Abstract
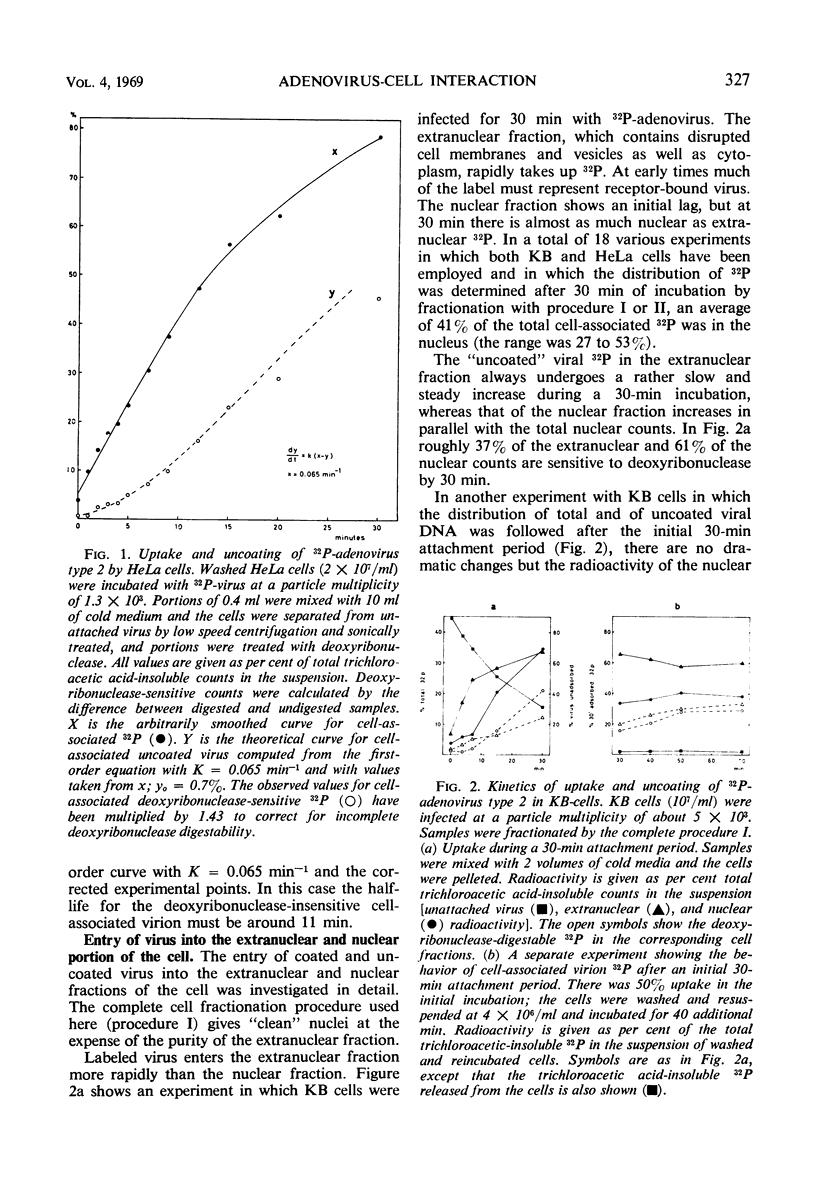
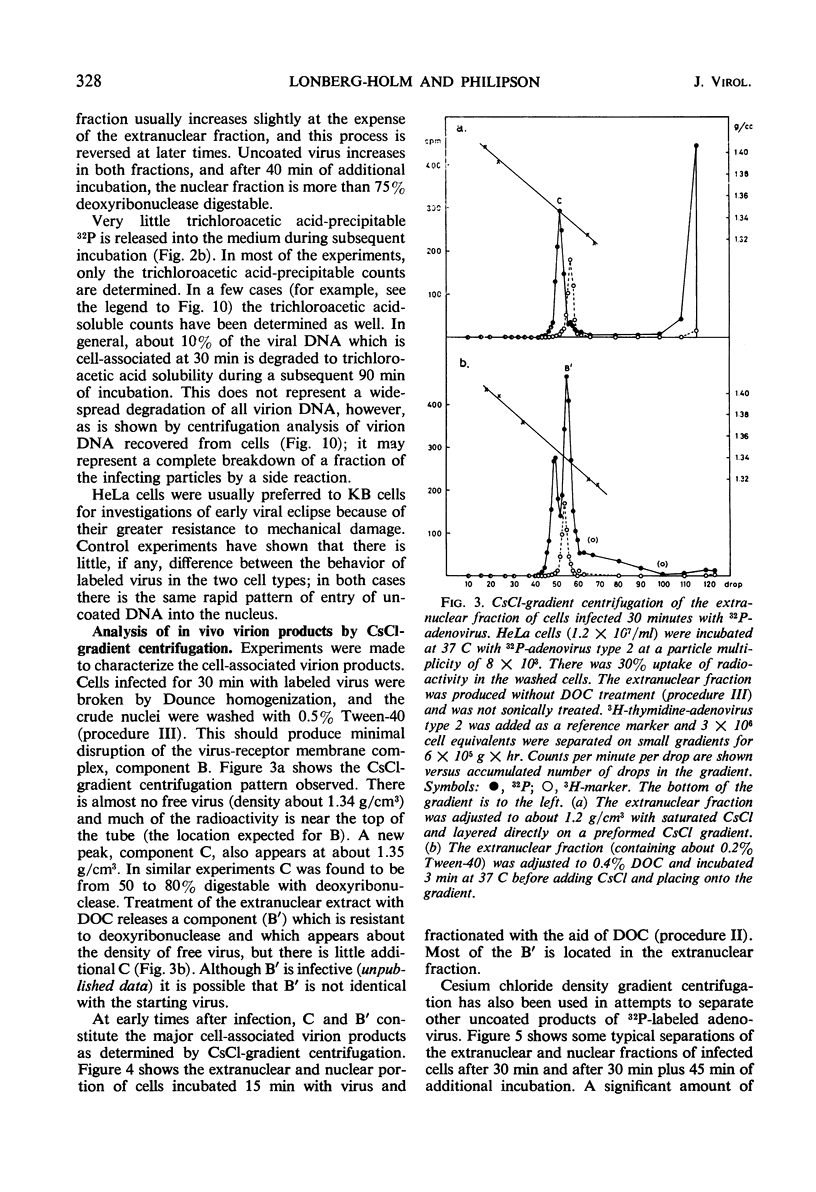
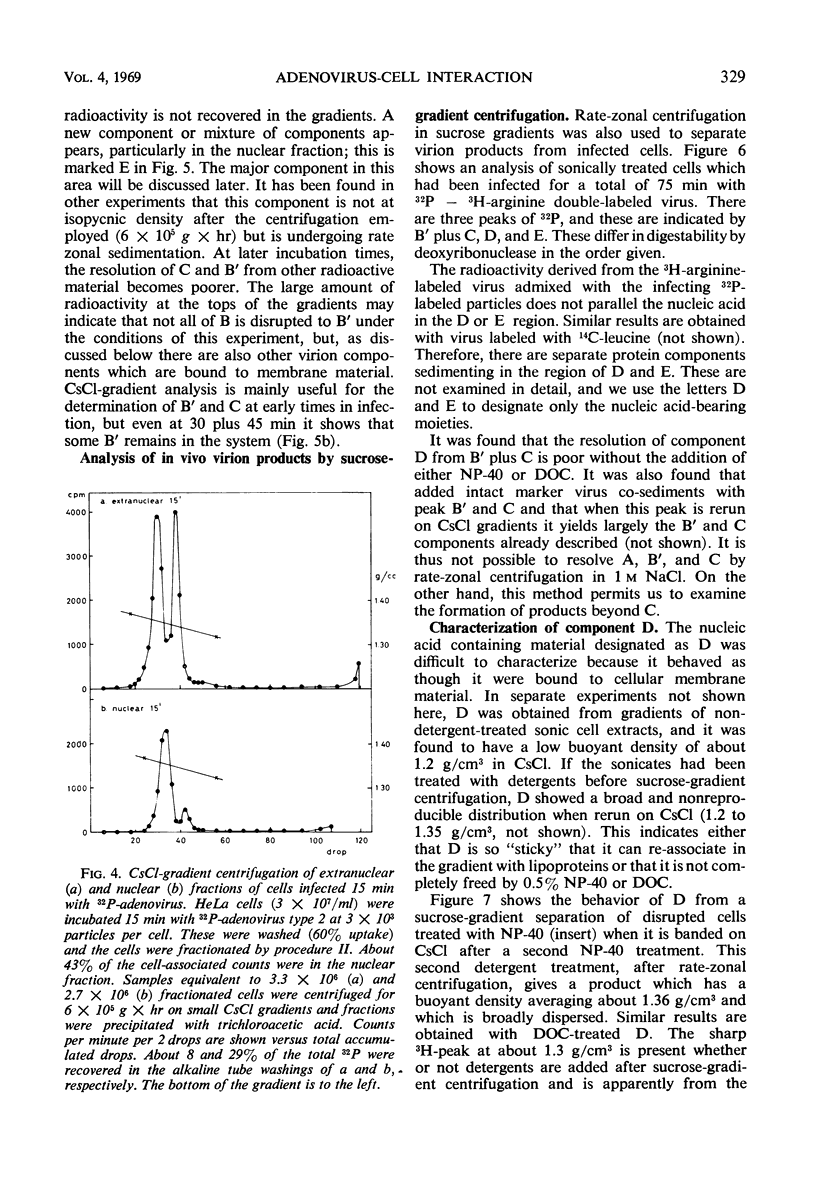
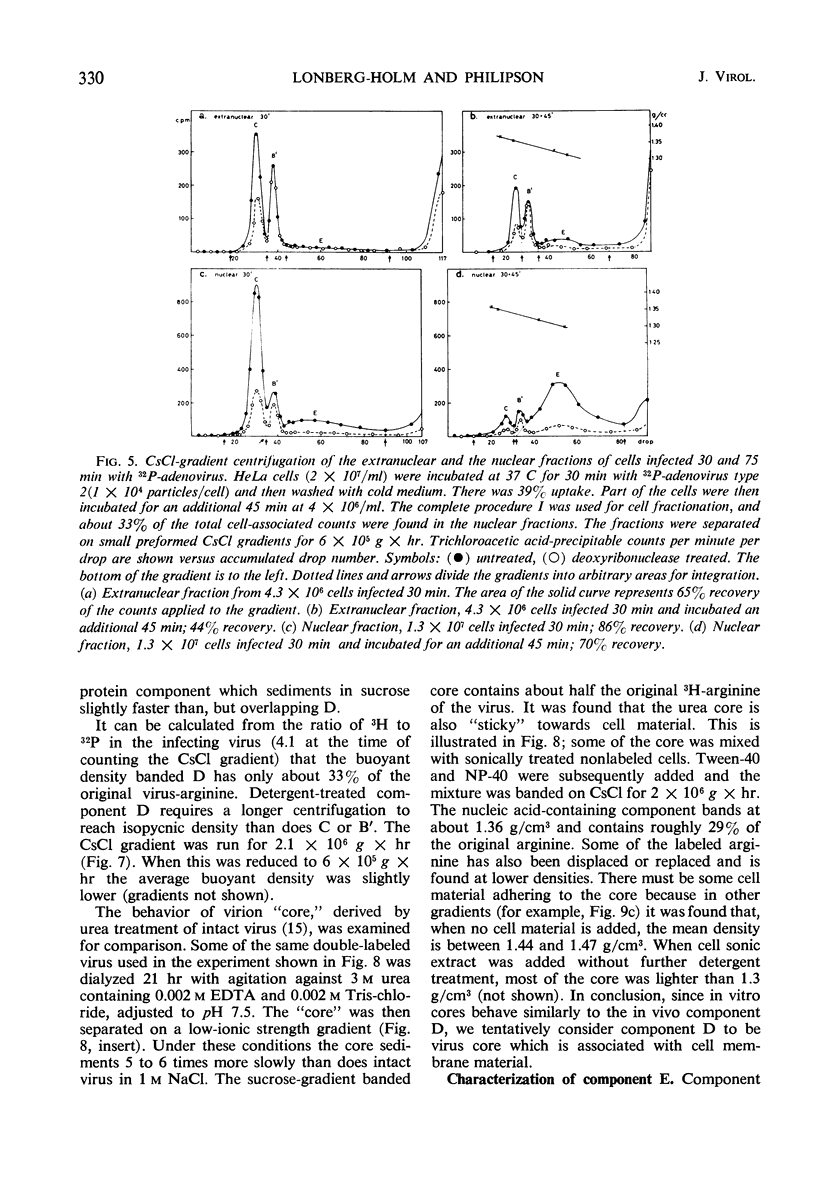
The interaction of 32P-labeled adenovirus type 2 and HeLa or KB cells has been examined during early infection. The kinetics of virus uncoating to deoxyribonuclease-sensitive products, the partial characterization of three such products by gradient centrifugation, and the distribution of these products in the extranuclear and nuclear portions of infected cells are reported. The results are compatible with the following model. Extracellular virus attaches to a receptor on the plasma membrane. The membrane-bound virus has a half-life of less than 15 min and is transformed to a partly uncoated product which is free inside the cell and about half of which rapidly enters the cell nucleus. This is rapidly transformed, in both cytoplasm and nucleus, to a membrane-bound virion “core.” The proteins of the bound “core” are then removed from the intact virus deoxyribonucleic acid (DNA). In the nucleus, viral DNA is the main product and there the overall sequence is completed in about 2 hr.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg E., Becker Y. Adsorption, penetration and uncoating of herpes simplex virus. J Gen Virol. 1968 Mar;2(2):231–241. doi: 10.1099/0022-1317-2-2-231. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Smith I., Penman S. Electron microscopic studies of detergent-treated HeLa cell nuclei. J Mol Biol. 1966 May;17(1):131–135. doi: 10.1016/s0022-2836(66)80099-2. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The molecular basis of the viral eclipse phase. Prog Med Virol. 1965;7:44–96. [PubMed] [Google Scholar]
- LACY S., GREEN M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. VII. HOMOLOGY BETWEEN DNA'S OF TUMORIGENIC AND NONTUMORIGENIC HUMAN ADENOVIRUSES. Proc Natl Acad Sci U S A. 1964 Oct;52:1053–1059. doi: 10.1073/pnas.52.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G., Pereira H. G., Russell W. C., Valentine R. C. Isolation of an internal component from adenovirus type 5. J Mol Biol. 1968 Nov 14;37(3):379–386. doi: 10.1016/0022-2836(68)90109-5. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968 Sep;36(1):126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Hartzell R. W., Rubin B. A. Solubilization and some properties of the erythrocyte receptor for adenovirus type 7 haemagglutinin. Nature. 1969 Mar 15;221(5185):1069–1071. doi: 10.1038/2211069a0. [DOI] [PubMed] [Google Scholar]
- Norrby E. Biological significance of structural adenovirus components. Curr Top Microbiol Immunol. 1968;43:1–43. doi: 10.1007/978-3-642-46118-7_1. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E. Ribosomal RNA synthesis and processing in a particulate site in the HeLa cell nucleus. Science. 1966 Nov 11;154(3750):786–789. doi: 10.1126/science.154.3750.786. [DOI] [PubMed] [Google Scholar]
- Philipson L. Attachment and eclipse of adenovirus. J Virol. 1967 Oct;1(5):868–875. doi: 10.1128/jvi.1.5.868-875.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Philipson L. Internal basic proteins in adenovirus. Virology. 1968 Nov;36(3):508–511. doi: 10.1016/0042-6822(68)90178-5. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Laver W. G., Sanderson P. J. Internal components of adenovirus. Nature. 1968 Sep 14;219(5159):1127–1130. doi: 10.1038/2191127a0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Valentine R. C., Pereira H. G. The effect of heat on the anatomy of the adenovirus. J Gen Virol. 1967 Oct;1(4):509–522. doi: 10.1099/0022-1317-1-4-509. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. W. Adenoviruses: the nature of the virion and of controlling factors in productive or abortive infection and tumorigenesis. Adv Virus Res. 1969;14:1–61. doi: 10.1016/s0065-3527(08)60556-4. [DOI] [PubMed] [Google Scholar]
- Thiel J. F., Smith K. O. Fluorescent focus assay of viruses on cell monolayers in plastic Petri plates. Proc Soc Exp Biol Med. 1967 Jul;125(3):892–895. doi: 10.3181/00379727-125-32232. [DOI] [PubMed] [Google Scholar]



