Abstract
Over the years, the proteasome has been extensively investigated due to its crucial roles in many important signaling pathways and its implications in diseases. Two proteasome inhibitors—bortezomib and carfilzomib—have received FDA approval for the treatment of multiple myeloma, thereby validating the proteasome as a chemotherapeutic target. As a result, further research efforts have been focused on dissecting the complex biology of the proteasome to gain the insight required for developing next-generation proteasome inhibitors. It is clear that chemical probes have made significant contributions to these efforts, mostly by functioning as inhibitors that selectively block the catalytic activity of proteasomes. Analogues of these inhibitors are now providing additional tools for visualization of catalytically active proteasome subunits, several of which allow real-time monitoring of proteasome activity in living cells as well as in in vivo settings. These imaging probes will provide powerful tools for assessing the efficacy of proteasome inhibitors in clinical settings. In this review, we will focus on the recent efforts towards developing imaging probes of proteasomes, including the latest developments in immunoproteasome-selective imaging probes.
The proteasome is a key component of the ubiquitin proteasome pathway, which mediates the tightly controlled degradation of proteins involved in a myriad of cellular processes, including cell cycle regulation, apoptosis, immune responses, and malignant transformation [1, 2]. This multiprotease complex was validated as a chemotherapeutic target by the FDA approval of the first-generation proteasome inhibitor bortezomib (Velcade®). Since bortezomib’s approval in 2003, the second-generation proteasome inhibitor carfilzomib (Kyprolis®) has also been approved, and several others are in various stages of preclinical and clinical development.
Each eukaryotic proteasome contains three distinct types of catalytically active subunits. The constitutive proteasome, containing catalytic subunits β1, β2, and β5, is expressed in all eukaryotic cells. Based on their ability to hydrolyze fluorogenic peptide substrates, these subunits have been assigned caspase-like activity, trypsin-like activity, and chymotrypsin-like activity, respectively [3]. The immunoproteasome, an alternate form of the proteasome, is also expressed in hematopoietic cells and can be induced in other cell types by cytokines such as interferon-γ and tumor necrosis factor-α. During immunoproteasome assembly, β1i, β2i, and β5i are incorporated in place of their constitutive proteasome counterparts, thereby altering the catalytic activity of the resulting proteasome complex [4, 5] (Figure 1). A third proteasome subtype, known as the thymoproteasome, has also been discovered in cortical thymic epithelial cells. Thymoproteasomes contain the immunoproteasome catalytic subunits β1i and β2i along with the thymoproteasome-specific catalytic subunit subunit β5t, and are suggested to function in the positive selection of T cells [6]. Proteasome catalytic subunits are synthesized as inactive zymogens containing N-terminal propeptides. Removal of these propeptides upon completion of proteasome assembly is required to expose their catalytic residues [7, 8]. Since the discovery of proteasomes decades ago, our knowledge about the constitutive proteasome has been remarkably increased by extensive studies, which have revealed its crucial roles in many important cellular processes. More recently, the importance of the immunoproteasome in cells has gained recognition [9].
Figure 1.
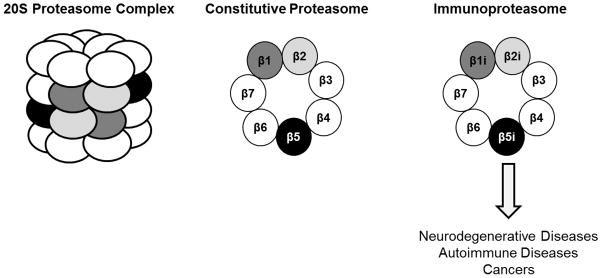
Representation of the 20S proteasome complex, which is comprised of two outer α-rings and two inner β-rings, and an aerial view of β-rings containing either constitutive proteasome or immunoproteasome catalytic subunits. Aberrant regulation of the immunoproteasome is implicated in neurodegenerative diseases, autoimmune diseases, and cancers.
While the immunoproteasome is suggested to primarily function in generating optimal peptides for MHC class I antigen presentation, other studies have suggested its involvement in additional processes as well [9–13]. Additionally, expression levels of immunoproteasome catalytic subunits are aberrantly regulated in a variety of diseases, including neurodegenerative and autoimmune diseases as well as cancers [14–18] [19]. These findings have prompted the development of immunoproteasome-targeting inhibitors for treating these diseases, as well as for use as molecular probes to investigate immunoproteasome functions [17, 19–22]. However, much about the detailed cellular roles of immunoproteasomes remains to be understood. These mysterious immunoproteasome functions have led efforts to develop chemical tools to facilitate the selective visualization of its active subunits [23]. In this report, we describe current advances in proteasome imaging probes.
From Activity-Based Proteasome Inhibitors to Imaging Probes
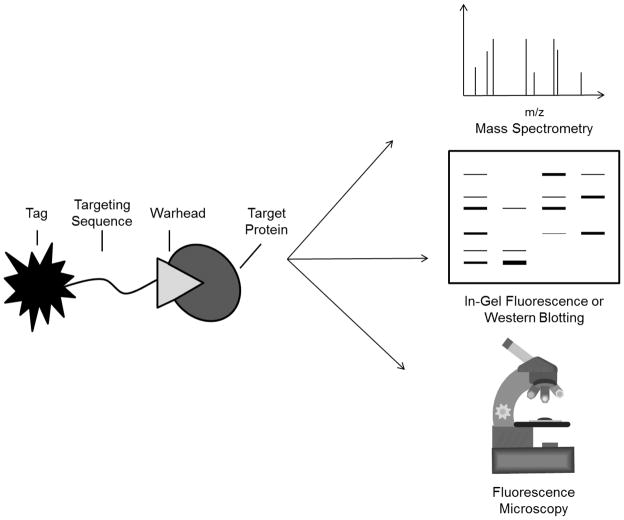
The mechanism-based binding of small molecule inhibitors to active site residues of enzymes has been exploited in the development of activity-based probes (ABPs), which facilitate functional studies of catalytically active proteasomes in complex biological samples. APBs can be generated by attaching a reporter group to a proteasome inhibitor which binds to the proteasome’s catalytic threonine residues covalently and irreversibly. The reporter group facilitates the affinity purification of probe-bound proteins, or their visualization via in-gel fluorescence, western blotting, or fluorescence microscopy (Figure 2). ABP-based approaches are superior to evaluating expression levels of proteasome subunits due to the presence of both inactive and active forms of these subunits within cells, which are detected indiscriminately using antibody-based approaches. For this reason, researchers have made considerable efforts to develop ABPs targeting the proteasome in recent years.
Figure 2.
Structural representation of an activity-based probe (ABP), which is comprised of a targeting sequence that directs the probe to the targeted enzyme, a reactive chemical warhead which binds irreversibly to the enzyme’s catalytic residues, and a tag for identification or visualization of probe-bound enzymes. Biotin-tagged ABPs can be used for affinity purification and mass spectrometry-based identification of probe targets, as well as for visualization of these targets by streptavidin affinity blotting. Radiolabeled and fluorescent ABPs can be used to visualize target proteins by in-gel fluorescence. Additionally, cell-permeable fluorescent ABPs can be used to monitor enzyme activity in living cells or tissue samples via fluorescence microscopy.
Most of the proteasome-targeting ABPs reported to date are derived from broad-spectrum proteasome inhibitors that generally inhibit all active proteasome subunits. In addition, several subunit-selective ABPs have been developed. Achieving a better understanding of how proteasome activity is regulated as well as the functions of distinct catalytic subunits of the proteasome will likely lead to improved clinical responses in patients treated with proteasome inhibitors, as well as to the identification of new therapeutic applications of these drugs.
The first peptide-based proteasome-targeting ABPs were reported by Bogyo and colleagues. Equipped with a vinyl-sulfone pharmacophore, which reacts with the proteasome’s catalytic threonine residues via a Michael addition, along with a 125I radiolabel, the ABP 125I-NIP-L3VS paved the way for the subsequent use of peptide vinyl-sulfone-based probes to study the proteasome’s catalytic activities [24]. In early studies, radiolabeled vinyl sulfone-based probes were used, in combination with fluorogenic peptide substrates, to assign distinct catalytic activities to individual proteasome subunits [25, 26]. They were also used to gain insight into the impact of inhibitor substituents on their subunit binding preferences. These studies have led to the design of subunit-specific or broad-spectrum proteasome inhibitors [25–27]. In addition, a visualization marker such as biotin was added to these irreversible inhibitors to identify the composition of catalytically active subunits of proteasomes in cell extracts. For example, ε-biotinyl lysine was added to the broad-spectrum peptide vinyl sulfone inhibitor AdaAhx3L3VS to generate the biotin-labeled proteasome-targeting ABP AdaK(Bio)Ahx3L3VS [27]. This probe has been used for mass spectrometry-based identification of the active proteasome subunits modified by Ahx3L3VS-based probes in Arabidopsis leaf extracts [28], and in primary PBMCs isolated from a CLL patient in a study of proteasome activity patterns in samples from bortezomib-treated patients [29]. Additionally, this probe was used to confirm the β2/β2i-selectivity of the azide-containing ABP az-NC-002 [30].
Exchanging the N-terminal segment of AdaK(Bio)Ahx3L3VS [27] for the small dansyl-sulfonamidohexanoyl hapten gave rise to a cell-permeable analogue of this potent, broad-spectrum ABP [31]. Proteins bound by this probe can be detected by western blotting using antibodies against the dansyl moiety. This probe was the first of its kind to demonstrate the subunit binding preferences of bortezomib and the peptide aldehyde inhibitor MG132 in living cells [31], and has subsequently been used to study the subunit binding selectivities of these inhibitors and others in living cells and in cell extracts [32, 33]. Interestingly, results using this probe consistently demonstrate that MG132 fully inhibits β2i but not β2, suggesting differences in the active site chemistry between these two highly homologous subunits [31, 33]. DansylAhx3L3VS was also used to show the concentration-dependent inhibition of active subunits in primary monocytes following incubation with bortezomib, to show the subunits targeted by bortezomib in PBMCs isolated from a bortezomib-treated patient, and to show that the levels of activity of proteasome catalytic subunits are variable in primary human leukemia samples [29]. In the same study, this probe was further used to examine whether proteasome activity patterns are associated with sensitivity to the cytotoxic effects of bortezomib [29]. DansylAhx3L3VS labeling also demonstrated the proteasome inhibitory activity of disease-associated prion protein aggregates [34], and provided the first evidence of a relationship between Bcr-Abl transformation of cells and proteasome activity in cell line models of CML, in support of a proteasome-targeting therapeutic approach for treating CML [35].
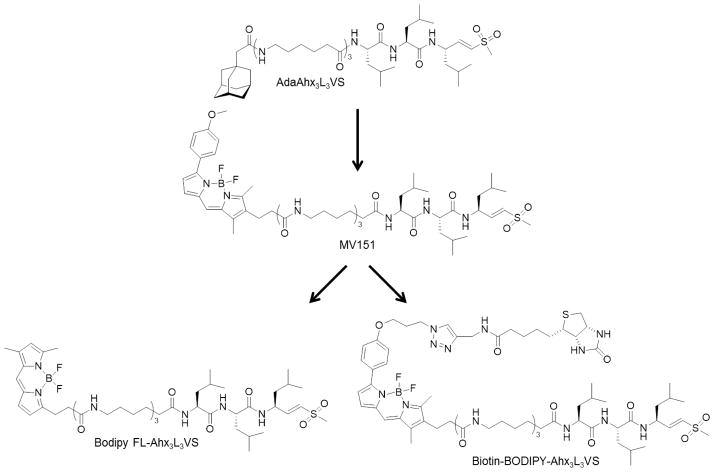
Finally, replacement of the dansyl moiety with the bright Bodipy TMR fluorophore afforded the cell-permeable activity-based fluorescent probe MV151 (Bodipy TMR-Ahx3L3VS) [36] (Figure 3). The Bodipy fluorophore allows sensitive in-gel detection of proteasome catalytic subunits modified by this probe as well as the ability to monitor proteasome activity in living cells by fluorescence microscopy. Additionally, MV151 effectively labels active proteasome subunits in vivo following intraperitoneal administration to mice. The utility of MV151 in examining the biodistribution of the boronic acid proteasome inhibitor MG262 was also demonstrated [36]. Like the previous radiolabeled and dansylated peptide vinyl sulfones, MV151 has also been used to evaluate the subunit binding preferences of both established and novel proteasome inhibitors, the latter of which has resulted in the development of subunit-selective and broad-spectrum proteasome inhibitors and ABPs [28, 30, 37–40]. Results from one of these studies indicated that caspase-3 inhibitors target the β1 subunit of the Arabidopsis proteasome, indicating the proteasome’s involvement in programmed cell death in plants. This study also used MV151 to monitor proteasome activities during defense responses of Arabidopsis plants following exposure to benzothiadiazole, an activator of salicylic acid signaling [28]. Corroborating results obtained with the dansylated probe [29], labeling of active proteasome subunits with MV151 demonstrated their elevated activity in bortezomib-adapted cell lines in comparison with the parental cell lines from which they were generated, as well as the residual activity of the targeted β1 and β5 subunits in the presence of a clinically relevant concentration of bortezomib in the bortezomib-adapted cells. This was the first report describing quantitative analysis of altered proteasome activity patterns in intact cells following adaptation to bortezomib [41].
Figure 3.
Development of broad-spectrum proteasome ABPs based on the extended peptide vinyl sulfone inhibitor AdaAhx3L3VS.
Based on the successful development of MV151 and its broad applications, the same research group subsequently synthesized a series of fluorescent proteasome-targeting ABPs containing the H2NAhx3L3VS sequence to identify additional novel probes suitable for use in clinical settings. While the incorporation of different fluorescent dyes influenced the cell permeability and subunit binding specificities of the resulting probes, incorporation of the Bodipy FL fluorophore afforded a broad-spectrum ABP suitable for proteasome labeling in living cells [42] (Figure 3). Like MV151, this probe was shown to be amenable to microscopy and flow cytometry-based readouts. Probing proteasome activity of different mouse tissues using this probe revealed differential activities of individual active subunits among these tissues, demonstrating the organ-specific regulation of proteasome activity and supporting the presence of different proteasome subtypes. Bodipy FL-Ahx3L3VS was also used to evaluate the cellular effects of proteasome inhibitors in intact tissue segments [42]. Results from this study suggested that MV151 and the Bodipy FL-labeled probe could be used to assess the inhibitory action and efficacy of proteasome inhibitors in clinical settings. In fact, the use of a close analogue of this probe in a comparative study of bortezomib and the orally-bioavailable second-generation boronic acid inhibitor delanzomib showed that, in a preclinical model of human multiple myeloma, bortezomib and delanzomib similarly inhibit the proteasome in normal tissues, while inhibition in tumor tissue was significantly enhanced by delanzomib in comparison with bortezomib [43, 44].
From Natural Product Proteasome Inhibitors to Imaging Probes
The natural product proteasome inhibitor syringolin A (SylA) belongs to a class of proteasome inhibitors known as the syrbactins. Similar to peptide vinyl sulfone-based inhibitors, the syrbactins bind irreversibly to the proteasome’s catalytic threonine residue in a Michael-type addition with their α,β-unsaturated amide. The fluorescent derivative, Rh-SylA, was generated by derivatization of SylA’s free carboxyl group, a position at which SAR studies indicated could be modified without significantly reducing the potency of the resulting rhodamine-tagged probe [45, 46] (Figure 4). In investigating the potential utility of SylA as a therapeutic agent, Rh-SylA was used to further explore the proteins bound by SylA. In EL4 lysates, Rh-SylA was shown to specifically label both constitutive and immunosubunits through mechanism-dependent binding, with a high signal-to-noise ratio. The β5 subunit was found to be the preferential target of this probe, followed by the β2 subunit, while binding to the β1 subunit required higher concentrations. This subunit selectivity differs from that of the β5/β1-selective inhibitor bortezomib—a property that could prove useful in overcoming bortezomib resistance mechanisms. Like other proteasome-targeting ABPs, Rh-SylA demonstrated utility in determining the subunit binding selectivities of proteasome inhibitors and, like dansylAhx3L3VS and MV151 [29, 41], Rh-SylA can be used to visualize differences in activities of proteasome catalytic subunits in lysates from parental and bortezomib-adapted cell lines [46]. This probe was also used to investigate the subcellular targeting of SylA via confocal microscopy and in-gel fluorescence readouts [47]. An inactive Rh-SylA derivative lacking the Michael ring system was used to demonstrate that nuclear accumulation of Rh-SylA resulted from its mechanism-based proteasome binding in this organelle [47].
Figure 4.
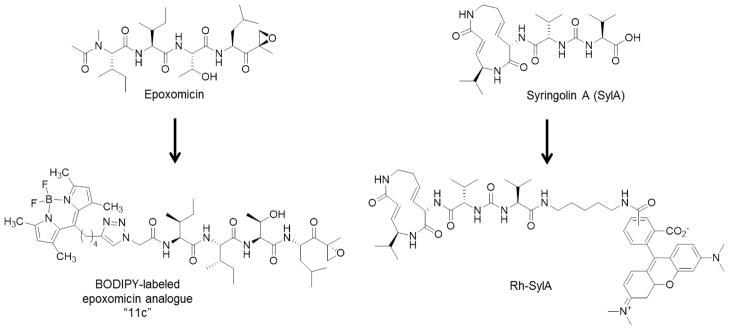
Development of fluorescent epoxomicin and syringolin-A analogues.
Epoxomicin is another natural product proteasome inhibitor that has been successfully derivatized to produce imaging probes of proteasomes. Initially, biotin-tagged epoxomicin was created to investigate the antitumor mechanism of epoxomicin. Subsequent to the identification of the proteasome as the selective target of epoxomicin, this peptide epoxyketone natural product has been extensively exploited as a molecular probe [48]. For example, biotinylated epoxomicin was used to screen a small library of peptide epoxyketones, leading to the identification of the first β1i-selective inhibitor, UK-101 [20]. In addition, three epoxomicin-derived fluorescent ABPs were reported. These probes, which were generated by the chemoselective labeling of an azido-functionalized epoxomicin analogue with three different acetylene-functionalized BODIPY dyes via the copper (I) catalyzed Huisgen 1,3-dipolar cycloaddition, or “click” reaction, were shown to label active proteasome subunits in EL4 lysates [46] (Figure 4). All of these probes were also shown to be cell-permeable and therefore capable of labeling proteasomes in intact EL4 cells [38]. In a more recent study, one of these probes was used to demonstrate Rh-SylA’s specificity toward the proteasome and mechanism-dependent binding [45]. Biotin-BODIPY-epoxomicin and BODIPY-epoxomicin, together with biotin-BODIPY-Ahx3-L3VS and MV151, have also been used to show that the thymoproteasome subunit β5t is catalytically active, and to demonstrate that the substrate/inhibitor sequence preferences of this subunit differ from those of β5 and β5i. This provided evidence in support of the involvement of β5t in producing peptides for positive selection of T cells [49].
Additionally, BODIPY-epoxomicin was used to study the inhibition of Arabidopsis proteasomes by syringolin A (SylA) [47]. This probe, as well as its azide-containing and biotin-containing analogues, is more specific for catalytic proteasome subunits than MV151, which also targets papain-like cysteine proteases [28, 47]. Both MV151 and BODIPY-epoxomicin have been shown to preferentially target β5 but label all catalytic subunits of the Arabidopsis proteasome. Biotinylated BODIPY-epoxomicin was used to demonstrate that SylA preferentially binds β2 and β5 in Arabidopsis leaf extracts. These results demonstrated the value of epoxomicin-based imaging probes in demonstrating that the Michael ring system plays a critical role in the activity of SylA, as expected based on the proposed binding mechanism [50], and that the L-configuration of the valine at position 1 of SylA is required for its β2 selectivity. Together with Rh-SylA, BODIPY-epoxomicin was used to investigate the subcellular targeting of SylA via confocal microscopy and in-gel fluorescence readouts [47].
Proteasome Imaging Probes from Two-Step Reactions
To circumvent the potential cell-impermeability and altered binding affinities resulting from direct attachment of biotin or fluorophores to inhibitory compounds, two-step labeling strategies have been developed (Figure 5). In these approaches, probes tagged with small functional groups (e.g. azide, alkyne) are first incubated with living cells, resulting in covalent modification of the targeted proteins. Following cell lysis and denaturation of cellular proteins, this functional group is utilized as a ligation handle to attach a reporter group in a bioorthogonal reaction. A cell-permeable azide-containing active site probe, “azide-containing proteasome inhibitor 2” (AdaAhx(α-N3)Ahx2L3VS), targeting all six catalytic subunits of the constitutive proteasome and immunoproteasome, was the first proteasome-targeting activity-based probe designed for use in a two-step labeling procedure [51]. Treatment of cells with this probe resulted in covalent modification of all active proteasome subunits, and, following lysis and denaturation of cellular proteins, addition of a biotinylated phosphane reagent allowed selective modification of the azido groups in a Staudinger-Bertozzi ligation [51]. Similarly, using a bifunctional azido-BODIPY acid derivative, vinyl sulphone pharmacophore-based imaging probes were prepared for one- and two-step labeling. While both the one-step probe, biotin-BODIPY-Ahx3-L3VS (Figure 3), and the two-step probe, N3-BODIPY-Ahx3-L3VS, appeared to be about equally effective in labeling active subunits in EL4 cell lysates, differences were observed in labeling in living EL4 cells, with N3-BODIPY-Ahx3L3VS being more efficient, most likely reflecting its enhanced cell permeability. This demonstrates the benefit of the two-step labeling approach. Furthermore, the efficiency of two-step labeling was shown to solely depend on the interaction of the probe with the active proteasome subunits and not on the second ligation step [52]. The targets of the two-step probe were determined in living plant cells. Extracts from probe-treated cells were incubated with biotin-alkyne, and subsequent purification of biotinylated proteins revealed that, aside from proteasome catalytic subunits, the papain-like cysteine proteases RD21 and RD19A and RD19B were also targets. Competition assays with N3-BODIPY-Ahx3L3VS confirmed epoxomicin as a proteasome-selective inhibitor and E-64d as an inhibitor selectively targeting papain-like cysteine proteases, but the alleged proteasome-selective inhibitor MG132 was found to preferentially inhibit papain-like cysteine proteases rather than the proteasome [53].
Figure 5.
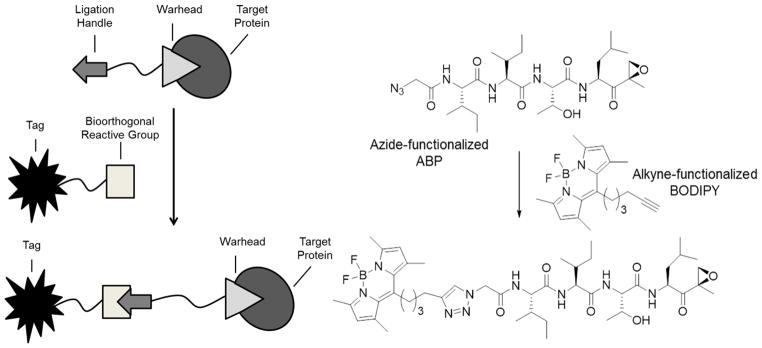
A) Schematic representation of a two-step labeling approach. Living cells are first treated with an ABP containing a ligation handle for subsequent post-lysis labeling of probe-bound proteins by a bioorthogonal ligation reagent containing a reporter tag. B) An example of a proteasome-targeting two-step ABP [38, 61].
Subunit-Selective Imaging Probes
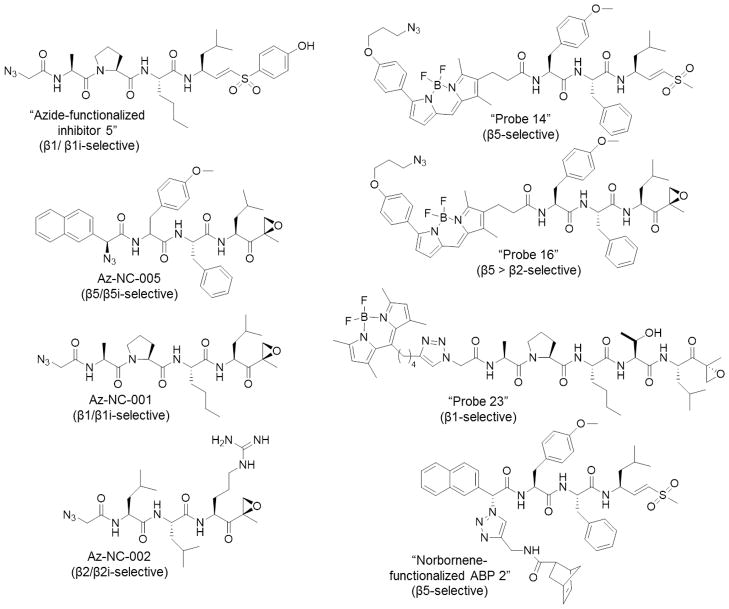
As expression levels of catalytic proteasome subunits in cancer cell lines are highly variable, recent research efforts have been made to dissect the function of each catalytic subunit. Over the past few years, several subunit-specific chemical probes have been developed (Figure 6). For example, “azide-functionalized inhibitor 5” (N3AcAPNleLVSPhOH), a cell-permeable, C-L-specific inhibitor, was developed to facilitate two-step labeling of the subunits responsible for this activity in living cells via a Staudinger-Bertozzi ligation. In RPMI 8226 human multiple myeloma cells, detection of proteasome subunits labeled by this method via fluorescently labeled streptavidin revealed preferential labeling of β1i at low inhibitor concentrations, and labeling of both β1i and β1 at higher concentrations. At the concentrations used, no off-target proteins were detected on a 2D gel. This probe can be used for functional studies of C-L subunits [54]. In a similar approach, incorporation of an azido group into a β5/β5i-selective epoxyketone-based inhibitor gave rise to a two-step ABP targeting these subunits. Treatment of RPMI 8226 multiple myeloma cells with az-NC-005, followed by cell lysis and incubation with an azido-reactive biotinylated phosphane to facilitate a Staudinger-Bertozzi ligation, demonstrated that this probe selectively labels β5/β5i. At higher concentrations, az-NC-005 also labels β2, β2i, β1, and β1i [55]. Similarly, a β1/β1i-selective probe was developed based on the inhibitor NC-001. Az-NC-001 was shown to be highly selective for these subunits in RPMI 8226 cells, both based on streptavidin affinity blotting in whole cell lysates and immunoblotting with subunit-specific antibodies following affinity purification of probe-bound proteins [55]. Az-NC-001 was also used, along with a norbornene-functionalized β5-selective probe and an alkyne-functionalized epoxomicin-based ABP, to independently label β1, β2, and β5 with different reporter groups (BodipyTMR, BodipyFL, and biotin) in a single sample. This labeling strategy employed a tetrazine ligation, Staudinger-Bertozzi ligation, and copper(I)-catalyzed Huisgen [2+3] cycloaddition (“click” reaction), and was the first reported application of a tetrazine ligation to such an approach[56].
Figure 6.
Subunit-selective ABPs.
To further evaluate the selectivity of newly developed inhibitors of the proteasome’s trypsin-like activity, the two-step ABP az-NC-002 was synthesized. Following incubation of NCI-H929 cells with this probe, cell lysis, and introduction of the azido-reactive biotinylated phosphane to initiate Staudinger-Bertozzi ligation, western blotting showed that the major target labeled by az-NC-002 was β2, and β2i was labeled more weakly [30]. Likewise, β1- and β5-selective activity-based fluorescent probes (Figure 6) containing BODIPY fluorophores were demonstrated to selectively label their targeted subunits in both HEK293T cell lysates and living HEK293T cells, which express only constitutive proteasomes. Importantly, these subunit-selective imaging probes have the ability of simultaneously labeling their targeted subunits in cell lysates, allowing two-color imaging. This improved resolution over labeling with broad-spectrum proteasome ABPs, following which the β1 and β5 subunits are typically difficult to resolve on an SDS-PAGE gel [39].
Immunoproteasome-Selective Imaging Probes
While the subunit-selective ABPs described thus far selectively target β1-type, β2-type, or β5-type subunits, they are limited by their inability to discriminate between the targeted constitutive proteasome subunit and its immunoproteasome counterpart. Probes that selectively target the constitutive or immunoproteasome subunits are essential for gaining insight into the functions of these distinct proteasome subtypes.
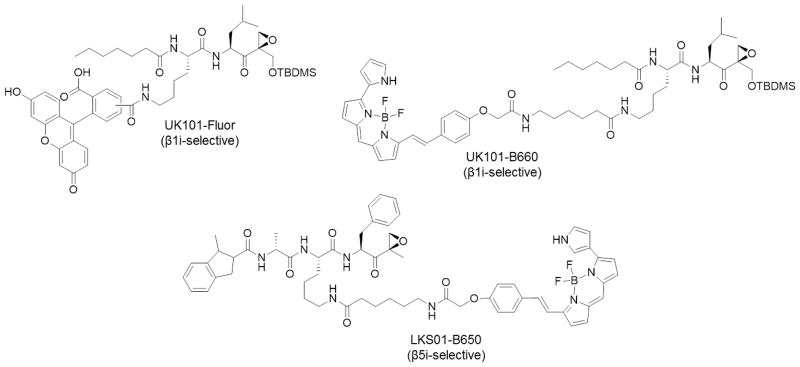
The first fluorescent, β1i-selective ABP, UK101-Fluor, was developed by derivatization of the β1i-selective inhibitor UK-101 [20] to introduce a fluorescein moiety at the P2 position [57] (Figure 7). This position was chosen based on molecular modeling studies, which indicated that the P2 alanine side chain of UK-101 does not interact directly with the substrate binding pocket of the β1i subunit [58]. Indeed, the P2 position was successfully modified to allow introduction of fluorescent groups while maintaining the β1i binding selectivity of the parent compound. Competition assays with unlabeled UK-101, as well as the markedly reduced fluorescent signal observed following shRNA-mediated knockdown of β1i, demonstrated that UK101-Fluor maintained its β1i selectivity. This probe enabled the subcellular localization of catalytically active β1i to be assessed using fluorescence microscopy.
Figure 7.
Immunoproteasome-selective ABPs.
Due to the high expression levels of this subunit in multiple types of cancer [17, 20, 59], we also developed a near-infrared (NIRF) probe by replacing the fluorescein group of UK101-Fluor with the BODIPY 650/665 fluorophore for use as a non-invasive imaging probe in animal studies [57] (Figure 7). The utility of this probe in allowing the selective detection of β1i in living cells was demonstrated by both in-gel fluorescence and fluorescence microscopy. This probe may therefore be used to evaluate β1i levels in tumor tissues. Additionally, with β1i serving as a potential tumor biomarker, UK101-B660 may serve as an imaging probe for cancer screening or for monitoring disease progression.
Using a similar approach, the β5i-selective inhibitor IPSI served as a lead compound in developing the β5i-selective fluorescent ABP LKS01-B650 [60] (Figure 7). Western blotting, in-gel fluorescence, and fluorescence microscopy analysis demonstrated that LKS01-B650 selectively targets β5i and can be utilized to monitor the subcellular localization of this immunoproteasome subunit. Additionally, substantial colocalization of fluorescent signals from LKS01-B650 and UK101-Fluor further demonstrated that LKS01-B650 selectively targets catalytically active β5i in the context of the fully assembled immunoproteasome complex in living cells. This probe can therefore be used in functional studies of the immunoproteasome in disease states. Like UK101-B660, the NIRF fluorophore of LKS01-B650 enables the use of this probe in monitoring the subcellular localization of catalytically active β5i in in vivo studies.
Perspective
Our understanding of the function of proteasomes has advanced over past two decades due, in large part, to the available proteasome inhibitors. Despite these advances, there is a remaining need for imaging probes that allow proteasome functions to be monitored in living cell models as well as in in vivo settings. In recent years, a new class of probes that selectively target the immunoproteasome has provided powerful tools for investigating immunoproteasome biology. It is clear that imaging probes selectively targeting the constitutive proteasome or immunoproteasome are valuable for investigating the distinct functions of these subtypes in both in vitro and in vivo settings. Functional studies with immunoproteasome-targeting imaging probes are expected to make great contributions towards deciphering the cellular roles of immunoproteasomes.
Acknowledgments
We gratefully acknowledge the National Institutes of Health for their financial support (R01 CA128903).
Footnotes
Conflict of interest All authors declare that they have no con ict of interest.
References
- 1.Konstantinova IM, Tsimokha AS, Mittenberg AG. Role of proteasomes in cellular regulation. Int Rev Cell Mol Biol. 2008;267:59–124. doi: 10.1016/S1937-6448(08)00602-3. [DOI] [PubMed] [Google Scholar]
- 2.Marques AJ, Palanimurugan R, Matias AC, Ramos PC, Dohmen RJ. Catalytic mechanism and assembly of the proteasome. Chem Rev. 2009;109:1509–1536. doi: 10.1021/cr8004857. [DOI] [PubMed] [Google Scholar]
- 3.Navon A, Ciechanover A. The 26 S proteasome: from basic mechanisms to drug targeting. J Biol Chem. 2009;284:33713–33718. doi: 10.1074/jbc.R109.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 5.Hallermalm K, Seki K, Wei C, Castelli C, Rivoltini L, Kiessling R, Levitskaya J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood. 2001;98:1108–1115. doi: 10.1182/blood.v98.4.1108. [DOI] [PubMed] [Google Scholar]
- 6.Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 7.De M, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. Beta 2 subunit propeptides influence cooperative proteasome assembly. J Biol Chem. 2003;278:6153–6159. doi: 10.1074/jbc.M209292200. [DOI] [PubMed] [Google Scholar]
- 8.Murata S, Yashiroda H, Tanaka K. Molecular mechanisms of proteasome assembly. Nat Rev Mol Cell Biol. 2009;10:104–115. doi: 10.1038/nrm2630. [DOI] [PubMed] [Google Scholar]
- 9.Yewdell JW. Immunoproteasomes: regulating the regulator. Proc Natl Acad Sci U S A. 2005;102:9089–9090. doi: 10.1073/pnas.0504018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groettrup M, Kirk CJ, Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010;10:73–78. doi: 10.1038/nri2687. [DOI] [PubMed] [Google Scholar]
- 11.Rockwell CE, Monaco JJ, Qureshi N. A Critical Role for the Inducible Proteasomal Subunits LMP7 and MECL1 in Cytokine Production by Activated Murine Splenocytes. Pharmacology. 2012;89:117–126. doi: 10.1159/000336335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schroter F, Prozorovski T, Lange N, Steffen J, Rieger M, Kuckelkorn U, Aktas O, Kloetzel PM, Kruger E. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Angeles A, Fung G, Luo H. Immune and non-immune functions of the immunoproteasome. Front Biosci. 2012;17:1904–1916. doi: 10.2741/4027. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Hernandez M, Hernandez F, Martin-Aparicio E, Gomez-Ramos P, Moran MA, Castano JG, Ferrer I, Avila J, Lucas JJ. Neuronal induction of the immunoproteasome in Huntington’s disease. J Neurosci. 2003;23:11653–11661. doi: 10.1523/JNEUROSCI.23-37-11653.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M, Licastro F, Sorbi S, Pession A, Ohm T, Grune T, Franceschi C. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol Aging. 2006;27:54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick LR, Khare V, Small JS, Koltun WA. Dextran sulfate sodium-induced colitis is associated with enhanced low molecular mass polypeptide 2 (LMP2) expression and is attenuated in LMP2 knockout mice. Dig Dis Sci. 2006;51:1269–1276. doi: 10.1007/s10620-006-8047-2. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn DJ, Hunsucker SA, Chen Q, Voorhees PM, Orlowski M, Orlowski RZ. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood. 2009;113:4667–4676. doi: 10.1182/blood-2008-07-171637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn DJ, Orlowski RZ. The immunoproteasome as a target in hematologic malignancies. Seminars in hematology. 2012;49:258–262. doi: 10.1053/j.seminhematol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C, Sylvain C, Ring ER, Shields J, Jiang J, Shwonek P, Parlati F, Demo SD, Bennett MK, Kirk CJ, Groettrup M. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 20.Ho YK, Bargagna-Mohan P, Wehenkel M, Mohan R, Kim KB. LMP2-specific inhibitors: chemical genetic tools for proteasome biology. Chem Biol. 2007;14:419–430. doi: 10.1016/j.chembiol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parlati F, Lee SJ, Aujay M, Suzuki E, Levitsky K, Lorens JB, Micklem DR, Ruurs P, Sylvain C, Lu Y, Shenk KD, Bennett MK. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- 22.Singh AV, Bandi M, Aujay MA, Kirk CJ, Hark DE, Raje N, Chauhan D, Anderson KC. PR-924, a selective inhibitor of the immunoproteasome subunit LMP-7, blocks multiple myeloma cell growth both in vitro and in vivo. Br J Haematol. 2011;152:155–163. doi: 10.1111/j.1365-2141.2010.08491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W, Kim KB. The immunoproteasome: an emerging therapeutic target. Current Topics in Medicinal Chemistry. 2011;11:2923–2930. doi: 10.2174/156802611798281348. [DOI] [PubMed] [Google Scholar]
- 24.Bogyo M, McMaster JS, Gaczynska M, Tortorella D, Goldberg AL, Ploegh H. Covalent modification of the active site threonine of proteasomal beta subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proc Natl Acad Sci U S A. 1997;94:6629–6634. doi: 10.1073/pnas.94.13.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogyo M, Shin S, McMaster JS, Ploegh HL. Substrate binding and sequence preference of the proteasome revealed by active-site-directed affinity probes. Chem Biol. 1998;5:307–320. doi: 10.1016/s1074-5521(98)90169-7. [DOI] [PubMed] [Google Scholar]
- 26.Nazif T, Bogyo M. Global analysis of proteasomal substrate specificity using positional-scanning libraries of covalent inhibitors. Proc Natl Acad Sci U S A. 2001;98:2967–2972. doi: 10.1073/pnas.061028898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler BM, Tortorella D, Altun M, Kisselev AF, Fiebiger E, Hekking BG, Ploegh HL, Overkleeft HS. Extended peptide-based inhibitors efficiently target the proteasome and reveal overlapping specificities of the catalytic beta-subunits. Chem Biol. 2001;8:913–929. doi: 10.1016/s1074-5521(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 28.Gu C, Kolodziejek I, Misas-Villamil J, Shindo T, Colby T, Verdoes M, Richau KH, Schmidt J, Overkleeft HS, van der Hoorn RA. Proteasome activity profiling: a simple, robust and versatile method revealing subunit-selective inhibitors and cytoplasmic, defense-induced proteasome activities. Plant J. 2010;62:160–170. doi: 10.1111/j.1365-313X.2009.04122.x. [DOI] [PubMed] [Google Scholar]
- 29.Kraus M, Ruckrich T, Reich M, Gogel J, Beck A, Kammer W, Berkers CR, Burg D, Overkleeft H, Ovaa H, Driessen C. Activity patterns of proteasome subunits reflect bortezomib sensitivity of hematologic malignancies and are variable in primary human leukemia cells. Leukemia. 2007;21:84–92. doi: 10.1038/sj.leu.2404414. [DOI] [PubMed] [Google Scholar]
- 30.Mirabella AC, Pletnev AA, Downey SL, Florea BI, Shabaneh TB, Britton M, Verdoes M, Filippov DV, Overkleeft HS, Kisselev AF. Specific cell-permeable inhibitor of proteasome trypsin-like sites selectively sensitizes myeloma cells to bortezomib and carfilzomib. Chemistry & biology. 2011;18:608–618. doi: 10.1016/j.chembiol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, Ploegh HL, Ovaa H, Galardy PJ. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, Ovaa H, Berkers C, Nicholson B, Chao TH, Neuteboom ST, Richardson P, Palladino MA, Anderson KC. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Crawford LJ, Walker B, Ovaa H, Chauhan D, Anderson KC, Morris TC, Irvine AE. Comparative selectivity and specificity of the proteasome inhibitors BzLLLCOCHO, PS-341, and MG-132. Cancer Res. 2006;66:6379–6386. doi: 10.1158/0008-5472.CAN-06-0605. [DOI] [PubMed] [Google Scholar]
- 34.Kristiansen M, Deriziotis P, Dimcheff DE, Jackson GS, Ovaa H, Naumann H, Clarke AR, van Leeuwen FW, Menendez-Benito V, Dantuma NP, Portis JL, Collinge J, Tabrizi SJ. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell. 2007;26:175–188. doi: 10.1016/j.molcel.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Crawford LJ, Windrum P, Magill L, Melo JV, McCallum L, McMullin MF, Ovaa H, Walker B, Irvine AE. Proteasome proteolytic profile is linked to Bcr-Abl expression. Exp Hematol. 2009;37:357–366. doi: 10.1016/j.exphem.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Verdoes M, Florea BI, Menendez-Benito V, Maynard CJ, Witte MD, van der Linden WA, van den Nieuwendijk AM, Hofmann T, Berkers CR, van Leeuwen FW, Groothuis TA, Leeuwenburgh MA, Ovaa H, Neefjes JJ, Filippov DV, van der Marel GA, Dantuma NP, Overkleeft HS. A fluorescent broad-spectrum proteasome inhibitor for labeling proteasomes in vitro and in vivo. Chem Biol. 2006;13:1217–1226. doi: 10.1016/j.chembiol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Verdoes M, Berkers CR, Florea BI, van Swieten PF, Overkleeft HS, Ovaa H. Chemical proteomics profiling of proteasome activity. Methods Mol Biol. 2006;328:51–69. doi: 10.1385/1-59745-026-X:51. [DOI] [PubMed] [Google Scholar]
- 38.Verdoes M, Hillaert U, Florea BI, Sae-Heng M, Risseeuw MD, Filippov DV, van der Marel GA, Overkleeft HS. Acetylene functionalized BODIPY dyes and their application in the synthesis of activity based proteasome probes. Bioorg Med Chem Lett. 2007;17:6169–6171. doi: 10.1016/j.bmcl.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Verdoes M, Willems LI, van der Linden WA, Duivenvoorden BA, van der Marel GA, Florea BI, Kisselev AF, Overkleeft HS. A panel of subunit-selective activity-based proteasome probes. Org Biomol Chem. 2010;8:2719–2727. doi: 10.1039/c001036g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Screen M, Britton M, Downey SL, Verdoes M, Voges MJ, Blom AE, Geurink PP, Risseeuw MD, Florea BI, van der Linden WA, Pletnev AA, Overkleeft HS, Kisselev AF. Nature of pharmacophore influences active site specificity of proteasome inhibitors. The Journal of biological chemistry. 2010;285:40125–40134. doi: 10.1074/jbc.M110.160606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruckrich T, Kraus M, Gogel J, Beck A, Ovaa H, Verdoes M, Overkleeft HS, Kalbacher H, Driessen C. Characterization of the ubiquitin-proteasome system in bortezomib-adapted cells. Leukemia. 2009;23:1098–1105. doi: 10.1038/leu.2009.8. [DOI] [PubMed] [Google Scholar]
- 42.Berkers CR, van Leeuwen FW, Groothuis TA, Peperzak V, van Tilburg EW, Borst J, Neefjes JJ, Ovaa H. Profiling Proteasome Activity in Tissue with Fluorescent Probes. Molecular pharmaceutics. 2007 doi: 10.1021/mp0700256. [DOI] [PubMed] [Google Scholar]
- 43.Berkers CR, Leestemaker Y, Schuurman KG, Ruggeri B, Jones-Bolin S, Williams M, Ovaa H. Probing the specificity and activity profiles of the proteasome inhibitors bortezomib and delanzomib. Molecular pharmaceutics. 2012;9:1126–1135. doi: 10.1021/mp2004143. [DOI] [PubMed] [Google Scholar]
- 44.de Jong A, Schuurman KG, Rodenko B, Ovaa H, Berkers CR. Fluorescence-based proteasome activity profiling. Methods Mol Biol. 2012;803:183–204. doi: 10.1007/978-1-61779-364-6_13. [DOI] [PubMed] [Google Scholar]
- 45.Clerc J, Groll M, Illich DJ, Bachmann AS, Huber R, Schellenberg B, Dudler R, Kaiser M. Synthetic and structural studies on syringolin A and B reveal critical determinants of selectivity and potency of proteasome inhibition. Proc Natl Acad Sci U S A. 2009;106:6507–6512. doi: 10.1073/pnas.0901982106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clerc J, Florea BI, Kraus M, Groll M, Huber R, Bachmann AS, Dudler R, Driessen C, Overkleeft HS, Kaiser M. Syringolin A selectively labels the 20 S proteasome in murine EL4 and wild-type and bortezomib-adapted leukaemic cell lines. Chembiochem. 2009;10:2638–2643. doi: 10.1002/cbic.200900411. [DOI] [PubMed] [Google Scholar]
- 47.Kolodziejek I, Misas-Villamil JC, Kaschani F, Clerc J, Gu C, Krahn D, Niessen S, Verdoes M, Willems LI, Overkleeft HS, Kaiser M, van der Hoorn RA. Proteasome activity imaging and profiling characterizes bacterial effector syringolin A. Plant physiology. 2011;155:477–489. doi: 10.1104/pp.110.163733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Florea BI, Verdoes M, Li N, van der Linden WA, Geurink PP, van den Elst H, Hofmann T, de Ru A, van Veelen PA, Tanaka K, Sasaki K, Murata S, den Dulk H, Brouwer J, Ossendorp FA, Kisselev AF, Overkleeft HS. Activity-based profiling reveals reactivity of the murine thymoproteasome-specific subunit beta5t. Chem Biol. 2010;17:795–801. doi: 10.1016/j.chembiol.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groll M, Schellenberg B, Bachmann AS, Archer CR, Huber R, Powell TK, Lindow S, Kaiser M, Dudler R. A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature. 2008;452:755–758. doi: 10.1038/nature06782. [DOI] [PubMed] [Google Scholar]
- 51.Ovaa H, van Swieten PF, Kessler BM, Leeuwenburgh MA, Fiebiger E, van den Nieuwendijk AM, Galardy PJ, van der Marel GA, Ploegh HL, Overkleeft HS. Chemistry in living cells: detection of active proteasomes by a two-step labeling strategy. Angew Chem Int Ed Engl. 2003;42:3626–3629. doi: 10.1002/anie.200351314. [DOI] [PubMed] [Google Scholar]
- 52.Verdoes M, Florea BI, Hillaert U, Willems LI, van der Linden WA, Sae-Heng M, Filippov DV, Kisselev AF, van der Marel GA, Overkleeft HS. Azido-BODIPY acid reveals quantitative Staudinger-Bertozzi ligation in two-step activity-based proteasome profiling. Chembiochem. 2008;9:1735–1738. doi: 10.1002/cbic.200800231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaschani F, Verhelst SH, van Swieten PF, Verdoes M, Wong CS, Wang Z, Kaiser M, Overkleeft HS, Bogyo M, van der Hoorn RA. Minitags for small molecules: detecting targets of reactive small molecules in living plant tissues using ‘click chemistry’. Plant J. 2009;57:373–385. doi: 10.1111/j.1365-313X.2008.03683.x. [DOI] [PubMed] [Google Scholar]
- 54.van Swieten PF, Samuel E, Hernandez RO, van den Nieuwendijk AM, Leeuwenburgh MA, van der Marel GA, Kessler BM, Overkleeft HS, Kisselev AF. A cell-permeable inhibitor and activity-based probe for the caspase-like activity of the proteasome. Bioorg Med Chem Lett. 2007;17:3402–3405. doi: 10.1016/j.bmcl.2007.03.092. [DOI] [PubMed] [Google Scholar]
- 55.Britton M, Lucas MM, Downey SL, Screen M, Pletnev AA, Verdoes M, Tokhunts RA, Amir O, Goddard AL, Pelphrey PM, Wright DL, Overkleeft HS, Kisselev AF. Selective inhibitor of proteasome’s caspase-like sites sensitizes cells to specific inhibition of chymotrypsin-like sites. Chem Biol. 2009;16:1278–1289. doi: 10.1016/j.chembiol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willems LI, Li N, Florea BI, Ruben M, van der Marel GA, Overkleeft HS. Triple bioorthogonal ligation strategy for simultaneous labeling of multiple enzymatic activities. Angew Chem Int Ed Engl. 2012;51:4431–4434. doi: 10.1002/anie.201200923. [DOI] [PubMed] [Google Scholar]
- 57.Carmony KC, Lee DM, Wu Y, Lee NR, Wehenkel M, Lee J, Lei B, Zhan CG, Kim KB. A bright approach to the immunoproteasome: development of LMP2/beta1i-specific imaging probes. Bioorg Med Chem. 2012;20:607–613. doi: 10.1016/j.bmc.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lei B, Abdul Hameed MD, Hamza A, Wehenkel M, Muzyka JL, Yao XJ, Kim KB, Zhan CG. Molecular basis of the selectivity of the immunoproteasome catalytic subunit LMP2-specific inhibitor revealed by molecular modeling and dynamics simulations. The journal of physical chemistry B. 2010;114:12333–12339. doi: 10.1021/jp1058098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wehenkel M, Ban JO, Ho YK, Carmony KC, Hong JT, Kim KB. A selective inhibitor of the immunoproteasome subunit LMP2 induces apoptosis in PC-3 cells and suppresses tumour growth in nude mice. Br J Cancer. 2012;107:53–62. doi: 10.1038/bjc.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma LK, Lee NR, Jang ER, Lei B, Zhan CG, Lee W, Kim KB. Activity-Based Near-Infrared Fluorescent Probe for LMP7: A Chemical Proteomics Tool for the Immunoproteasome in Living Cells. Chembiochem. 2012;13:1899–1903. doi: 10.1002/cbic.201200307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willems LI, Li N, Florea BI, Ruben M, van der Marel GA, Overkleeft HS. Triple Bioorthogonal Ligation Strategy for Simultaneous Labeling of Multiple Enzymatic Activities. Angewandte Chemie International Edition. 2012;51:4431–4434. doi: 10.1002/anie.201200923. [DOI] [PubMed] [Google Scholar]