Abstract
Reversible Ser/Thr phosphorylation of cytoskeletal and adherent junction (AJ) proteins has a critical role in the regulation of endothelial cell (EC) barrier function. We have demonstrated earlier that protein phosphatase 2A (PP2A) activity is important in EC barrier integrity. In the present work, macro- and microvascular EC were examined and we provided further evidence on the significance of PP2A in the maintenance of EC cytoskeleton and barrier function with special focus on the Bα (regulatory) subunit of PP2A. Immunofluorescent staining revealed that inhibition of PP2A results in changes in the organization of EC cytoskeleton as microtubule dissolution and actin re-arrangement were detected. Depletion of Bα regulatory subunit of PP2A had similar effect on the cytoskeleton structure of the cells. Furthermore, transendothelial electric resistance measurements demonstrated significantly slower barrier recovery of Bα depleted EC after thrombin treatment. AJ proteins, VE-cadherin and β-catenin, were detected along with Bα in pull-down assay. Also, inhibition of PP2A (by okadaic acid or fostriecin) or depletion of Bα caused β-catenin translocation from the membrane to the cytoplasm in parallel with its phosphorylation on Ser552. In conclusion, our data suggest that the A/Bα/C holoenzyme form of PP2A is essential in EC barrier integrity both in micro- and macrovascular EC.
Keywords: endothelial cytoskeleton structure, protein phosphatase 2A, adherent junction, β-catenin
INTRODUCTION
PP2A is a ubiquitously expressed Ser/Thr protein phosphatase responsible for dephosphorylation and regulation of many molecular targets involved in the regulation of numerous cellular processes (Janssens and Goris, 2001; Zolnierowicz, 2000). PP2A holoenzymes are heterotrimers, consisting of a core dimer (PP2AD) which contains a 65 kDa structural subunit (PP2A A) and a 36 kDa catalytic subunit (PP2A C). The dimer is associated with a third variable regulatory B subunit. Three unrelated families of B subunits, B, B′ and B″, have been identified, all encoded by multiple genes and with multiple splice variants creating a huge diversity of these regulatory subunits and their isoforms (Csortos et al., 1996; Hendrix et al., 1993; McCright and Virshup, 1995; Strack et al., 1999). They have a crucial role in the determination of substrate specificity and in the targeting of PP2A to different subcellular localizations (Ferrigno et al., 1993; McCright and Virshup, 1995). The methylation and phosphorylation on Tyr and Thr residues in the C terminal region of the catalytic subunit selectively affect the binding of the B subunit to PP2AD (Janssens et al., 2008). Distinct B families have two separate A subunit binding domains which are conserved (Li and Virshup, 2002). These facts are in agreement with the large number of cellular functions recognized so far to be regulated by PP2A and support that the different subunit compositions define specific functions for distinct PP2A heterotrimer complexes (Silverstein et al., 2002).
ABαC is the most abundant and ubiquitous PP2A holoenzyme, and Bα is the most widespread regulatory subunit of PP2A (Hendrix et al., 1993; Mayer et al., 1991), while the localization of Bβ and Bγ are limited to neuronal tissues. In contrast to Bα, which is expressed at a constant level, the expression of Bβ and Bγ are developmentally controlled (Strack et al., 1998). Bα was demonstrated to have functional role in the cytoskeleton, cytoplasm, nucleus, plasma membrane, Golgi, and in the endoplasmic reticulum (Sontag, 2001). The ABαC trimer has several specific functions in the cytoskeleton as it has been shown to associate with microtubules in epithelial cells, fibroblasts, and neurons (Sontag et al., 1999).
The vascular endothelium dynamically regulates the liquid and macromolecule transport between the blood and the interstitial space. Protein phosphorylation and dephosphorylation is a principal regulating mechanism in the endothelial barrier function because Ser, Thr, and Tyr residues of many cytoskeletal proteins and cytoskeleton associated proteins, as well as cell junction proteins are modulated by reversible phosphorylation (Csortos et al., 2007). The role of PP2A in macrovascular endothelial barrier function has been studied previously by our group. It was demonstrated that PP2A and its substrates are implicated in the maintenance of normal barrier function and PP2A protects ECs from thrombin or nocodazole induced gap formation and barrier dysfunction (Tar et al., 2004; Tar et al., 2006).
Endothelial cells communicate through junctional structures such as tight junctions (TJs), adherent junctions (AJs), and gap junctions (GJs) (Dejana et al., 1999; Wallez and Huber, 2008) formed by transmembrane proteins, as part of the paracellular pathway (Lum and Malik, 1994). AJs play a dominant role in EC barrier function, and their regulation depends on the phosphorylation state of the adherent proteins (Huber and Weis, 2001). AJs consist of the transmembrane VE-cadherin and its intracellular components β-catenin and plakoglobin which bind α-catenin, supporting the linkage between the AJ complex and the actin cytoskeleton (Ben-Ze’ev and Geiger, 1998). In addition, β-catenin is a key component of the Wnt-signaling pathway, it plays an important role in embryonic development and tumorigenesis (Nusslein-Volhard and Wieschaus, 1980). Phosphorylation of β-catenin by casein kinase I and GSK3β on Ser45 and subsequently on Ser33/37, Thr41, respectively, leads to its ubiquitination and proteosomal degradation (Aberle et al., 1997; Rubinfeld et al., 1996). AKT and PKA phosphorylate β-catenin on Ser552 and Ser675 residues (Fang et al., 2007; Taurin et al., 2008) and the phosphorylation modulates the transcriptional activity of β-catenin and promotes proliferation of vascular smooth muscle cells, respectively. However, Ser/Thr protein phosphatases involved in the dephosphorylation of the adherent junction proteins are not characterized in EC. Here we provide evidence that the ABαC holoenzyme form of PP2A is involved in the regulation of pulmonary EC cytoskeleton organization. Furthermore, we demonstrate that Bα controls the barrier function of EC via direct or non-direct regulation of the dephosphorylation of β-catenin.
MATERIALS AND METHODS
Proteins and reagents were obtained as follows: Protease Inhibitor Cocktail Set III EMD Biosciences (San Diego, CA); TRIZOL Applied Biosystems (Foster City, CA); M-MLV reverse transcriptase Promega (Madison, WI); Fostriecin Tocris Bioscience (Bristol, UK) anti-V5 antibody Invitrogen (Carlsbad, CA); antibodies against PP2A B, rabbit β-catenin, β-catenin (Ser552), - Ser675, VE-cadherin Cell Signaling Technology, Inc. (Beverly, MA); mouse anti-β-catenin and anti-actin antibodies SIGMA (St Louis, MO); anti-β-tubulin antibody Millipore (Billerica, MA). Alexa 488-, 594-conjugated antibodies, Texas Red-phalloidin, ProLong Gold Antifade medium with DAPI Molecular Probes (Eugene, OR). Substances for cell culturing were from Invitrogen. All other chemicals were from Sigma (St Louis, MO).
Cell cultures
Human pulmonary artery endothelial cells (HPAEC) and Human lung microvascular endothelial cells (HLMVEC) obtained from Lonza Group Ltd. (Walkersville, MD), were propagated in culture medium EGM-2-MV (Lonza) supplemented with 5% (v/v) fetal bovine serum (FBS; HyClone, Waltham, MA) and used at passages 3–7. Bovine pulmonary artery endothelial cells (BPAEC) (culture line-CCL209) from American Type Tissue Culture Collection (Rockville, MD) were maintained in MEM with 10% FBS, 1% sodium pyruvate, 1% MEM non-essential amino acids, 1% antibiotic-antimycotic mixture. Human Embryonic Kidney 293T (HEK 293T) cells from European Collection of Cell Cultures (Salisbury, UK) were cultured in DMEM with 2 mM glutamine, 10% FBS and 1% antibiotic-antimycotic mixture. All cells were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Immunofluorescent staining was performed as described in (Csortos et al., 2008). The coverslips were observed with an Olympus Fluoview FV1000 confocal microscope using UPLSAPO 60 x 1.35 NA oil immersion objective or with Zeiss Axiolab microscope using 63 x oil immersion objective. Images were processed with FV10-ASW v1.5 software.
Image analysis of stress fiber formation
Texas Red-stained EC monolayers treated with either thrombin or PP2A Bα siRNA were observed under Zeiss Axiolab microscope using 63 x oil immersion objective. 8 bit images were analyzed using Image J 1.46R as described previously (Birukova et al., 2004). Briefly, the ratio to the cell area covered by stress fibers to the whole cell area was determined. Statistical analysis of the data was done by GraphPad Prism 5.
Pull-down assay
HEK cells transfected with PP2A Bα/pcDNA3.1/V5-His construct using 10 μg DNA: 20 μl PEI ratio were lysed with lysis buffer (10 mM Tris-HCl pH 7.5; 140 mM NaCl; 1% Triton-X-100; protease inhibitor cocktail (1:200); 10 mM EDTA; 0.1% SDS), scraped and centrifuged for 10 min at 8200g. The supernatant was applied onto anti-V5-affinity gel and incubated for 4 h at 4°C to bind recombinant Bα. Next the resin was centrifuged for 30 s at 8200g. BPAEC were washed with PBS and scraped in lysis buffer followed by sonication and centrifugation. These extracts were added to the resin to which the recombinant Bα was bonded in advance, and incubated for 4 h at 4°C, centrifuged for 30 min at 8200 g, washed with PBS, then boiled with 2x sample buffer.
Depletion of endogenous PP2A Bα
Cells (HLMVEC, HPAEC and BPAEC) were treated with SMART selection-designed PP2A Bα-specific and nontargeting (#1) siRNA duplexes (50 nM) (Thermo Fisher Scientific, Lafayette, CO).Cells were transfected at 70% confluence using DharmaFECT1 (Thermo Fisher Scientific) and were utilized after 48–72 h.
RNA isolation, RT-PCR, and PP2A Bα expression construct preparation
RNA isolated from HPAEC and HLMVEC with TRIZOL was transcribed using M-MLV reverse transcriptase and oligo dT. Primers for PCR: PP2A Bα (partial coding sequence to check silencing) 5′CAGCACCTTCCAGAGCCA3′; 5′GGCAGATGCCCTCATGTC3′; full length PP2A Bα 5′ATGGCAGGAGCTGGAG3′; 5′ATTCACTTTGTCTTGAAATATATACAG3′. EcoRI was used for subcloning Bα into pcDNA3.1/V5-His from the initial Bα/pCR2.1-TOPO construct.
Western blot
Cells were lysed in 2x sample buffer. Proteins separated by SDS-PAGE were transferred to nitrocellulose membrane, and probed with specific antibodies.
Measurement of transendothelial electrical resistance
Transendothelial electrical resistance (TER) was measured in response to EC barrier disruptive agent (thrombin) using an electrical cell substrate impedance sensing system (ECIS; Applied Biophysics, Troy, NY) as previously described (Bogatcheva et al., 2007; Kolosova et al., 2005). HPAEC and HLMVEC transfected with small interfering RNA (siRNA) specific to the regulatory subunit of PP2A were plated on gold microelectrodes. TER was measured 72 hours later.
RESULTS
The lack of PP2A activity changes the cytoskeleton structure of pulmonary EC
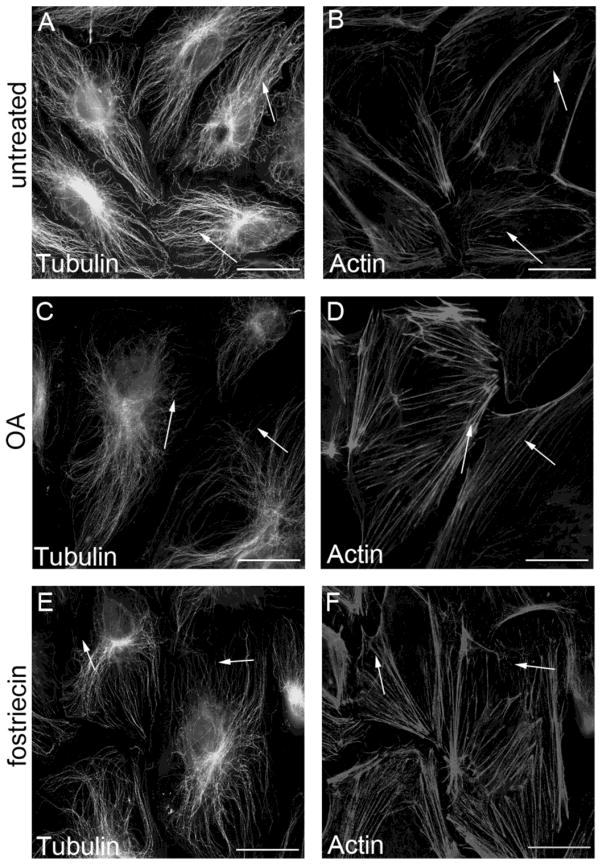
PP2A activity was inhibited by challenging macro- and microvascular lung endothelial cells with okadaic acid (OA) (5 nM, 90 min), a specific inhibitor of PP2A in this concentration (Wera and Hemmings, 1995) or with fostriecin (100 nM, 1 hr) a more selective inhibitor of PP2A (Swingle et al., 2007; Walsh et al., 1997). The treatment affected the distribution and the assembly both of the microtubules and the microfilaments (Fig.1 and SupFig1). Texas red-phalloidin staining (Fig.1B,D,F and SupFig1B,D) indicated cortical F-actin dissolution and stress fiber formation reflecting a more contractile phenotype. In addition, partial depolymerization of the microtubules can be observed (Fig.1A,C,E and SupFig1A,C). These data support previous findings from our group that PP2A regulates the organization of endothelial cytoskeleton structure (Tar et al., 2006).
Figure 1. PP2A inhibition affects the organization of cytoskeleton structure.
HLMVEC (A–F) monolayers were treated either with 0.1% DMSO (A,B), with 5 nM OA for 90 min (C,D), or with 100 nM fostriecin for 1 hr (E,F), then the cells were double stained as described in Materials and Methods with anti-β-tubulin primary antibody (A,C,E) and with Texas Red–phalloidin (B,D,F) to visualize the microtubules and microfilaments, respectively. Pictures were taken with an Olympus Fluoview FV1000 confocal microscope, scale bars: 200 μm. A and B, C and D, E and F are parallel images. Shown are representative data of three independent experiments.
As expected, immunofluorescent staining of EC with an antibody specific for the B regulatory subunit of PP2A revealed that the majority of PP2A B localizes in the cytoplasm (Fig.2A). However, a portion of PP2A B was observed at the cell periphery (Fig. 2C) suggesting that PP2A B may play a role in the cortical actin – cell junction assembly.
Figure 2. Subcellular localization of regulatory B subunit.
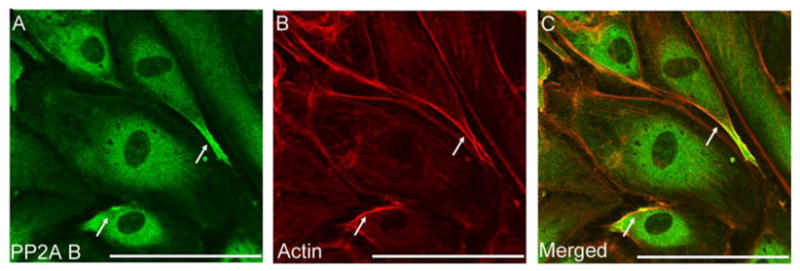
Immunofluorescent staining of BPAEC monolayer was performed using rabbit polyclonal antibody against PP2A B (A). To visualize F-actin Texas Red-phalloidin (B) was applied. Pictures were taken with an Olympus Fluoview FV1000 confocal microscope, scale bars: 200 μm. A and B are parallel images of double-stained cells, panel C is the merged image of A and B. Shown are representative data of three independent experiments.
PP2A Bα depletion affects the organization of EC microfilaments and barrier
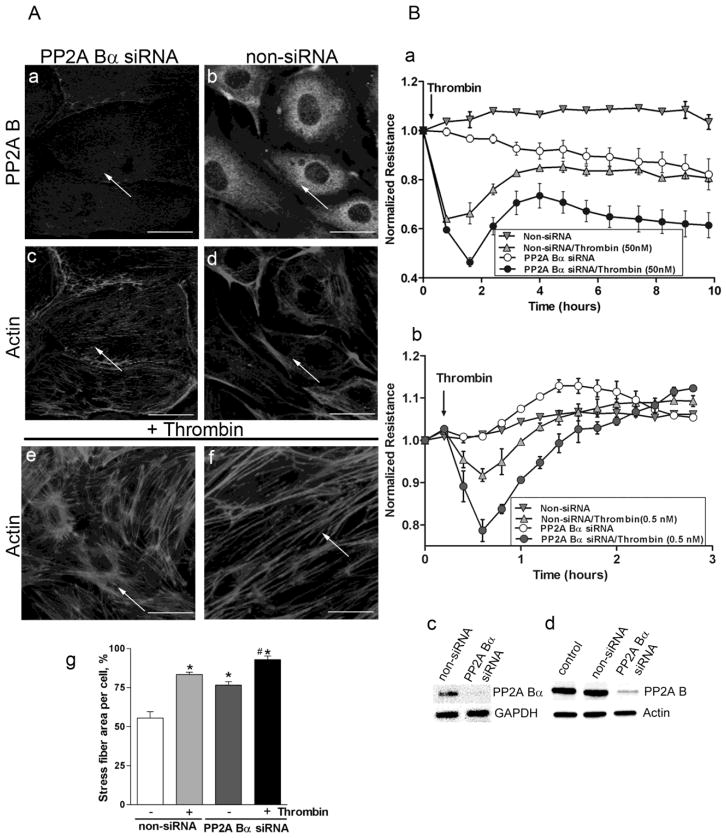
To further examine the function of B subunit in the regulation of cytoskeleton structure, PP2A Bα silencing was utilized. The efficiency of depletion (about 70–80 %) was confirmed by RT-PCR, Western blot and by immunofluorescence (Fig.3). Bα depleted cells showed increase in the amount of total F-actin and cortical actin detected by Texas Red-phalloidin staining (Fig.3A/c) compared to EC transfected with non-siRNA (Fig.3A/d). Importantly, thrombin treatment (50 nM, 30 min) enhanced the effect of Bα depletion on F-actin stress fiber formation (Fig.3A/e) compared to the undepleted cells (Fig.3A/f), simultaneously leading to the dissolution of the cortical actin ring suggesting a more contractile phenotype. Quantitative analysis (Fig.3A/g) confirmed the increased stress fiber formation in thrombin treated or PP2A Bα depleted cells.
Figure 3. PP2A Bα depletion affects endothelial cytoskeleton organization and barrier function.
Cells were transfected with small interfering RNA (siRNA) specific for PP2A Bα or with non-silencing (non-si) RNA. Panel A: Immunofluorescent staining of BPAEC transfected with PP2A Bα specific siRNA (a,c,e) and non-silencing siRNA (b,d,f). Transfected monolayers without any further treatment were double-stained with PP2A B specific antibody (a,b) and with Texas Red-phalloidin (c,d). Actin staining of transfected cells challenged with thrombin (50 nM, 30 min) (e,f) is also shown. Stress fiber formation induced by thrombin was evaluated by morphometric analysis using Image J program as described in Materials and Methods (g). The results are presented as means n=14, ± SEM. Significant changes are indicated by * (P<0.05 vs control), and # (P<0.05 vs non-si/thrombin). Pictures were taken with a Zeiss Axiolab microscope, scale bars: 200 μm. Panel B: HPAEC (a) and HLMVEC (b) plated on gold microelectrodes were transfected with non-targeting or specific PP2A Bα siRNA. 72 hours later TER was measured. Arrow indicates the time point when thrombin (a:50 nM or b:0.5 nM) or vehicle was added to the medium. The depletion was verified by RT-PCR (Bc) using specific primer pairs; and by Western blot (Bd) of the lysate of control cells and lysates of cells transfected with non-si or PP2A B specific siRNA using B subunit specific antibody. GAPDH and actin signals were used as inner and loading controls, respectively. RT-PCR and Western blot verification was done for all cell types investigated. The efficiency of depletion was about the same, results shown (Bc-d) were acquired on HLMVEC.
Shown are representative data of three independent experiments.
Published data from our group strongly suggested the involvement of PP2A in EC barrier protection against edemagenic agonists, thrombin and nocodazole (Tar et al., 2004). Therefore the effect of PP2A Bα depletion on TER of control and thrombin-challenged macro- and microvascular EC monolayers was studied (Fig. 3B). Silencing of PP2A Bα significantly exacerbated thrombin-induced EC permeability increase and delayed or abolished TER recovery after thrombin. We detected a significantly longer time period from addition of thrombin until 50% recovery of PP2A Bα silenced HMLVEC compared to non-siRNA transfected HMLVEC (0.42h vs 0.27h, respectively, P<0.05; Fig.3Bb). These results indicate that the regulatory B subunit of PP2A is critical for EC barrier maintenance.
PP2A Bα regulatory subunit associates with adherent junction proteins
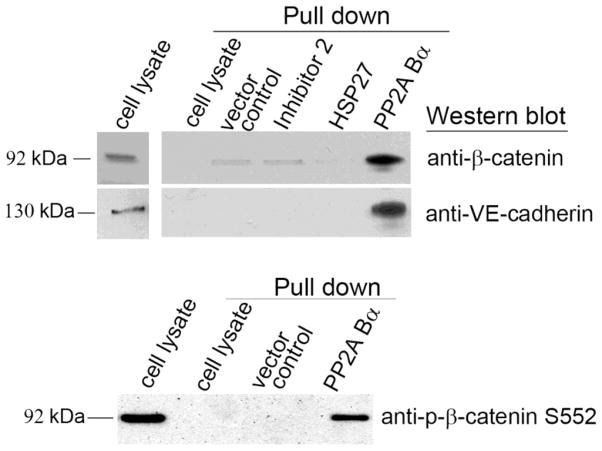
Endothelial cell-cell contacts and the regulation of their assembly are closely connected with EC permeability. Localization of B subunit at the cell periphery lead us to examine possible binding of PP2A Bα to adherent junction proteins with pull-down experiments. Since the efficiency of EC transfection is quite low in general, we generated a PP2A Bα/pcDNA3.1/V5-His construct as described in “Materials and Methods”, which was transfected into HEK cells (SupFig. 2). Over-expressed Bα and control proteins were immobilized on anti-V5-agarose resin, and then BPAEC lysate was loaded onto the resin. We could identify adherent junction proteins along with the eluted Bα using specific antibodies against β-catenin, VE-cadherin, and phosphorylated β-catenin Ser552 by Western blot (Fig.4) proving the association of Bα and EC adherent junction proteins. Importantly, neither the known PP2A binding protein, HSP27, nor the PP1 binding heat-stable phosphatase inhibitor 2 was able to bind the studied AJ proteins (Fig.4) in parallel pull-down assays confirming the specificity of the detected direct or non-direct binding of AJ proteins to Bα.
Figure 4. Investigation of PP2A Bα - adherent junction protein interactions.
Pull-down experiment was performed as described in Materials and Methods. Briefly, lysates of transfected (pcDNA3.1/V5-His vector control or expression constructs in the same vector) HEK cells were loaded onto anti-V5 agarose in order to immobilize V5 or V5-tagged inhibitor 2, HSP27, and PP2A Bα on the resin. After a washing step the resin was incubated with BPAEC cell lysates. In addition, BPAEC lysate was incubated with anti-V5 agarose without any immobilized V5-tagged protein, as a negative control of the pull-down. After another washing step the protein complexes were eluted by boiling the resin in 2x SDS sample buffer and analyzed by Western blot using β-catenin, VE-cadherin and phospho-β-catenin Ser552 specific antibodies. Untreated BPAEC cell lysate was also loaded in the first lane as a positive control. Shown are representative data of three independent experiments.
PP2A inhibitors or Bα depletion evokes redistribution of adherent junction proteins
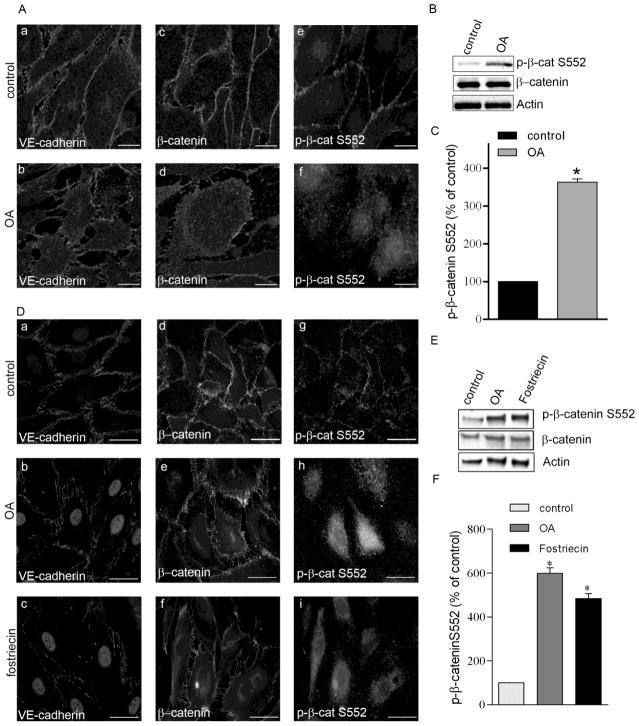
As shown in Fig.5 OA (5 nM, 90 min) or fostriecin (100 nM, 1 hr) induced interruption of continuous VE-cadherin staining at the cell periphery suggesting disruption of AJs (Fig.5Aa,b and Da–c). At the same time, β-catenin staining at the edge of the cells was less pronounced after OA treatment (Fig. 5Ac,d and Dd–f) without evident decrease of total protein amount (Fig. 5B,E) suggesting some cellular redistribution of β-catenin. We also examined the effect of OA or fostriecin on cellular distribution of phospho-β-catenin. Immunofluorescent staining (A,D) and Western blot (B,C,E,F) of macro- and microvascular EC using a specific antibody revealed that β-catenin phosphorylated on Ser552 relocated to the cytoplasm (Fig. 5Ae,f and Dg–i).
Figure 5. Okadaic acid and fostriecin treatment evokes redistribution of adherent junction proteins.
BPAEC (A,B) and HLMVEC (D,E) monolayers were treated with vehicle (Panel A: a,c,e; Panel D: a,d,g), 5 nM okadaic acid (OA) for 90 min (Panel A: b,d.f; Panel D:b,e,h), or with 100 nM fostriecin for 1 hr (Panel D: c,f,i). Afterwards the cells were fixed for immunofluorescent staining (A, D) or lysed for Western blot analysis (B,C) as described in Materials and Methods. Antibodies against VE-cadherin, β-catenin and phospho-β-catenin Ser552 were used as indicated in the immunofluorescent pictures to detect the subcellular distribution of adherent junction proteins. Pictures were taken with a Zeiss Axiolab microscope, scale bars: 200 μm. (B, E) Phosphorylation level of β-catenin on Ser552 was analyzed by Western blot using specific antibody against phospho-β-catenin Ser552. β-catenin and actin were also detected as loading controls. (C, F) Phosphorylation level of β-catenin was normalized to total β-catenin and quantified by densitometry of Western blots (n=3, p<0.05 vs control). Shown are representative data of three independent experiments.
The effect of PP2A Bα depletion on localization and the phosphorylation level of β-catenin in EC (Fig.6) was similar to the effect of OA or fostriecin on both cell types. Depletion of PP2A Bα led to disruption of AJs. In addition, we observed an inverse correlation between the protein levels of Bα and the phosphorylation level of phospho-Ser552-β-catenin. In the depleted cells, the phosphorylation of β-catenin at Ser552 increased, while significant decrease was detected in PP2A Bα (Fig.6B,C). Interestingly, almost the entire amount of phospho-Ser552-β-catenin in PP2A Bα-depleted cells seems to be located in the cytoplasm, but not at the cell edges. These data suggest that PP2A Bα may be important for the integrity of AJs, consistent with our data indicating that depletion of this subunit leads to EC barrier compromise (Fig 3).
Figure 6. Depletion of PP2A Bα evokes redistribution of β-catenin and p-β-catenin coinciding with the disruption of cell-cell contacts.
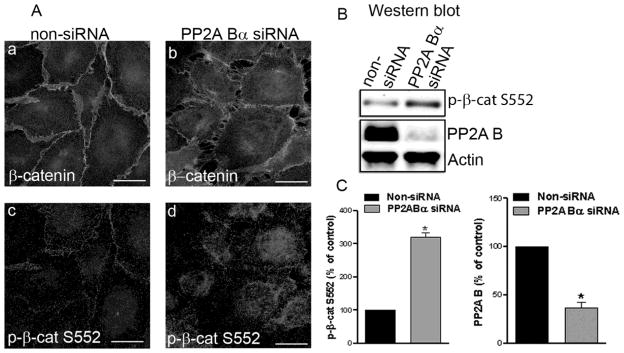
BPAEC were transfected with specific PP2A Bα siRNA and with non-silencing RNA. (A) Immunofluorescent staining with anti-β-catenin (a,b) and anti-phospho-β-catenin Ser552 (c,d) antibodies as described in Materials and Methods. Pictures were taken with a Zeiss Axiolab microscope, scale bars: 200 μm. (B) Western blotting of cell lysates using phospho-β-catenin Ser552, PP2A B, and actin specific antibodies. (C) Densitometric quantification of data demonstrated on panel B (n=3, p<0.05). Shown are representative data of three independent experiments.
Also, these data suggest the involvement of PP2A in the regulation of β-catenin phosphorylation and cellular distribution, which affect AJs assembly and EC permeability.
DISCUSSION
PP2A, a major Ser/Thr phosphatase, is involved in many physiological processes in the cell. Our previous results have proved that PP2A plays a critical role in the endothelial barrier protection and in the arrangement of cytoskeletal proteins (Tar et al., 2004; Tar et al., 2006). To better understand the functional role of PP2A in the regulation of endothelial cytoskeleton organization we employed PP2A inhibitors, okadaic acid (OA), a well-known cell-permeable inhibitor of PP2A (Haystead et al., 1989), and fostriecin, an antitumor antibiotic produced by Streptomyces pulveraceus (Walsh et al., 1997). OA and fostriecin inhibit PP2A more efficiently (Ki=0.2 nM and 3.2 nM, respectively) compared to PP1 (Ki=2 μM and 131 μM, respectively) (Cohen et al., 1990; Walsh et al., 1997). Several earlier studies demonstrated considerable effect of okadaic acid on the cytoskeleton of different cell types. It has been shown that OA causes depolymerization of interphase microtubules and abnormalities in the mitotic spindle in LLC-PK cells (Vandre and Wills, 1992) and also promotes PP2A-mediated microtubule destabilization and phosphorylation of PP2A-sensitive microtubule-associated proteins (Sontag et al., 1996). In our experiments, inhibition of PP2A affected the actin and tubulin organization in EC suggesting a pivotal role of PP2A in the maintenance of cytoskeleton structures. However, our group previously showed that OA (5 nM) had no significant effect on BPAEC permeability (Verin et al., 1995). In addition, staining of F-actin showed that OA (5 nM) treatment did not cause any detectable change of the actin cytoskeleton in HPAEC (Tar et al., 2004). This apparent controversy can be resolved by the differing culturing conditions used. In both of the published works the human and bovine ECs were maintained in M199 containing 20% bovine serum, and endothelial cell growth supplement. In the present work we used MEM, according to the recommendation of ATCC, to maintain BPAEC supplemented with only 10% of bovine serum for better comparability to serum starved silencing conditions. This serum concentration was routinely used for maintaining the very same cell type (CCL-209) in other laboratories as reported in (Drew et al., 2010; Duthu and Smith, 1980; Ludwig et al., 2005; Wu et al., 2010).
PP2A is one of the most abundant phospho-Ser/Thr-specific protein phosphatases. The large families of PP2A holoenzymes have wide substrate specificity; therefore PP2A is involved in many basic processes of the cell. Since we have previously shown that the over-expression of the catalytic C, and structural A subunits significantly attenuated thrombin or nocodazole- induced barrier dysfunction and cytoskeleton rearrangement (Tar et al., 2006), to narrow the affected processes, the present study was rather focused on the possible regulatory role of the Bα subunit of PP2A in endothelial cells. Endogenous localization and distribution of the B subunits have been described in several different tissues and cell types, but endothelium (Janssens et al., 2008; Mayer et al., 1991). In agreement with the earlier findings in other cell types, immunofluorescent staining of the endogenous Bα demonstrated mainly cytoplasmic localization in BPAEC. Nevertheless, B also localizes at the cell periphery, seemingly with the cortical actin ring. Moreover, alteration of PP2A activity by reduction (about 80%) of Bα protein level affected the organization of F-actin and cortical actin; both were detected in an increased level in Bα depleted cells, and thrombin-induced stress fiber formation became more prominent in Bα silenced cells compared to controls transfected with non-silencing RNA. Importantly, our TER measurements also support that Bα plays a role in the barrier maintaining function of PP2A. Based on these findings, we hypothesized that PP2A activity may be required not only in the regulation of the phosphorylation level of cytoskeleton associated proteins, as we suggested earlier, but it may have an important role in the intercellular junctions as well.
Adherent junctions are the most abundant communicating structures among endothelial cells. β-catenin was originally identified associating with the cytoplasmic domains of cadherins and found to have a crucial role in Ca2+ dependent cell adhesion (Aberle et al., 1996). Besides being a structural component of AJ, β-catenin has several functions, which are regulated via phosphorylation of Ser/Thr and Tyr residues (Valenta et al., 2012). It is well known that β-catenin is a component of the Wnt signaling pathway (Kikuchi, 2003). It is interesting to note that during Wnt-signaling in Drosophila the interaction of PP2A Bα with β-catenin regulates the phosphorylation state of Ser33/37 and Thr41 residues and degradation of β-catenin (Zhang et al., 2009). The phosphorylation state of the VE-cadherin and β-catenin determines the stability of their complex. Phosphorylation of β-catenin at Tyr654 and the Tyr860 residue in cadherin prevents their interaction. Phosphorylation of Ser residues in cadherin were also shown to inhibit the interaction. On the other hand, phosphorylation of specific Ser residues in cadherin may enhance binding of β-catenin (Bazzoni and Dejana, 2004; Kemler, 1993; Lilien and Balsamo, 2005). We investigated the possible interaction between the Bα regulatory subunit of PP2A and adherent junction proteins in BPAEC to see whether PP2A activity is significant in the stability of the VE-cadherin- β-catenin complex. Recombinant Bα was utilized as bait in pull-down experiments and adherent proteins, VE-cadherin and β-catenin, from EC were detected in the bound protein complex. To learn whether PP2A activity triggers dephosphorylation of β-catenin and/or VE-cadherin or dephosphorylates them directly, and identification of the concerned amino acid residues requires further studies.
Inhibition of PP2A with OA or fostriecin challenge, or depletion of Bα resulted in a redistribution of VE-cadherin and β-catenin from the cell membranes (to the cytoplasm or nuclei) and the loss of cell-cell contacts, demonstrating the critical role of PP2A activity in well-functioning adherent junctions. Consistent with our observations, OA treatment caused re-localization of VE-cadherin from the membrane to the cytoplasm in human keratinocytes (Serres et al., 2000) and thrombin induced dissociation of β-catenin and p120 from the cell membrane has been described recently in human umbilical vein ECs (Beckers et al., 2008). It was also suggested that the catalytic subunit of PP2A is important in the stabilization of E-cadherin/β-catenin complex at the plasma membrane (Gotz et al., 2000). Interestingly, PP2A activity was shown to increase paracellular permeability of epithelial cells by dephosphorylating tight junction proteins (Nunbhakdi-Craig et al., 2002). The differing cell- and junction types examined could explain this apparently opposing result. It may also suggest that adherent and tight junctions might be differently regulated via the phosphorylation state of their components. It should also be noted, that the authors reported no noticeable change in the F-actin structure upon OA treatment of the epithelial cells, while our results reflected changes in the cytoskeleton arrangement after the inhibition of PP2A in EC.
It is known that Ser552 phosphorylation site of β-catenin is not related to the degradation of the protein. Different kinases may phosphorylate β-catenin at Ser552, and it was reported that it caused dissociation of β-catenin from cell-cell contacts and an increase in its transcriptional activity (Fang et al., 2007; Taurin et al., 2008). We observed that the phosphorylation level of Ser552 in EC is low, and appears mostly at the cell membrane. After OA or fostriecin treatment, or silencing of Bα the Ser552 residue of β-catenin was phosphorylated, and this phosphorylated form appeared to be present in the cytoplasm. These findings indicate that Bα and most likely PP2A as well, have a pivotal role in regulating the phosphorylation level of the Ser552 side chain of β-catenin. However, the role of this site-specific Ser phosphorylation of β-catenin on EC cytoskeleton/barrier/AJ assembly and the exact role of PP2A in β-catenin dephosphorylation remains to be determined. A recent paper published during the preparation of this manuscript also claims that PP2A activity is necessary to maintain brain EC monolayer integrity (Le Guelte et al., 2012).
Conclusions
Taken together these results further strengthen our previous findings that PP2A is a critical component in the maintenance of the endothelial cytoskeleton structure, and suggest that Bα is at least one among the regulatory B subunits of the PP2A holoenzyme having a significant role in this aspect. Furthermore, association of Bα and AJ proteins was detected suggesting that the activity of the ABαC holoenzyme form of PP2A might be necessary for functional adherent junctions in EC. However, our results do not exclude the possibility of other PP2A holoenzyme forms containing a different B subunit being also involved in EC barrier maintenance through the regulation of the phosphorylation state of cytoskeleton associated and cell junction proteins via opposing or parallel effects of the different PP2A activities.
Supplementary Material
BPAEC (A–D) monolayers were treated either with 0.1% DMSO (A,B), or with 5 nM OA for 90 min (C,D), then the cells were double stained as described in Materials and Methods with anti-β-tubulin primary antibody (A,C) and with Texas Red–phalloidin (B,D) to visualize the microtubules and microfilaments, respectively. Pictures were taken with an Olympus Fluoview FV1000 confocal microscope, scale bars: 200 μm. A and B, C and D are parallel images. Shown are representative data of three independent experiments.
HEK cells were transfected with PP2A Bα/pcDNA3.1/V5/His, inhibitor 2/pcDNA3.1/V5/His or HSP27/pcDNA3.1/V5/His mammalian expression constructs. Empty pcDNA3.1/V5His were also utilized as a negative control. Lysates of the transfected cells were analyzed with Western blot using anti-V5 antibody.
Highlights.
Protein phosphatase (PP) 2A is critical in endothelial cytoskeleton structure.
Loss of Bα subunit of PP2A weakens the endothelial barrier.
Interaction of Bα and adherent junction proteins were detected.
Bα supports functional adherent junctions.
Acknowledgments
The authors are most thankful to Dr. György Vámosi (University of Debrecen, Department of Biophysics and Cell Biology) for his help in confocal microscopy.
This work was supported by the Hungarian Science Research Fund (CNK80709), UD Faculty of Medicine Research Fund (Bridging Fund 2012) and TÁMOP-4.2.2.A-11/1/KONV-2012-0025 project (co-financed by the European Union and the European Social Fund), and NIH (PO1-HL101902 and R01-HL67307).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, et al. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H, et al. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J Biol Chem. 1996;271:1520–6. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Beckers CM, et al. Nuclear targeting of beta-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res. 2008;79:679–88. doi: 10.1093/cvr/cvn127. [DOI] [PubMed] [Google Scholar]
- Ben-Ze’ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–39. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- Birukova AA, et al. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, et al. Involvement of microtubules, p38, and Rho kinases pathway in 2-methoxyestradiol-induced lung vascular barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2007;292:L487–99. doi: 10.1152/ajplung.00217.2006. [DOI] [PubMed] [Google Scholar]
- Cohen P, et al. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Csortos C, et al. TIMAP is a positive regulator of pulmonary endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2008;295:L440–50. doi: 10.1152/ajplung.00325.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csortos C, et al. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am J Physiol Lung Cell Mol Physiol. 2007;293:L843–54. doi: 10.1152/ajplung.00120.2007. [DOI] [PubMed] [Google Scholar]
- Csortos C, et al. High complexity in the expression of the B′ subunit of protein phosphatase 2A0. Evidence for the existence of at least seven novel isoforms. J Biol Chem. 1996;271:2578–88. doi: 10.1074/jbc.271.5.2578. [DOI] [PubMed] [Google Scholar]
- Dejana E, et al. The role of endothelial cell-to-cell junctions in vascular morphogenesis. Thromb Haemost. 1999;82:755–61. [PubMed] [Google Scholar]
- Drew CP, et al. Bluetongue virus infection alters the impedance of monolayers of bovine endothelial cells as a result of cell death. Vet Immunol Immunopathol. 2010;136:108–15. doi: 10.1016/j.vetimm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthu GS, Smith JR. In vitro proliferation and lifespan of bovine aorta endothelial cells: effect of culture conditions and fibroblast growth factor. J Cell Physiol. 1980;103:385–92. doi: 10.1002/jcp.1041030303. [DOI] [PubMed] [Google Scholar]
- Fang D, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–9. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno P, et al. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol Biol Cell. 1993;4:669–77. doi: 10.1091/mbc.4.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, et al. Distinct role of protein phosphatase 2A subunit Calpha in the regulation of E-cadherin and beta-catenin during development. Mech Dev. 2000;93:83–93. doi: 10.1016/s0925-4773(00)00267-7. [DOI] [PubMed] [Google Scholar]
- Haystead TA, et al. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337:78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hendrix P, et al. Analysis of subunit isoforms in protein phosphatase 2A holoenzymes from rabbit and Xenopus. J Biol Chem. 1993;268:7330–7. [PubMed] [Google Scholar]
- Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–39. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, et al. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–21. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–21. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225–9. doi: 10.1111/j.1349-7006.2003.tb01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova IA, et al. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res. 2005;97:115–24. doi: 10.1161/01.RES.0000175561.55761.69. [DOI] [PubMed] [Google Scholar]
- Le Guelte A, et al. Semaphorin 3A elevates endothelial cell permeability through PP2A inactivation. J Cell Sci. 2012;125:4137–46. doi: 10.1242/jcs.108282. [DOI] [PubMed] [Google Scholar]
- Li X, Virshup DM. Two conserved domains in regulatory B subunits mediate binding to the A subunit of protein phosphatase 2A. Eur J Biochem. 2002;269:546–52. doi: 10.1046/j.0014-2956.2001.02680.x. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–65. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ludwig A, et al. Effect of statins on the proteasomal activity in mammalian endothelial and vascular smooth muscle cells. Biochem Pharmacol. 2005;70:520–6. doi: 10.1016/j.bcp.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267:L223–41. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- Mayer RE, et al. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991;30:3589–97. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- McCright B, Virshup DM. Identification of a new family of protein phosphatase 2A regulatory subunits. J Biol Chem. 1995;270:26123–8. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, et al. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–78. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Serres M, et al. The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res. 2000;257:255–64. doi: 10.1006/excr.2000.4895. [DOI] [PubMed] [Google Scholar]
- Silverstein AM, et al. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci U S A. 2002;99:4221–6. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13:7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- Sontag E, et al. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–7. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- Sontag E, et al. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem. 1999;274:25490–8. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- Strack S, et al. Cloning and characterization of B delta, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett. 1999;460:462–6. doi: 10.1016/s0014-5793(99)01377-0. [DOI] [PubMed] [Google Scholar]
- Strack S, et al. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392:515–27. [PubMed] [Google Scholar]
- Swingle M, et al. Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol Biol. 2007;365:23–38. doi: 10.1385/1-59745-267-X:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tar K, et al. Phosphatase 2A is involved in endothelial cell microtubule remodeling and barrier regulation. J Cell Biochem. 2004;92:534–46. doi: 10.1002/jcb.20036. [DOI] [PubMed] [Google Scholar]
- Tar K, et al. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J Cell Biochem. 2006;98:931–53. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- Taurin S, et al. Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2008;294:C1169–74. doi: 10.1152/ajpcell.00096.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, et al. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–36. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandre DD, Wills VL. Inhibition of mitosis by okadaic acid: possible involvement of a protein phosphatase 2A in the transition from metaphase to anaphase. J Cell Sci. 1992;101(Pt 1):79–91. doi: 10.1242/jcs.101.1.79. [DOI] [PubMed] [Google Scholar]
- Verin AD, et al. Regulation of endothelial cell gap formation and barrier function by myosin-associated phosphatase activities. Am J Physiol. 1995;269:L99–108. doi: 10.1152/ajplung.1995.269.1.L99. [DOI] [PubMed] [Google Scholar]
- Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Walsh AH, et al. Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett. 1997;416:230–4. doi: 10.1016/s0014-5793(97)01210-6. [DOI] [PubMed] [Google Scholar]
- Wera S, Hemmings BA. Serine/threonine protein phosphatases. Biochem J. 1995;311(Pt 1):17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, et al. Statin post-treatment provides protection against simulated ischemia in bovine pulmonary arterial endothelial cells. Eur J Pharmacol. 2010;636:114–20. doi: 10.1016/j.ejphar.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. PR55 alpha, a regulatory subunit of PP2A, specifically regulates PP2A-mediated beta-catenin dephosphorylation. J Biol Chem. 2009;284:22649–56. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60:1225–35. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BPAEC (A–D) monolayers were treated either with 0.1% DMSO (A,B), or with 5 nM OA for 90 min (C,D), then the cells were double stained as described in Materials and Methods with anti-β-tubulin primary antibody (A,C) and with Texas Red–phalloidin (B,D) to visualize the microtubules and microfilaments, respectively. Pictures were taken with an Olympus Fluoview FV1000 confocal microscope, scale bars: 200 μm. A and B, C and D are parallel images. Shown are representative data of three independent experiments.
HEK cells were transfected with PP2A Bα/pcDNA3.1/V5/His, inhibitor 2/pcDNA3.1/V5/His or HSP27/pcDNA3.1/V5/His mammalian expression constructs. Empty pcDNA3.1/V5His were also utilized as a negative control. Lysates of the transfected cells were analyzed with Western blot using anti-V5 antibody.