Abstract
Electroacupuncture has been shown to induce a preconditioning effect in the brain. The mechanisms for this protection are not fully elucidated. We hypothesize that this protection is mediated by excitatory amino acid transporters (EAATs) that have been shown to be neuroprotective. To test this hypothesis, two-month old male Sprague-Dawley rats and EAAT type 3 (EAAT3) knockout mice received or did not receive 30-min electroacupuncture once a day for 5 consecutive days. They were subjected to a 120-min middle cerebral arterial occlusion (MCAO) at 24 h after the last electroacupuncture. Neurological outcome was assessed 2 days after the MCAO. Brain tissues were harvested at 24 h after the last electroacupuncture for Western blotting. Rats subjected to electroacupuncture at the Baihui acupoint had smaller brain infarct volumes and better neurological deficit scores than control rats. Electroacupuncture increased EAAT type 2 (EAAT2) in the cerebral cortex, tended to increase EAAT3 in the hippocampus, and had no effect on EAAT type 1 expression. Dihydrokainate, an EAAT2 inhibitor, worsened the neurological outcome of rats with electroacupuncture pretreatment. Electroacupuncture pretreatment at the Baihui acupoint increased EAAT2 in the cerebral cortex and improved the neurological outcome of EAAT3 knockout mice. Together, our results suggest that EAAT2 may mediate the electroacupuncture preconditioning-induced neuroprotection.
Keywords: brain, electroacupuncture, glutamate transporter, ischemia, preconditioning
1. Introduction
Stroke is a leading cause of death and disability (Hoyert and Xu, 2012). The major pathophysiology for stroke to lead to these poor outcomes is ischemic brain injury. It has been a focus for medical research to identify interventions to reduce ischemic brain injury. However, very few clinically practical and effective interventions have been developed so far.
One of the promising approaches to reduce ischemic brain injury is to induce endogenous protective mechanisms. Preconditioning in which a prior exposure of tissues or organs to a stimulus or a drug reduces ischemia- or severe hypoxia-induced injury to the tissues or organs is such an approach (Dirnagl et al., 2003). It has been shown that electroacupuncture can induce a preconditioning effect in the brain (Ma et al., 2011; Wang et al., 2009). Acupuncture is a critical component of traditional Chinese medicine. Electroacupuncture is a modern technique that involves application of currents to a needle inserted at an acupoint to improve and diversify the stimulations and to reduce the requirement of accurate placement of the needle. This is because various forms of currents can be applied and the currents can travel a short distance to stimulate the acupoint.
The mechanisms for electroacupuncture preconditioning-induced neuroprotection are not fully understood. It is known that preconditioning-induced neuroprotection often requires the synthesis of protective proteins (Dirnagl et al., 2003). Glutamate transporters (also called excitatory amino acid transporters, EAATs), a group of proteins that transport the excitatory amino acid neurotransmitters including glutamate from extracellular space to intracellular compartments (Danbolt, 2001), have been shown to provide neuroprotection (Li and Zuo, 2011; Rao et al., 2001; Rothstein et al., 1996). In addition, studies have implicated the involvement of EAATs in the neuroprotection induced by various preconditioning stimuli (Bigdeli et al., 2008; Romera et al., 2007; Wang et al., 2007; Zheng and Zuo, 2003). Thus, we hypothesize that EAATs mediate the electroacupuncture preconditioning-induced neuroprotection. There are five EAATs. EAAT1 and EAAT2 are glial. EAAT3 and EAAT4 are neuronal. EAAT5 is mainly distributed in the retina. EAAT1 – 3 are widely expressed in the central nervous system; whereas EAAT4 mainly exists in the cerebrum. Quantitatively, EAAT2 and EAAT3 are the major glial and neuronal EAAT, respectively (Danbolt, 2001; Rothstein et al., 1996). Thus, we designed this study to mainly determine the role of EAAT2 and EAAT3 in the electroacupuncture preconditioning-induced neuroprotection. Our study showed that electroacupuncture increased EAAT2 in the cerebral cortex and that dihydrokainate, an EAAT2 inhibitor, inhibited electroacupuncture-induced neuroprotection.
2. Materials and methods
2.1. Animals
The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Virginia. All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised in 1996. All efforts were made to minimize the number of animals used and their suffering.
Two-month old male Sprague-Dawley rats (280 to 320 g) were from Charles River Laboratories Inc, Wilmington, MA. The EAAT3 knockout mice (28 – 32 g) were descendants of the strain established by Peghinni et al (Peghini et al., 1997). Their exon 1 of eaat3 gene is disrupted by a neomycin resistance cassette. These mice were backcrossed with wild-type CD-1 mice for more than 10 generations to produce a strain of EAAT3 knockout mice before our study. They have been backcrossed with wild-type CD-1 mice in our laboratory at least once every 8 generations to prevent genetic drift as recommended from the Banbury Conference (Silva et al., 1997). The CD-1 wild-type mice were from Charles River Laboratories. Male 8-week-old EAAT3 knockout mice (28 to 32 g) were used in our study. The lack of the EAAT3 protein expression in these mice has been confirmed by our previous studies (Lee et al., 2010; Li and Zuo, 2011).
2.2. Electroacupuncture pretreatment
Electroacupuncture pretreatment was performed as we previously described (Wang et al., 2009). Rats or mice were anesthetized with intraperitoneal sodium pentobarbital (40 mg/kg) and inhaled oxygen by face mask gassed with 100% oxygen at 1 L/min. An electroacupuncture needle (0.25 mm in diameter at the tip and 13 mm long; Suzhou Medical Appliance Ltd, Suzhou, China) was inserted at the acupoint “Baihui (GV 20)” that is located at the intersection of the sagittal midline and the line drawn to connect two ears. The needle was connected to a generator (SDZ-V Electronic Acupuncture Treatment Instrument, Suzhou Medical Appliance Ltd) that produced electric currents with the intensity of 1 mA and frequency of 2/15 Hz. The stimulation was for 30 min per day for 5 consecutive days. To assess whether the induced effects are specific to the stimulation of the Baihui acupoint, animals were stimulated 1 cm lateral to the Baihui acupoint with the same current setting for 30 min per day for 5 consecutive days (Li et al., 2012). This treatment was called para-electroacupuncture. Another group of rats received pentobarbital every day but without electroacupuncture for 5 consecutive days. This group was called pentobarbital group. Temporalis muscle temperature was monitored and maintained at 37.0°C ± 0.2°C by warming blanket during the anesthesia. The heart rate, arterial blood oxygen saturation, respiration rate were monitored during electroacupuncture treatment. The control animals were placed in a chamber gassed with 100% oxygen for 30 min per day for 5 consecutive days. The numbers of rats in each study group were as follows: 8 for the control group, 8 for the pentobarbital group, 7 for the para-electroacupuncture group, 17 for the electroacupuncture group, 7 for the dihydrokainate group and 6 for the dihydrokainate plus electroacupuncture group.
2.3. Administration of dihydrokainate
Dihydrokainate (10 mg/kg, Tocris, Bristol, UK), a specific inhibitor of EAAT2, dissolved in normal saline was intraperitoneally injected at 30 min before the onset of brain ischemia as described before in rats (Chu et al., 2007).
2.4. Transient middle cerebral arterial occlusion
Focal cerebral ischemia was induced at 24 h after the last electroacupuncture by middle cerebral artery occlusion (MCAO) as we previously described (Li and Zuo, 2009, 2011). Briefly, rats were anesthetized with isoflurane and then intubated and mechanically ventilated with pure O2 containing 2% isoflurane. Mice were anesthetized with 1.5% isoflurane carried by pure O2 through a mask. The right common carotid artery, external carotid artery, and internal carotid artery were isolated via a ventral midline neck incision. A nylon monofilament with rounded tip (3-0 for rats and 6-0 for mice) (Beijing Sunbio Biotech Co. Ltd., Beijing, China) was introduced into the right internal carotid artery via a puncture in the external carotid artery and advanced until slight resistance was felt. Isoflurane anesthesia was stopped immediately once the suture was in place. After recovery from anesthesia, animals were placed back into their cages with ad libitum access to food and water. They were re-anesthetized by isoflurane for ~ 2 min at 120 min (rat) or 90 min (mice) after the placement of the monofilament to remove this filament. During the surgery, temporalis muscle temperature in rats or rectal temperature in mice was strictly maintained at 37 ± 0.2 °C by a warming blanket. The inhaled and exhaled gases were also monitored with a Datex infrared analyzer (Capnomac, Helsinki, Finland) and normal end-tidal carbon dioxide concentrations were maintained. Their heart rates, breathing rates, and pulse oximeter oxygen saturations were monitored continuously and noninvasively using a MouseOX Murine Plus Oximeter System (Starr Life Sciences Corporation, Oakmont, PA, USA) as we did before (Li and Zuo, 2011). After recovery from anesthesia, animals were placed back in their cages with ad libitum access to food and water.
2.5. Evaluation of infarct volumes and neurological deficit scores
Neurological deficit scores of rats and mice were evaluated at 48 h and 24 h, respectively, after the transient MCAO based on an eight-point scale by a person who was blind to the group assignment of the animals. Rats and mice were scored as follows: 0, no apparent deficits; 1, failure to extend left forepaw fully; 2, decreased grip of the left forelimb; 3, spontaneous movement in all directions, contralateral circling only if pulled by the tail; 4, circling or walking to the left; 5, walking only if stimulated; 6, unresponsiveness to stimulation and with depressed level of consciousness; and 7, dead.
Cerebral infarct volumes were evaluated at 48 h (rats) and 24 h (mice) after the MCAO as we described before (Li and Zuo, 2009, 2011). Briefly, brain was sliced into coronal 2-mm (rats) or 1-mm (mice) thick slices. These slices were subjected to 2,3,5-triphenyltetrazolium chloride staining. The infarct areas were analyzed using NIH Image 1.60 (NIH, Bethesda, MD, USA). The sum of the infarct areas in the rostral and caudal sides of each brain slice was divided by 2 to get the average infarct area of the brain slice. The infarct volume of the brain slice was calculated by multiplying the average infarct area of the slice by the thickness of the slice. The total infarct volume in the brain was the sum of infarct volume in each brain slice. The ipsilateral hemisphere volume was measured in the same way. The percentage of infarct volumes in the ipsilateral hemisphere volumes was calculated to account for cerebral edema resulting from brain ischemia and tissue processing and to correct for the individual difference in brain volumes (Swanson et al., 1990).
2.6. Western analysis
Brain cortices and hippocampus of rats or mice were collected at 24 h after the last electroacupuncture without the MCAO. In another study, the right frontal cortex area 1 (Fr1), an ischemic penumbral brain region after MCAO (Li and Zuo, 2011; Zheng and Zuo, 2004), was harvested from rats at 2 h after MCAO. The brain tissues were homogenized on ice in a lysis buffer containing 200 mM mannitol, 80 mM HEPES (pH 7.4), protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) and 1 mM phenylmethanesulfonylfluoride. The tissue lysates were centrifuged at 1000 g for 10 min at 4°C and the supernatant was aspirated. The supernatant was centrifuged again at 100,000 g for 1 h at 4°C. The supernatant was removed and the pellet was re-suspended in the lysis buffer for Western blot (50 μg protein per lane). The following primary antibodies were used in this study: the rabbit polyclonal anti-EAAT1 antibody (1:1000, Cell Signaling Technology Inc, Danvers, MA, USA), the rabbit polyclonal anti-EAAT2 antibody (1:1000, Cell Signaling Technology Inc), the rabbit polyclonal anti-EAAT3 antibody (1:500, Alpha Diagnostic International Inc., San Antonio, TX, USA), the rabbit polyclonal anti-glyceraldehydes 3-phosphate dehydrogenase (GAPDH) antibody (1:1000, Cell Signaling Technology Inc.) and the rabbit polyclonal anti-β-actin antibody ((1:1000, Cell Signaling Technology Inc.). The protein bands were visualized with the enhanced chemoluminescence methods. Quantitative analysis of the protein bands was performed using an Image-Quant 5.0 GE Healthcare Densitometer (GE Healthcare, Sunnyvale, CA). The densities of EAAT1, EAAT2, and EAAT3 protein bands were normalized to those of GAPDH or β-actin in the same samples to control for errors in protein sample loading and transferring during western blotting. The results of EAATs under various conditions were normalized by the mean values of the corresponding results in the control animals.
2.7. Statistical analysis
All data, except for neurological deficit scores, are presented as mean ± S.E.M. and were analyzed by Student’s t-test or one-way analysis of variance followed by Student-Newman-Keuls method as appropriate. Neurological deficit scores were analyzed with rank sum test or one way analysis of variance on ranks with Dunn’s post-hoc test as appropriate. A P < 0.05 was considered statistically significant. All statistical analysis was performed with SigmaStat software (SYSTAT Software, Inc., Point Richmond, CA, USA).
3. Results
There were no significant differences in the heart rates, pulse oxygen saturation, and respiratory rates among the various groups (data not shown). One, three and two rats died in the para-electroacupuncture, dihydrokainate and dihydrokainate plus electroacupuncture groups, respectively, before the end of the 2-day observation period. These deaths occurred at 16 h or longer after the MCAO. There was no death in other three groups of rats. The death rates of the three groups in which death occurred were not different from that of control group. The data of the dead rats contributed to the final data of neurological deficit scores but did not contribute to the infarct volumes.
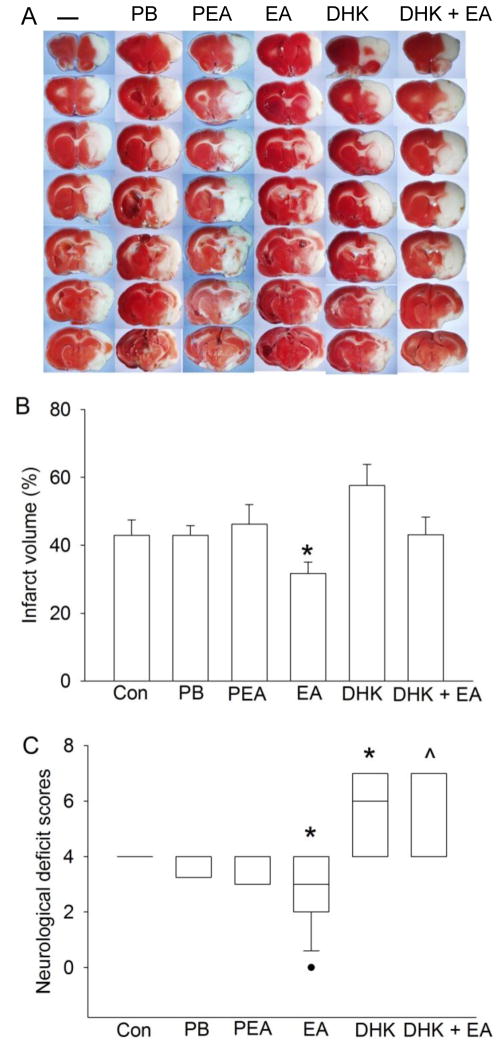
Similar to the previous studies (Ma et al., 2011; Xiong et al., 2003), rats pretreated with electroacupuncture had smaller infarct volumes and better neurological deficit scores than control rats. The infarct volumes and neurological deficit scores were similar among the control, pentobarbital and para-electroacupuncture groups (Fig. 1). Dihydrokainate injected 30 min before the MCAO significantly worsened the neurological deficit scores when compared to control group. Dihydrokainate also significantly worsened the neurological deficit scores in rats with electroacupuncture pretreatment when compared to electroacupuncture alone. There were no differences in the infarct volumes and neurological deficit scores between the dihydrokainate alone and dihydrokainate plus electroacupuncture groups (Fig. 1).
Fig. 1. Electroacupuncture preconditioning improved histological and neurological function outcome after focal brain ischemia in adult rats.
A: Brain sections after 2,3,5-triphenyltetrazolium chloride staining from representative rats in various groups. B: Percentage of brain infarct volume in ipsilateral hemisphere volume. Results are the means ± S.E.M. (n = 5 – 17). C: Neurological deficit scores evaluated immediately before the animals were euthanized for the assessment of infarct volumes (data are presented in panel B) or assigned 7 to the animals that died before this end time point for observation. Results are presented in a box plot format (n = 6 – 17). ●: lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25 – 75% interval including the median of the data. * P < 0.05 compared with control group. ^ P < 0.05 compared with electroacupuncture preconditioning group. Con: animals inhaling oxygen for 30 min each day for 5 consecutive days; PB: pentobarbital; PEA: para-electroacupuncture; EA: electroacupuncture; DHK: dihydrokainate.
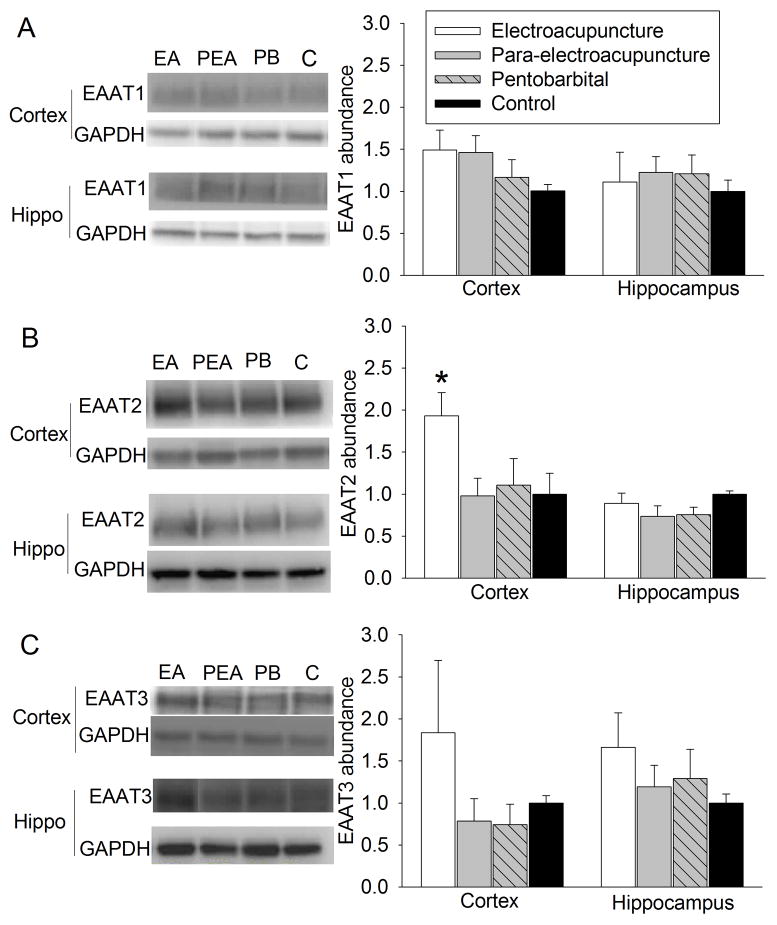
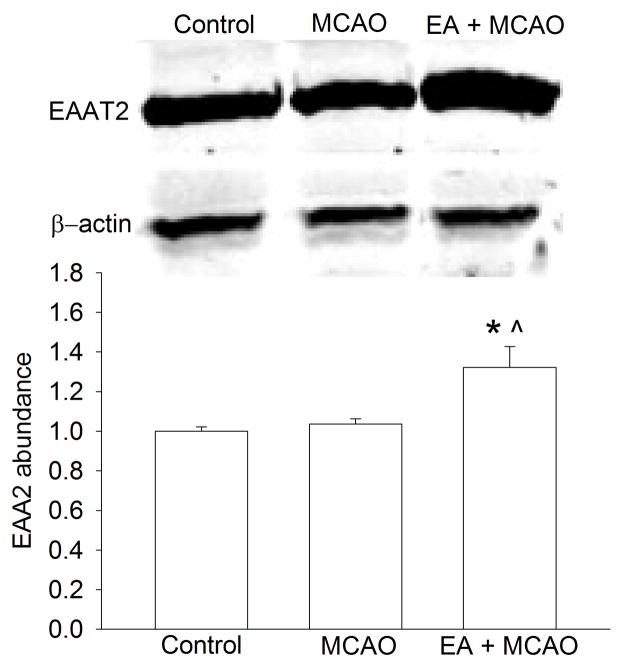
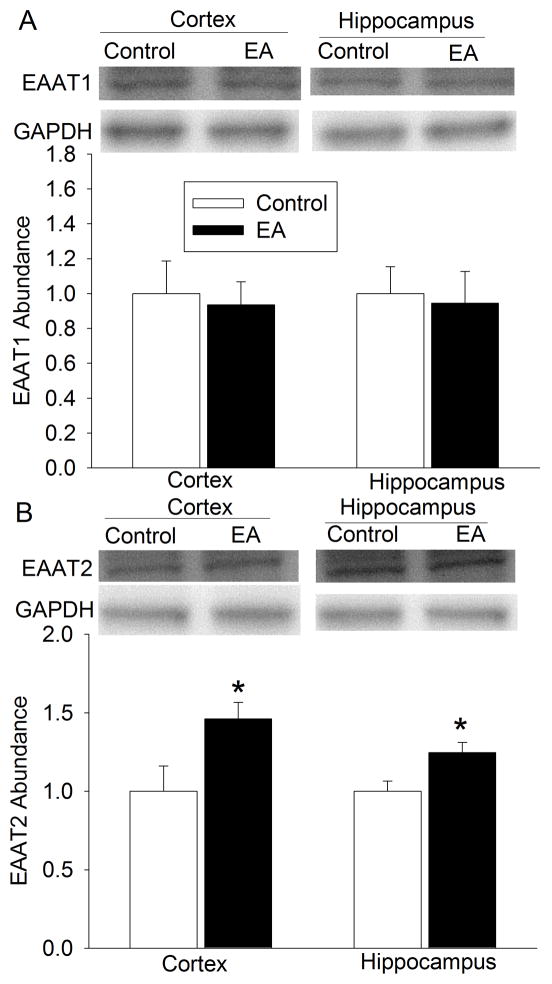
At 24 h after the last electroacupuncture without the MCAO, EAAT1 expression in the cerebral cortex and hippocampus was not significantly different among the control, pentobarbital, para-electroacupuncture and electroacupuncture groups. EAAT2 expression in the cerebral cortex of rats with electroacupuncture was significantly higher than that in the control rats. This difference was not observed in the hippocampus. Electroacupuncture tended to increase EAAT3 expression in the hippocampus (P = 0.158) when compared with control condition (Fig. 2). Similarly, rats with electroacupuncture preconditioning had a higher EAAT2 expression in the right Fr1 at 2 h after the right MCAO than control rats (Fig. 3).
Fig. 2. Effects of electroacupuncture on excitatory amino acid transporter (EAAT) expression in rats.
Cerebral cortex and hippocampus were harvested for Western blotting at 24 h after the last electroacupuncture without the MCAO. The EAAT1, EAAT2 and EAAT3 expression is presented in the panels A, B and C. Representative Western blots are shown in the left panel and the graphic presentation of the EAAT protein abundance quantified by integrating the volume of autoradiograms from 5 rats for each experimental condition is shown in the right panel. Values in graphs are the means ± S.E.M. * P < 0.05 compared with control rats inhaling oxygen 30 min each day for 5 days. GAPDH: glyceraldehydes 3-phosphate dehydrogenase; Hippo: hippocampus.
Fig. 3. Effects of electroacupuncture on excitatory amino acid transporter type 2 (EAAT2) expression in the frontal cortex area 1 of rats after the middle cerebral arterial occlusion (MCAO).
The brain tissues were harvested for Western blotting at 2 h after the MCAO. Representative Western blots are shown on the top panel and the graphic presentation of the EAAT2 protein abundance quantified by integrating the volume of autoradiograms from 4 rats for each experimental condition is shown in the low panel. Values in graphs are the means ± S.E.M. * P < 0.05 compared with control rats inhaling oxygen 30 min each day for 5 days. ^ P < 0.05 compared with rats after MCAO only. GAPDH: glyceraldehydes 3-phosphate dehydrogenase; EA: electroacupuncture.
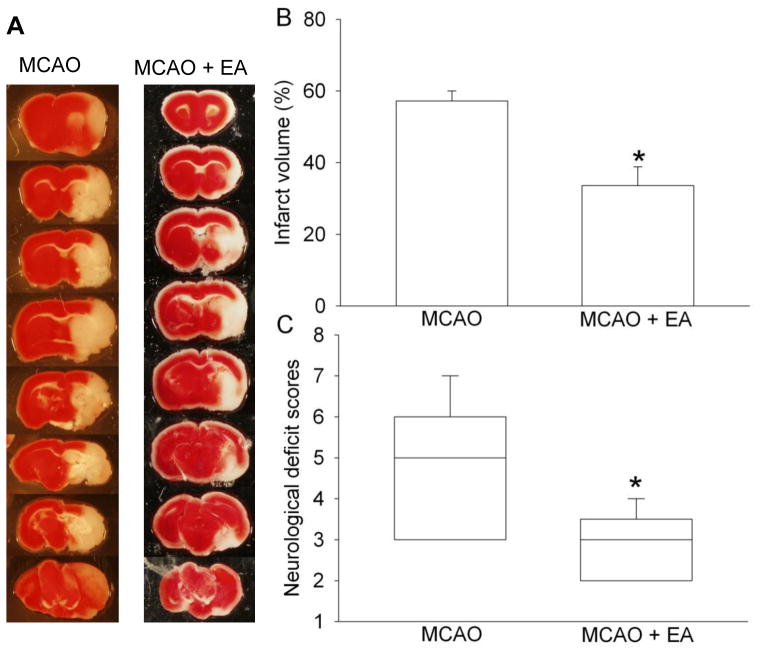
Eleven and nine EAAT3 knockout mice were used for the control and electroacupuncture groups, respectively. Two mice in the control group died before the end of the 24 h observation period. Electroacupuncture pretreatment at the Baihui acupoint significantly reduced the infarct volumes and improved the neurological deficit scores in the EAAT3 knockout mice (Fig. 4). Electroacupuncture also increased EAAT2 expression in the cerebral cortex and hippocampus. Electroacupuncture did not affect the expression of EAAT1 in the cerebral cortex or hippocampus (Fig. 5).
Fig. 4. Electroacupuncture preconditioning improved histological and neurological function outcome after focal brain ischemia in excitatory amino acid transporter type 3 (EAAT3) knockout mice.
A: Brain sections after 2,3,5-triphenyltetrazolium chloride staining from representative mice in various groups. B: Percentage of brain infarct volume in ipsilateral hemisphere volume. Results are the means ± S.E.M. (n = 9). C: Neurological deficit scores evaluated immediately before the animals were euthanized for the assessment of infarct sizes (data are presented in panel B) or assigned 7 to the animals that died before this end time point for observation. Results are presented in a box plot format (n = 9 – 11). ●: lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25 – 75% interval including the median of the data. * P < 0.05 compared with middle cerebral arterial occlusion (MCAO) only group that inhaled oxygen 30 min each day for 5 days before the MCAO.
Fig. 5. Effects of electroacupuncture on excitatory amino acid transporter (EAAT) expression in EAAT type 3 knockout mice.
Cerebral cortex and hippocampus were harvested for Western blotting at 24 h after the last electroacupuncture without the MCAO. The EAAT1 and EAAT2 expression is presented in the panels A and B, respectively. Representative Western blots are shown on the top panel and the graphic presentation of the EAAT protein abundance quantified by integrating the volume of autoradiograms from 5 mice for each experimental condition is shown on the bottom panel. Values in graphs are the means ± S.E.M. * P < 0.05 compared with control mice inhaling oxygen 30 min each day for 5 days. GAPDH: glyceraldehydes 3-phosphate dehydrogenase; EA: electroacupuncture.
4. Discussion
Similar to our previous finding (Ma et al., 2011; Xiong et al., 2003), we showed here that electroacupuncture at Baihui reduced brain infarct volumes and improved neurological function in rats. These effects are specific due to electronic current stimulation to the acupoint because rats received pentobarbital or para-electroacupuncture did not have these beneficial effects. These results clearly suggest that electroacupuncture at certain acupoints can induce a preconditioning effect against focal brain ischemia. We selected Baihui because stimulation of this acupoint affects neurochemical expression. For example, Baihui stimulation increases brain-derived neurotrophic factor in the hippocampus (Hwang et al., 2010). In addition, Baihui stimulation has been shown to induce preconditioning effect in the brain (Ma et al., 2011; Wang et al., 2009).
Our previous study suggests that electroacupuncture preconditioning involves cannabinoid receptors 2 to provide neuroprotection (Ma et al., 2011). However, it is not clear which protective proteins may be induced by electroacupuncture for the preconditioning effects in the brain. Multiple studies have shown the protective role of EAATs, especially EAAT2 and EAAT3, in the brain against ischemia (Li and Zuo, 2011; Rao et al., 2001; Rothstein et al., 1996). As the first step to determine whether EAATs are involved in the electroacupuncture preconditioning-induced neuroprotection, we quantified the EAAT expression in the presence and absence of electroacupuncture. Our results showed that electroacupuncture at Baihui significantly increased EAAT2 in the cerebral cortex and that this increase persisted after the MCAO. We also showed that dihydrokainate attenuated the electroacupuncture preconditioning-induced neuroprotection. Dihydrokainate is a non-transportable EAAT inhibitor and is more than 130-fold selective for EAAT2 (Ki = 23 μM) over EAAT1 (Ki > 3 mM) and EAAT3 (Ki > 3 mM) (Arriza et al., 1994). Together, our results suggest the involvement of EAAT2 in this neuroprotection. Our results do not support the role of EAAT3 in the electroacupuncture preconditioning-induced neuroprotection because electroacupuncture at Baihui did not significantly increase EAAT3 expression and electroacupuncture at Baihui induced an increase of EAAT2 expression in the brain of the EAAT3 knockout mice and a preconditioning effect in these mice.
We showed that rats received dihydrokainate only had worse neurological function than rats in the control group. This result is consistent with the previous finding that increased EAAT2 before brain ischemia reduce ischemic brain injury (Chu et al., 2007). Our result also suggests an important role of basal EAAT2 expression in providing neuroprotection against brain ischemia.
There is strong evidence for the protective effects of EAAT2 in the literature. An early study showed that EAAT2 knockdown in rats induced excitotoxicity (Rothstein et al., 1996). This result is consistent with our finding that dihydrokainate alone worsened neurological deficit scores after the MCAO. Rats with EAAT2 knockdown have poorer neurological outcome after focal brain ischemia (Rao et al., 2001). Increased EAAT2 using ceftriaxone before permanent or transient focal brain ischemia improves neurological outcome (Chu et al., 2007). Increased EEAT2 also plays a role in the neuroprotection induced by ischemic preconditioning and citicoline (Hurtado et al., 2008; Romera et al., 2007). More importantly, patients with a polymorphism in the EAAT2 promoter that decreases EAAT2 expression also have a higher frequency of early neurological worsening (Mallolas et al., 2006). Similarly, there is evidence to suggest a protective role of EAAT3 because EAAT3 knockout mice have worse neurological outcome after brain ischemia (Li and Zuo, 2011; Won et al., 2010).
One important question that needs to be addressed is how EAATs may provide neuroprotection. Ischemia and reperfusion significantly increase extracellular glutamate concentrations, which is a major secondary insult after brain ischemia to cause cell injury (Lipton, 1999). EAATs may uptake and bind extracellular glutamate in the ischemic penumbral brain tissue and, therefore, be protective. EAAT3 is the major mechanism in mature neurons to take up cysteine that is a substrate for glutathione synthesis. Glutathione is a critical component for antioxidant defenses and intracellular zinc binding. Thus, lack of EAAT3 worsens the oxidative stress and increases intracellular free zinc, which leads to cell injury after brain ischemia (Li and Zuo, 2011; Won et al., 2010).
The possible involvement of EAATs in the preconditioning-induced neuroprotection is based on mainly two lines of evidence in the literature. The first line of evidence is that preconditioning stimuli, such as normobaric hyperoxia, increase the expression of EAATs (Bigdeli et al., 2008). The second line of evidence is that general EAAT inhibitors reduce neuroprotection induced by preconditioning stimuli, such as volatile anesthetics (Wang et al., 2007; Zheng and Zuo, 2003). However, it is not known yet which subtype of EAATs may be involved in the preconditioning-induced neuroprotection. Our data suggest the role of EAAT2 in the electroacupuncture preconditioning-induced neuroprotection.
We showed in this study that electroacupuncture preconditioning increased EAAT2 expression in the cerebral cortex but not in the hippocampus. This result suggests a brain region-specific effect of electroacupuncture. The reasons for this finding are not clear. However, it is known that an acupoint has certain functions and stimulation of the acupoint may only affect certain regions or organs.
In summary, we have shown that electroacupuncture at Baihui induces brain ischemic tolerance in rats and mice. This effect may be mediated by EAAT2.
Research highlights.
Electroacupunture preconditioning reduce ischemic brain injury in rats and mice
Electroacupunture increases glutamate transporter type 2 expression in rat cerebral cortex
Electroacupunture preconditioning in the brain may be mediated by glutamate transporter type 2
Acknowledgments
Funding: This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, by a grant from the National Natural Science Foundation of China (Grant number 81028006 to Z Zuo and S Chen) and the Robert M. Epstein Professorship endowment, University of Virginia.
Abbreviations
- EA
electroacupuncture
- EAATs
excitatory amino acid transporters
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- MCAO
middle cerebral arterial occlusion
Footnotes
Conflict of interest: No.
Authors’ role: Study design: XZ, JY, LL, SC and ZZ; conduct of study: XZ, JY, LL, LM, HT and JD; data analysis: XZ, JY, LL, LM, HT and ZZ; manuscript preparation: XZ, JY and ZZ; securing funding: SC and ZZ.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigdeli MR, Hajizadeh S, Froozandeh M, Heidarianpour A, Rasoulian B, Asgari AR, Pourkhalili K, Khoshbaten A. Normobaric hyperoxia induces ischemic tolerance and upregulation of glutamate transporters in the rat brain and serum TNF-alpha level. Exp Neurol. 2008;212:298–306. doi: 10.1016/j.expneurol.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Hoyert D, Xu M. Deaths: Preliminary Data for 2011. Natl Vital Stat Rep. 2012;61:1–52. [PubMed] [Google Scholar]
- Hurtado O, Pradillo JM, Fernandez-Lopez D, Morales JR, Sobrino T, Castillo J, Alborch E, Moro MA, Lizasoain I. Delayed post-ischemic administration of CDP-choline increases EAAT2 association to lipid rafts and affords neuroprotection in experimental stroke. Neurobiol Dis. 2008;29:123–131. doi: 10.1016/j.nbd.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Chung JY, Yoo DY, Yi SS, Youn HY, Seong JK, Yoon YS. Effects of electroacupuncture at Zusanli and Baihui on brain-derived neurotrophic factor and cyclic AMP response element-binding protein in the hippocampal dentate gyrus. J Vet Med Sci. 2010;72:1431–1436. doi: 10.1292/jvms.09-0527. [DOI] [PubMed] [Google Scholar]
- Lee SN, Li L, Zuo Z. Glutamate transporter type 3 knockout mice have a decreased isoflurane requirement to induce loss of righting reflex. Neurosci. 2010;171:788–793. doi: 10.1016/j.neuroscience.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neurosci. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Luo P, Wang Q, Xiong L. Electroacupuncture Pretreatment as a Novel Avenue to Protect Brain against Ischemia and Reperfusion Injury. Evid Based Complement Alternat Med. 2012;2012:195397. doi: 10.1155/2012/195397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhu Z, Zhao Y, Hou L, Wang Q, Xiong L, Zhu X, Jia J, Chen S. Cannabinoid receptor type 2 activation yields delayed tolerance to focal cerebral ischemia. Curr Neurovasc Res. 2011;8:145–152. doi: 10.2174/156720211795495394. [DOI] [PubMed] [Google Scholar]
- Mallolas J, Hurtado O, Castellanos M, Blanco M, Sobrino T, Serena J, Vivancos J, Castillo J, Lizasoain I, Moro MA, Davalos A. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J Exp Med. 2006;203:711–717. doi: 10.1084/jem.20051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Simpson EM, Takahashi JS, Lipp HP, Nakanishi S, Wehner JM, Giese KP, Tully T, Abel T, Chapman PF, Fox K, Grant S, Itohara S, Lathe R, Mayford M, McNamara JO, Morris RJ, Picciotto M, John Roder Hee-Sup Shin, et al. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Wang C, Lee J, Jung H, Zuo Z. Pretreatment with volatile anesthetics, but not with the nonimmobilizer 1,2-dichlorohexafluorocyclobutane, reduced cell injury in rat cerebellar slices after an in vitro simulated ischemia. Brain Res. 2007;1152:201–208. doi: 10.1016/j.brainres.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, Lu Y, Xiong L. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009;40:2157–2164. doi: 10.1161/STROKEAHA.108.541490. [DOI] [PubMed] [Google Scholar]
- Won SJ, Yoo BH, Brennan AM, Shin BS, Kauppinen TM, Berman AE, Swanson RA, Suh SW. EAAC1 gene deletion alters zinc homeostasis and exacerbates neuronal injury after transient cerebral ischemia. J Neurosci. 2010;30:15409–15418. doi: 10.1523/JNEUROSCI.2084-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Lu Z, Hou L, Zheng H, Zhu Z, Wang Q, Chen S. Pretreatment with repeated electroacupuncture attenuates transient focal cerebral ischemic injury in rats. Chin Med J (Engl) 2003;116:108–111. [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning reduces Purkinje cell death in an in vitro model of rat cerebellar ischemia. Neurosci. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of P38 mitogen-activated protein kinases. Mol Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]