cell biology. 2009 Jun 15;185(6):1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.