Abstract
Objectives
To investigate the mechanism by which BRL37344, a β3-adrenergic receptor (β3-ARs) agonist, facilitate the inhibition of nerve-evoked contractions in human detrusor smooth muscle (DSM) isolated strips and to identify the role of large-conductance Ca2+-activated K+ (BK) channels in this process.
Methods
Human DSM specimens were obtained from open bladder surgeries on patients without preoperative history of overactive bladder (OAB) symptoms. Isometric DSM tension recordings were conducted using force-displacement transducers and thermostatically controlled tissue baths. Nerve-evoked contractions were generated by electrical field stimulation (EFS).
Results
BRL37344, a β3-AR agonist, significantly decreased the amplitude, muscle force, and duration of the DSM contractions induced by 20 Hz EFS, in a concentration-dependent manner. This BRL37344-mediated inhibition of the amplitude and muscle force of the nerve-evoked DSM contraction was significantly reduced by iberiotoxin, a highly selective inhibitor of the BK channel, revealing a role for BK channels in the β3-AR-induced inhibition of human DSM nerve-evoked contractions. We further used atropine, α,β-methylene-ATP, and suramin to separate the cholinergic and purinergic components of human DSM nerve-evoked contractions. We found that the β3-AR agonist, BRL37344, inhibited both components of the EFS-induced (0.5–50 Hz) DSM contractions.
Conclusions
This study supports the concept that β3-AR agonists inhibit nerve-evoked contractions in human DSM. We have further revealed that BK channels play a critical role in BRL37344-mediated relaxation of nerve-evoked contractions in human DSM. The study suggests that in addition to β3-ARs, BK channels may also represent promising pharmacological targets in the treatment of urinary bladder dysfunction.
Keywords: Urinary bladder, iberiotoxin, nerve-evoked contractions, atropine
INTRODUCTION
Overactive bladder (OAB) is a symptomatic condition characterized by urinary urgency, frequent urination, with or without urinary incontinence. The current therapeutic approaches for OAB, which rely primarily on behavioral therapies in combination with antimuscarinic drugs and a number of surgical techniques, have variable outcomes and numerous adverse effects. These drawbacks in treating OAB have led researchers to investigate alternative therapeutic approaches with novel mechanisms of action, better efficacy, and fewer unfavorable effects.
β-adrenergic receptors (β-ARs), β3-AR in particular, have emerged as targets for treatment of OAB as pharmacological activation of these receptors results in detrusor smooth muscle (DSM) relaxation(1, 2). β3-AR agonists including BRL37344 ((±)-(R*,R*)-[4-[2-[[2-(3-Chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetic acid sodium hydrate), mirabegron (YM178), solabegron (GW427353), and ritobegron (KUC-7483) have been demonstrated to induce relaxation of human and rodent DSM spontaneous, nerve-evoked, and pharmacologically-induced phasic contractions(2–5)_ENREF_4. In human DSM isolated strips, BRL37344 has been shown to inhibit carbachol-induced tone in a concentration-dependent manner (pEC50=6.25)(6). In fact, BRL37344 caused a 22% and 47% inhibition of human DSM carbachol-induced tone when used at a concentration of 10 μM and 100 μM, respectively(6). Based on numerous studies demonstrating the prominent role of β3-ARs in human DSM relaxation both in vitro and in vivo, mirabegron has received regulatory approval as the first β3-AR agonist for OAB treatment in Japan, Europe, and the United States. So far, the clinical use of mirabegron for OAB has not pointed toward substantial adverse effects as compared to the antimuscarinics.
The mechanism of β-AR-induced relaxation of DSM is thought to involve activation of the large-conductance Ca2+-activated K+ (BK) channel and reduction of DSM excitability(1, 7–13). In animal species, the BK channels are one of the most important physiologically relevant K+ channels that control DSM function(1, 2, 7, 9, 10, 14). Recent studies further demonstrated the critical role of BK channels in regulating human DSM excitability and contractility(15, 16)_ENREF_12. Collectively, such studies indicate a functional link between β-AR signaling and BK channels(1, 2, 9, 11–13).
However, the potential existence of such a functional link between β3-ARs and BK channels during nerve-evoked contractions has not been investigated. To investigate this mechanism, we used thermostatically-controlled tissue baths equipped with platinum electrodes for electrical field stimulation (EFS) to generate nerve-evoked contractions in human DSM isolated strips. The relationship between the effects of BRL37344 and iberiotoxin, a highly selective BK channel inhibitor, were examined to elucidate the underlying mechanisms involved in the β3-AR agonist-induced relaxation of human DSM and the role of the BK channel in this process. Having access to freshly-isolated and clinically-characterized human DSM tissues, the implications of the present study provide profound insight into the role of BK channels in the mechanism of action of β3-AR agonists in the treatment of OAB.
METHODS
Human DSM tissue collection
Human studies were conducted according to the reviewed and approved institutional review board protocol HR#16918 of the Medical University of South Carolina (MUSC). For these studies, DSM specimens isolated from 14 patients (11 males and 3 females, 49–76 years old) were used. Human samples were collected from patients without a preoperative history of OAB symptoms during surgeries such as radical cystectomy for bladder cancer and other open bladder surgeries for malignant or non-malignant conditions of the lower urinary tract. The human DSM specimens were stored in Ca2+-free dissection solution.
Isometric DSM tension recordings
Isometric DSM tension recordings were conducted as previously described(15, 16). Briefly, mucosa-free DSM tissues from humans were dissected into strips 5–7 mm long and 2–3 mm wide. DSM strips were clipped between a stationary mount and a force-displacement transducer then placed in tissue baths filled with Ca2+-containing physiological saline solution (PSS) (§Solutions and Drugs) thermostatically controlled at 37°C and aerated with 95% O2 and 5% CO2. Tissue baths were equipped with platinum electrodes for EFS. EFS pulses had 20 V amplitude, 0.75 ms width, 3 s stimulus duration, and polarity was reversed for alternating pulses. Then, DSM strips were stretched to 10 mN of initial tension and the bath solution was changed with fresh PSS every 15 min during an equilibration period of 45 to 60 min. Following the equilibration period, two different EFS protocols were generated using PHM-152I stimulator (Med Associates, Inc., Georgia, VT) and the DSM response to EFS was recorded using MyoMed software (Med Associates, Inc., Georgia, VT). Compounds were applied only to DSM strips with stable pre-compound controls following the equilibration period. In the first EFS protocol, a 20 Hz EFS frequency was applied continuously every minute to generate DSM nerve-evoked contractions. In the second EFS protocol, nerve-evoked DSM contractions were generated by applying increasing EFS frequencies (0.5, 2, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, 50 Hz) every 3 min. We evaluated the BRL37344 inhibitory effects on DSM contractions induced by EFS in the absence or presence of iberiotoxin, a selective BK channel blocker; atropine, a cholinergic blocker; suramin, a purinergic receptor blocker; and α,β-methylene-ATP, a purinergic receptor agonist.
Solutions and Drugs
The Ca2+-free dissection solution had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 N-2-hydroxyethylpiperazine-N'-2-ethanesulphonic acid (HEPES), 2 MgCl2, and pH 7.3 adjusted with NaOH. The Ca2+-containing physiological saline solution was prepared daily and contained (mM): 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 11 glucose, and aerated with 95% O2/5% CO2 to obtain pH 7.4. All drugs were purchased from Sigma-Aldrich Co. (St. Louis, MO), unless specified otherwise. BRL37344 was prepared daily in double-distillated water and heated at 60°C to be completely dissolved at the concentration of 10 mM as a stock solution as recommended by the manufacturer.
Data analysis and statistics
For the 20 Hz EFS-induced contractions, a 5 min period prior to the first BRL37344 application (10 nM) was taken as control (100%) and the responses to subsequent BRL37344 application (10 nM–100 μM) were normalized to that control. During cumulative addition of BRL37344, the effect of each BRL37344 concentration (10 nM–100 μM) on EFS-induced contraction amplitude, duration, and muscle force (determined by integrating the area under the curve of the nerve-evoked contractions) was evaluated by analyzing the 5 min period prior to the following BRL37344 concentration application. For the 0.5–50 Hz EFS-induced contractions, the contraction amplitude at EFS frequency of 50 Hz prior to BRL37344 application (control conditions) was taken to be 100% and the data were normalized. MiniAnalysis software version 6.0.7 (Synaptosoft, Inc., Decatur, GA) was used to analyze data of the EFS-induced contractions. GraphPad Prism 4.03 software (GraphPad Software Inc., La Jolla, CA, USA) was used for further statistical analysis. CorelDraw Graphic Suite X3 software (Corel Co., Ottawa, Canada) was used for data illustration. Results are summarized as mean ± SEM. Frequency-response curves were compared for statistical significance using paired or unpaired Student's t-test based on n, the number of DSM strips isolated from different patients (N=number of patients). A P-value <0.05 was considered statistically significant.
RESULTS
BRL37344 inhibits EFS-induced contractions of human DSM strips in a concentration-dependent manner
We investigated the effects of BRL37344 on the amplitude, duration, and muscle force of EFS-induced DSM contractions generated by 20 Hz EFS (applied each min) in human DSM isolated strips. BRL37344 (10 nM–100 μM) was very effective at inhibiting the amplitude, duration, and force of the 20 Hz EFS-induced contractions of human DSM isolated strips. BRL37344 (10 nM, 100 nM, 1 μM, 10 μM, and 100 μM) reduced the amplitude of the EFS-induced contractions by 11.6±3.9%, 22.9±8.2%, 33.3±10.3%, 45.5±10.4%, and 71.9±6.1%, respectively (n=4, N=3; Fig. 1A, C). BRL37344 (10 nM, 100 nM, 1 μM, 10 μM, and 100 μM) also decreased the force of the EFS-induced contractions by 7.0±5.2%, 33.2±4.9%, 46.2±9.1%, 57.7±7.9%, and 78.4±4.4%, respectively (n=4, N=3; Fig. 1A, D); and contraction duration by 3.6±5.4%, 5.9±0.4%, 15.0±1.8%, 21.3±2.6%, and 31.9±2.2%, respectively (n=4, N=3; Fig. 1A, E). These data suggest that BRL37344 effectively decreases nerve-evoked contractions in human DSM isolated strips.
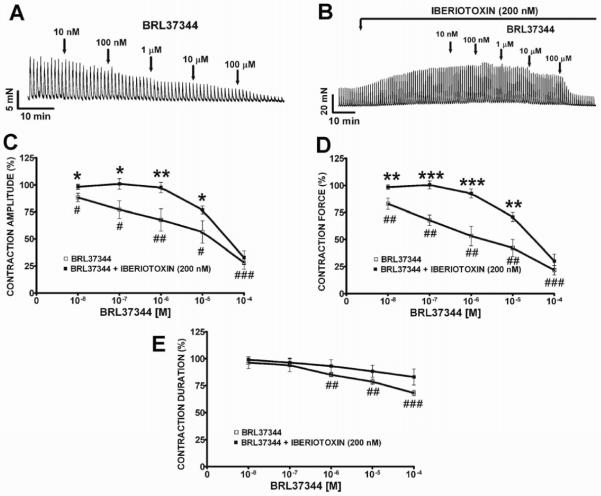
Figure 1. Iberiotoxin, a selective BK channel blocker, significantly reduced the BRL37344 inhibitory effects on 20 Hz EFS-induced contractions in human DSM isolated strips.
A) An original DSM tension recording illustrating BRL37344 (10 nM-100 μM) inhibitory effects on 20 Hz EFS-induced contractions in a human DSM isolated strip. B) An original DSM tension recording illustrating that iberiotoxin (200 nM) increased the 20 Hz EFS-induced contractions in human DSM and that the BRL37344 (10 nM-100 μM) inhibitory effects on the EFS-induced contraction amplitude, force, and duration were significantly attenuated in the presence of iberiotoxin (200 nM). C–E) Cumulative concentration-response curves illustrating iberiotoxin (200 nM) antagonistic action on BRL37344 inhibitory effects on EFS-induced contraction amplitude (C), force (D), and duration (E), respectively (n=4, N=3; #P<0.05, ##P<0.01, ###P<0.005 vs. control; *P<0.05, **P<0.01, ***P<0.005 vs. iberiotoxin).
BRL37344 inhibitory effect on EFS-induced contractions is reduced by iberiotoxin, a selective BK channel blocker
In this experimental series, we investigated the relationship between β3-ARs and BK channels in nerve-evoked DSM contractions. We found that in the presence of iberiotoxin (200 nM), BRL37344 (10 nM–100 μM) inhibitory effects on the 20 Hz EFS-induced contractions amplitude and force of human DSM isolated strips were significantly reduced (Fig. 1B, C, and D). These effects were statistically significant at lower BRL37344 concentrations (10 nM–10 μM), and not so obvious at higher concentrations (10 μM) at which BRL37344 may have some non-specific effects(17). The maximal antagonistic activity of iberiotoxin was observed at 1 μM BRL37344 where the BRL37344 inhibitory effects on the EFS-induced contraction amplitude and force were reduced from 32.4±10.3% to only 2.4±0.3% and from 46.2±9.1% to 7.4±4.2%, respectively (Fig. 1C and D). These findings suggest that BK channels and β3-ARs work in synergy to oppose nerve-evoked contractions in human DSM.
BRL37344 decreases the amplitude of the EFS-induced DSM contractions in a wide range of stimulation frequencies
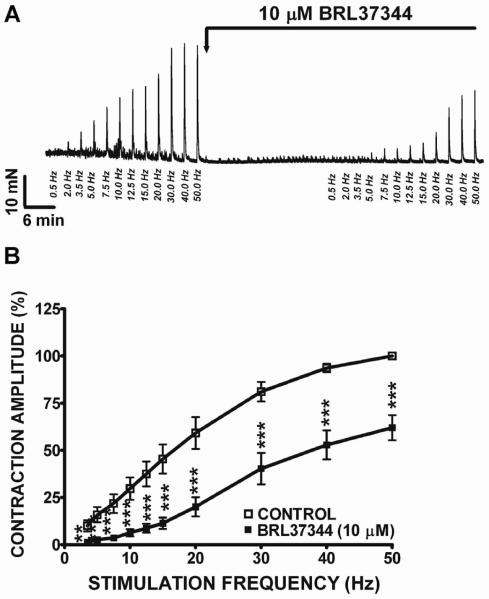
Here, we investigated how the β3-AR agonist, BRL37344, modulates DSM nerve-evoked contractions in response to a wide range of EFS frequencies in human DSM isolated strips. We first applied increasing EFS frequencies (0.5–50 Hz) as a control protocol, followed by the addition of a single concentration of BRL37344 (10 μM). Then, a second EFS protocol was applied to evaluate BRL37344 effects on the nerve-evoked contractions. In human DSM isolated strips, BRL37344 (10 μM) significantly decreased the amplitude of the EFS-induced contractions (Fig. 2A). At the maximum EFS frequency of 50 Hz, BRL37344 (10 μM) decreased human DSM EFS-induced contraction amplitude by 38.1±6.6% (n=8, N=4; Fig. 2B). We also applied a higher concentration of BRL37344 (100 μM) which caused almost a complete inhibition of the EFS-induced DSM contractions. At the maximum EFS frequency of 50 Hz, BRL37344 (100 μM) decreased human DSM EFS-induced contraction amplitude by 85.4±2.1% (n=11, N=3; data not illustrated). These data suggest that BRL37344 reduces human DSM nerve-evoked contractions induced by EFS at a wide range of stimulation frequencies.
Figure 2. BRL37344 decreases the amplitude of the EFS-induced contractions in human DSM isolated strips in a wide range of stimulation frequencies.
A) An original DSM tension recording illustrating the inhibitory effects of 10 μM BRL37344 on 0.5–50 Hz EFS-induced contractions of human DSM. B) Frequency-response curves showing 0.5–50 Hz EFS-induced contractions in response to 10 μM BRL37344 (n=8, N=4; **P<0.01, ***P<0.005).
BRL37344 attenuates both purinergic and cholinergic components of EFS-induced contractions in human DSM isolated strips
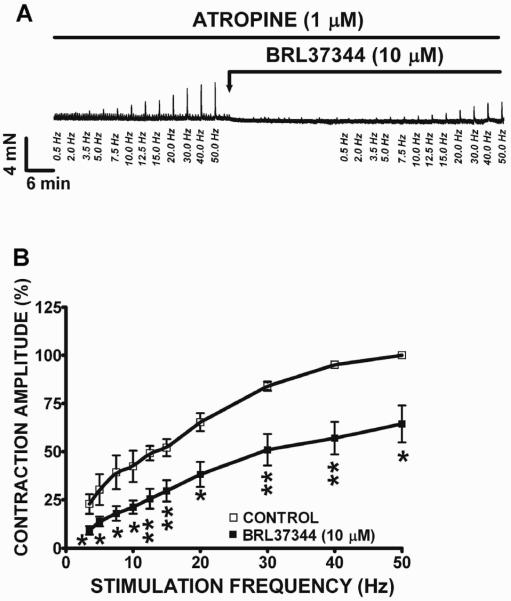
In this experimental series, we applied an experimental approach that allowed us to separate the cholinergic component from the purinergic component during EFS-induced DSM contractions. The cholinergic component of the EFS-induced DSM contractions was assessed by blocking purinergic receptors with α,β-methylene-ATP, a desensitizing agonist; and suramin, a purinergic receptor antagonist. Previous studies have shown that the combined use of these two compounds completely blocks the purinergic component of the nerve-evoked contractions in DSM(18, 19). Human DSM isolated strips were pre-incubated with suramin (10 μM) and α,β-methylene-ATP (10 μM) for 15 min prior to applying a 0.5–50 Hz EFS control protocol. Next, BRL37344 was added in the bath for 30 min followed by a second EFS protocol. The purinergic component of the EFS-induced contractions was assessed by pre-treating the DSM strips with 1 μM atropine for 15 min prior to applying a 0.5–50 Hz EFS control protocol in the presence or absence of BRL37344. In human DSM isolated strips, BRL37344 (10 μM) caused a significant decrease in the amplitude of the EFS-induced contraction in the presence of atropine at frequencies ranging from 3.5 to 50 Hz (Fig. 3A, B). At the maximal stimulation frequency of 50 Hz, BRL37344 (10 μM) caused a 35.6±9.6% decrease in the amplitude of DSM EFS-induced purinergic contractions in human DSM (n=4, N=3; Fig. 3B). BRL37344 (100 μM) had a more pronounced effect compared to BRL37344 (10 μM), and reduced the EFS-induced purinergic contraction amplitude of human DSM strips by 68.6±12.1% at 50 Hz-frequency (n=4, N=3; data not illustrated).
Figure 3. BRL37344 significantly inhibited the purinergic component of the 0.5–50 Hz EFS-induced contractions in human DSM isolated strips.
A) An original DSM tension recording illustrating BRL37344 (10 μM) inhibitory effects on EFS-induced contractions of human DSM isolated strips in the presence of atropine (1 μM). B) EFS frequency-response curves showing 10 μM BRL37344 inhibitory effects on nerve-evoked contractions of human DSM isolated strips (n=4, N=3; *P<0.05, **P<0.01).
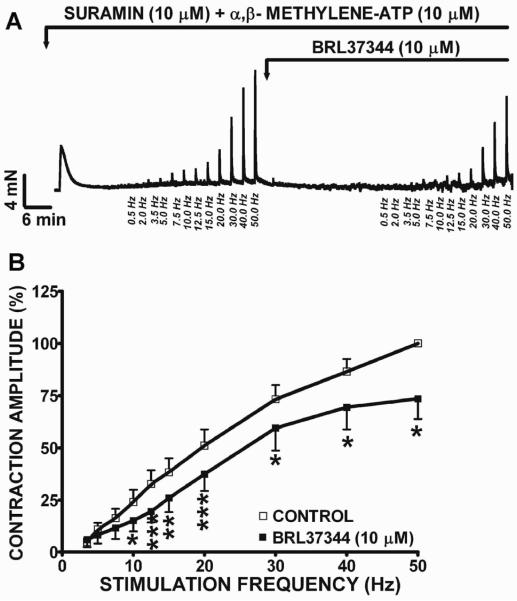
In the presence of suramin (10 μM) and α,β-methylene-ATP (10 μM), BRL37344 also significantly decreased the amplitude of the EFS-induced cholinergic contractions in human DSM isolated strips at EFS frequencies from 10 to 50 Hz (Fig. 4A and B). At the maximal frequency of 50 Hz, BRL37344 (10 μM and 100 μM) decreased the EFS-induced cholinergic contraction amplitude of human DSM strips by 26.5±9.7% (n=8, N=5, P<0.05; Fig. 4B) and 91.0±5.3% (n=4, N=3; P<0.05; data not illustrated), respectively. These data suggest that BRL37344 inhibits both the cholinergic and the purinergic components of the EFS-induced contractions in human DSM.
Figure 4. BRL37344 reduced the cholinergic component of the 0.5–50 Hz EFS-induced contractions of the human DSM.
A) An original DSM tension recording illustrating BRL37344 (10 μM) inhibitory effects on human DSM EFS-induced contractions in the presence of suramin (10 μM) and α,β-methylene-ATP (10 μM). B) Frequency-response curves illustrating BRL37344 (10 μM) inhibitory effects on EFS-induced contraction amplitude of human DSM isolated strips in the presence of suramin and α,β-methylene-ATP (n=8, N=5; *P<0.05, **P<0.01, ***P<0.005).
COMMENT
The present study demonstrates that the β3-AR agonist, BRL37344, effectively inhibits both the purinergic and cholinergic components of human DSM nerve-evoked contractions. This BRL37344 inhibitory effect was significantly reduced by iberiotoxin, a BK channel selective inhibitor suggesting that functional BK channels play a critical role in the β3-AR-mediated relaxation of human DSM nerve-evoked contractions.
In mouse, rat, guinea pig, and human DSM, it has been shown that physiological DSM nerve-evoked contractions are due to the combined action of two main excitatory neurotransmitters, acetylcholine and ATP, released from the parasympathetic nerves(18–22). ATP activates P2X receptors while acetylcholine stimulates M2/M3 muscarinic receptors to induce DSM contractions(18–25). Acetylcholine activation of M2 muscarinic receptors causes inhibition of adenylyl cyclase activity while activation of M3 receptors triggers the phospholipase-C and inositol 1,4,5-trisphosphate pathways(26, 27). This dual muscarinic receptor action ultimately leads to DSM contraction. We found that low concentrations of BRL37344 (10 nM–1 μM) decreased the amplitude of nerve-evoked contractions in human DSM consistent with previous studies demonstrating that BRL37344 could inhibit human DSM carbachol-induced tone(6). Our study suggests that β3-ARs play an important role in opposing human DSM nerve-evoked contractions. Our data are also consistent with previous findings showing that KUC-7322 and GW427353, two selective β3-AR agonists, also decreased carbachol-induced and nerve-evoked contractions in human DSM(3–5)_ENREF_6. Unlike the current study, these latter studies did not address the potential involvement of BK channels in this process. Previously, we demonstrated that BRL37344 effectively inhibits spontaneous phasic contractions of rat DSM isolated strips(2). This BRL37344 inhibitory effect was further demonstrated to be antagonized by SR59230A, a â3-AR antagonist, and H89, a protein kinase-A inhibitor(2).
Recently, our laboratory and others have demonstrated the critical role played by BK channels in regulating DSM spontaneous phasic contractions(1, 2, 10, 14–16). BK channels, which are activated by Ca2+ sparks released from the sarcoplasmic reticulum through the ryanodine receptors, regulate DSM function by opposing the phasic contractions induced by Ca2+ entry through L-type voltage-gated Ca2+ channels(2, 9, 10). Our group was the first to provide evidence for a functional link between β3-ARs and BK channels in rat DSM cells(2). Furthermore, in guinea pig, isoproterenol, a non-selective β-AR agonist, increases Ca2+ spark activity which activates BK channels and induces relaxation of the DSM(9). Iberiotoxin shifts the concentration-response curves for the BRL37344 inhibitory effects on the spontaneous phasic contraction amplitude, muscle force integral, and muscle tone, to the right suggesting that the pharmacological blockage of BK channels opposes β3-AR-mediated relaxation of rat DSM myogenic contractions(2). However, until now this mechanism has never been investigated in human DSM nerve-evoked contractions. Here, we reveal for the first time the role of BK channels in the β3-AR-induced relaxation of human DSM nerve-evoked contractions. BRL37344 decreased human DSM nerve-evoked contractions in a concentration-dependent manner (Fig. 1). At concentrations ranging between 10 nM and 10 μM, the BRL37344 inhibitory effect was significantly antagonized by iberiotoxin suggesting that functional BK channels play an important role in the β3-AR-mediated relaxation of human DSM nerve-evoked contractions (Fig. 1B, C and D). It has been shown that at sub-micromolar concentrations, BRL37344 may also activate β2-ARs(28). Regardless of the potential BRL37344 effect on β2-ARs, one would anticipate that the majority of the BRL37344 effect was mediated via activation of the β3-ARs since β3-ARs represent ~97% of all β-AR mRNA expressed in the human bladder(29) and numerous studies have demonstrated that relaxation of human DSM strips is mediated predominantly by the β3-ARs(17). It should be noted that β3-ARs and BK channels are expressed not only in the DSM cells but are widely present in the body. However, because the BK channels appear to be restricted to DSM cells with no detectable expression in the DSM nerves(19), the effects of iberiotoxin most likely occur at the level of the smooth muscle cells where BRL37344 acts directly and not at the level of the bladder nerves by modulating neurotransmitter release.
Using selective inhibitors, we further separated the purinergic and cholinergic pathways both of which contribute to DSM nerve-evoked contractions. Previous studies have established that at low stimulation frequencies (≤ 20 Hz), the purinergic pathway plays a greater role while at high stimulation frequencies (≥ 20 Hz), the cholinergic component predominates(18, 19). Inhibitory effects of nonselective β-AR agonists on various non-cholinergic stimuli have been reported in rat DSM(30). However, unlike our study on human DSM, this previous study on rat DSM used a non-selective β-AR agonist, isoproterenol, and did not apply EFS(30). A novel aspect of our study is that in the presence of atropine, which was used to block the cholinergic component of the nerve-evoked contractions, we found that β3-AR activation decreased the amplitude of the nerve-evoked contractions in human DSM in a wide range of stimulation frequencies (Fig. 3). While at higher concentrations BRL37344 may have some non-selective antimuscarinic properties(17), BRL37344 retained its ability to inhibit the EFS-induced DSM contraction in the presence of atropine (Fig. 3) indicating that the observed BRL37344 inhibitory effects were not due to its antimuscarinic properties. In the presence of suramin and α,β-methylene-ATP, which were used to block the purinergic component, BRL37344 also significantly decreased the amplitude of the nerve-evoked contractions in human DSM (Fig. 4). Taken together, these data suggest that BRL37344 inhibits both cholinergic and purinergic contractions of human DSM.
CONCLUSION
The present study reveals that the β3-AR agonist, BRL37344, is very effective in reducing human DSM nerve-evoked contractions. We reveal for the first time that the β3-AR-agonist-mediated relaxation of human DSM nerve-evoked contractions is BK channel-dependent, emphasizing the critical role of BK channels in human DSM physiology. Future studies using DSM tissue from patients with OAB and detrusor overactivity are anticipated to demonstrate a similar functional relationship between β3-ARs and the BK channel, which would provide further impetus for studying potential pharmacologic targets in this area for the treatment of OAB.
ACKNOWLEDGEMENTS
We would like to thank MUSC Urology staff surgeons: Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames and Jonathan Picard, and Ahmed M. El-Zawahry, as well as MUSC Residents: Matthew McIntyre, Jonathan N. Hamilton, Robin Bhavsar, Timothy R. Yoost, Vinh Q. Trang, Lydia Labocetta, Elizabeth Peacock, Matthew Young, Erin Burns, Vaughan Taylor, and Samuel Walker Nickles for their help with human tissue collection; We also thank Drs. John Malysz, Wenkuan Xin, Kiril Hristov, Shankar Parajuli, Ms. Amy Smith, Mr. Qiuping Cheng, and Mr. Ning Li for the critical evaluation of the manuscript.
GRANTS This study was supported by a grant from the National Institutes of Health DK084284 to Georgi V. Petkov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST The authors declare no conflict of interests.
REFERENCES
- 1.Brown SM, Bentcheva-Petkova LM, Liu L, et al. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol. 2008;295(4):F1149–57. doi: 10.1152/ajprenal.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hristov KL, Cui X, Brown SM, et al. Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2008;295(5):C1344–53. doi: 10.1152/ajpcell.00001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biers SM, Reynard JM, Brading AF. The effects of a new selective beta3-adrenoceptor agonist (GW427353) on spontaneous activity and detrusor relaxation in human bladder. BJU Int. 2006;98(6):1310–4. doi: 10.1111/j.1464-410X.2006.06564.x. [DOI] [PubMed] [Google Scholar]
- 4.Igawa Y, Schneider T, Yamazaki Y, et al. Functional investigation of beta-adrenoceptors in human isolated detrusor focusing on the novel selective beta3-adrenoceptor agonist KUC-7322. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(8):759–67. doi: 10.1007/s00210-012-0763-x. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi P, Thomas CA, Yoshimura N, Chancellor MB. Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol. 2009;35(1):76–83. doi: 10.1590/s1677-55382009000100012. [DOI] [PubMed] [Google Scholar]
- 6.Takeda M, Obara K, Mizusawa T, et al. Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther. 1999;288(3):1367–73. [PubMed] [Google Scholar]
- 7.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141(1):183–93. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakahira Y, Hashitani H, Fukuta H, et al. Effects of isoproterenol on spontaneous excitations in detrusor smooth muscle cells of the guinea pig. J Urol. 2001;166(1):335–40. [PubMed] [Google Scholar]
- 9.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288(6):C1255–63. doi: 10.1152/ajpcell.00381.2004. [DOI] [PubMed] [Google Scholar]
- 10.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol. 2012;9(1):30–40. doi: 10.1038/nrurol.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frazier EP, Peters SL, Braverman AS, et al. Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4–6):449–62. doi: 10.1007/s00210-007-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazier EP, Mathy M-J, Peters SLM, Michel MC. Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol? J Pharmacol Exp Ther. 2005;313(1):260–7. doi: 10.1124/jpet.104.077768. [DOI] [PubMed] [Google Scholar]
- 13.Takemoto J, Masumiya H, Nunoki K, et al. Potentiation of potassium currents by beta-adrenoceptor agonists in human urinary bladder smooth muscle cells: a possible electrical mechanism of relaxation. Pharmacology. 2008;81(3):251–8. doi: 10.1159/000114719. [DOI] [PubMed] [Google Scholar]
- 14.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R60–8. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- 15.Hristov KL, Parajuli SP, Soder RP, et al. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2012;302(11):C1632–41. doi: 10.1152/ajpcell.00417.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hristov KL, Chen M, Kellett WF, et al. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol. 2011;301(4):C903–12. doi: 10.1152/ajpcell.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl 2):S88–119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner TJ, Werner ME, Nausch B, et al. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol. 2009;587(Pt 21):5275–88. doi: 10.1113/jphysiol.2009.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner ME, Knorn AM, Meredith AL, et al. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R616–24. doi: 10.1152/ajpregu.00036.2006. [DOI] [PubMed] [Google Scholar]
- 20.Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol. 2000;524(Pt 2):565–79. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Reilly BA, Kosaka AH, Knight GF, et al. P2X receptors and their role in female idiopathic detrusor instability. J Urol. 2002;167(1):157–64. [PubMed] [Google Scholar]
- 22.Sibley GN. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984;354:431–43. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295(3):862–9. [PubMed] [Google Scholar]
- 24.Matsui M, Motomura D, Karasawa H, et al. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A. 2000;97(17):9579–84. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol. 2000;131(7):1489–95. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harriss DR, Marsh KA, Birmingham AT, Hill SJ. Expression of muscarinic M3-receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. J Urol. 1995;154(3):1241–5. [PubMed] [Google Scholar]
- 27.Matsui M, Griffin MT, Shehnaz D, et al. Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M2 receptor knockout mice. J Pharmacol Exp Ther. 2003;305(1):106–13. doi: 10.1124/jpet.102.044701. [DOI] [PubMed] [Google Scholar]
- 28.Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol. 2010;160(5):1048–61. doi: 10.1111/j.1476-5381.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi O. Beta3-adrenoceptors in urinary bladder. Urology. 2002;59(Supplement 5A):25–9. [Google Scholar]
- 30.Michel MC, Sand C. Effect of pre-contraction on beta-adrenoceptor-mediated relaxation of rat urinary bladder. World J Urol. 2009;27(6):711–5. doi: 10.1007/s00345-009-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]