Abstract
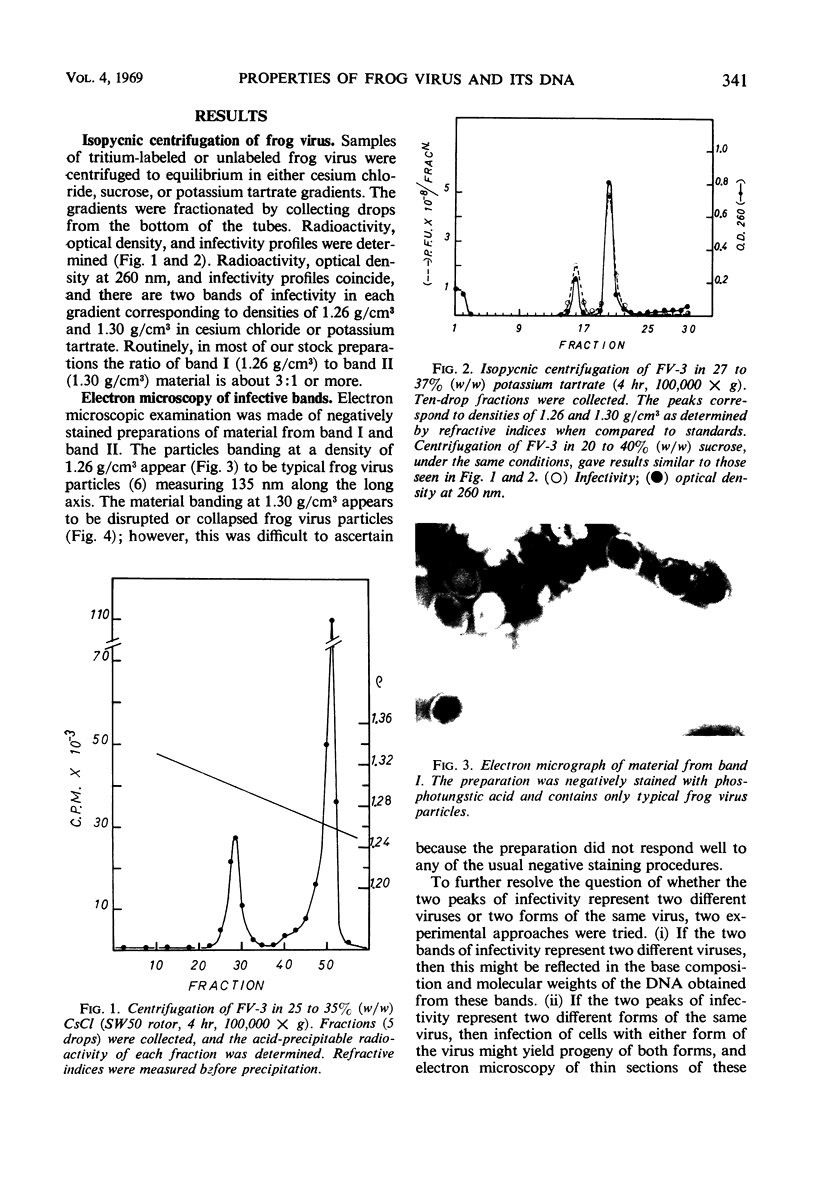
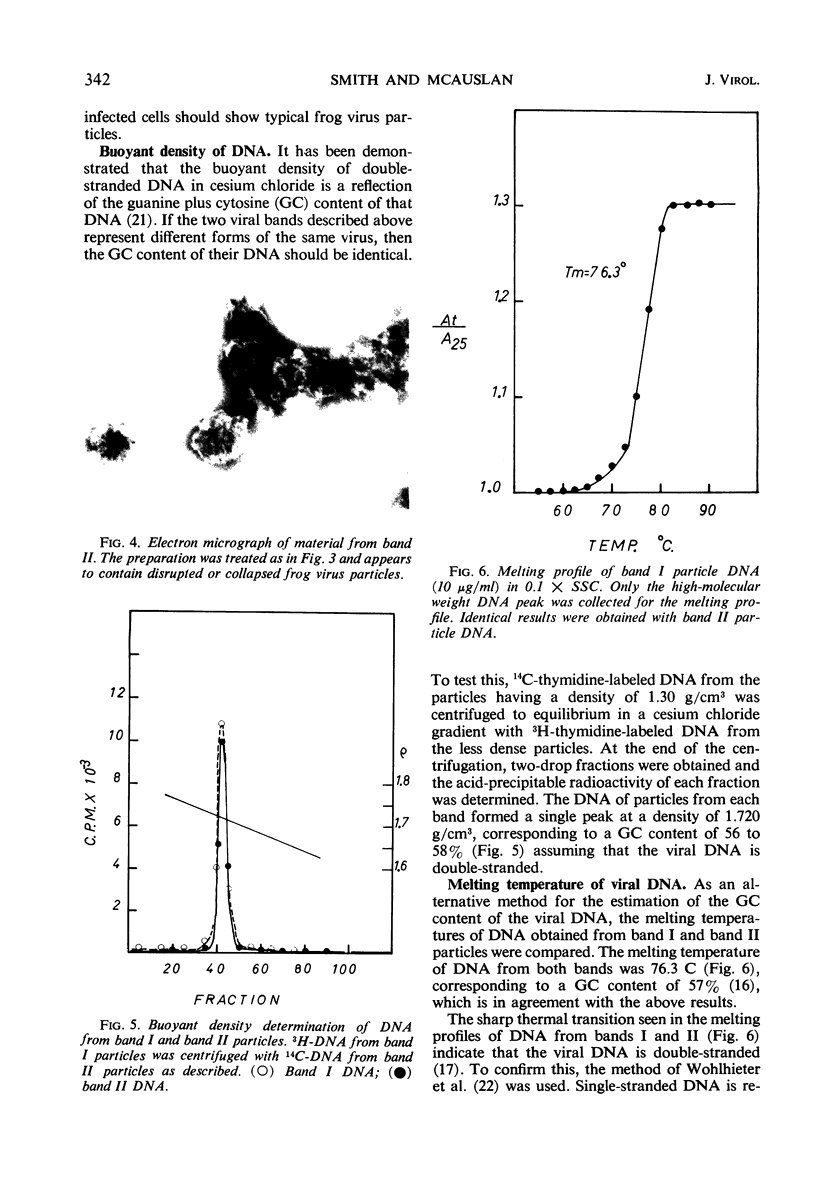
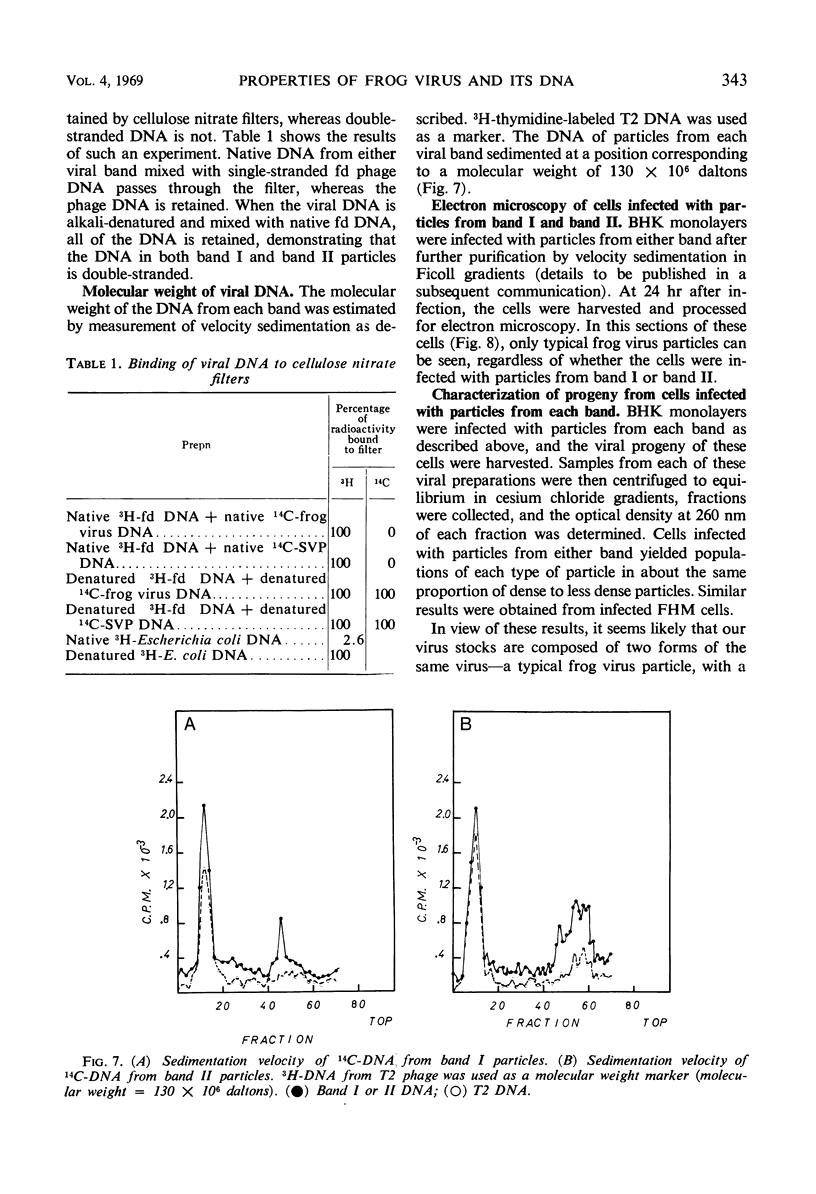
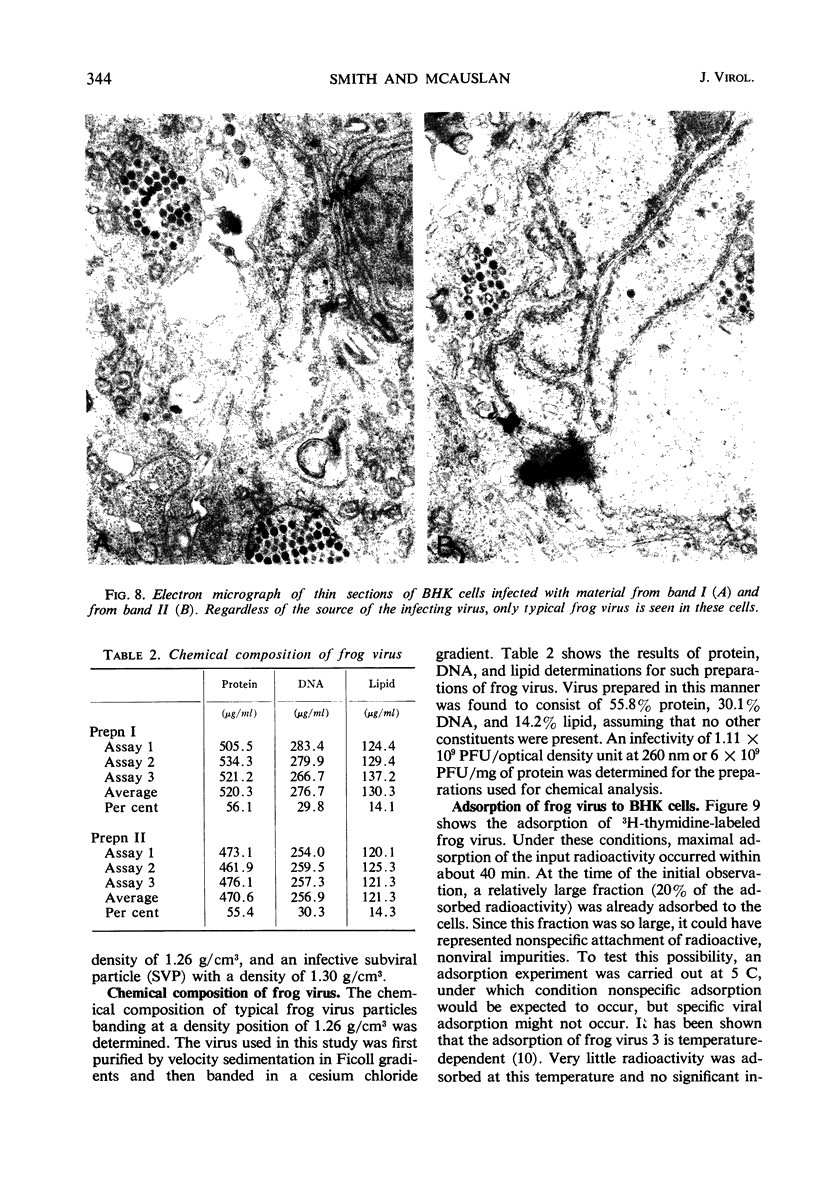
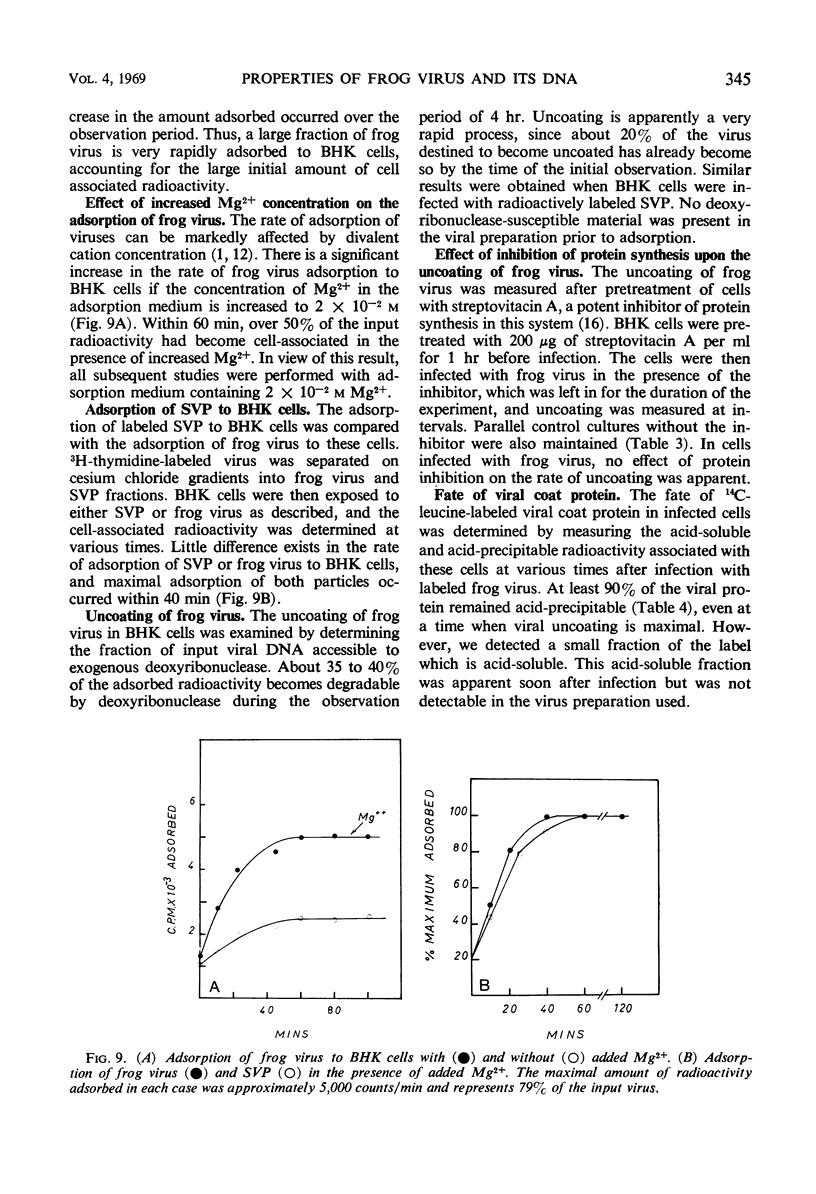
Frog virus (FV-3) was banded by isopycnic centrifugation in cesium chloride, sucrose, or potassium tartrate. Two bands of infectivity were regularly found at positions in cesium chloride corresponding to densities of 1.26 and 1.30 g/cm3, respectively. Deoxyribonucleic acid from either band had the following characteristics: double-stranded; a Tm of 76.3 C in 0.1 SSC (0.015 m NaCl plus 0.015 m sodium citrate) and a buoyant density of 1.720 g/cm3 in cesium chloride, corresponding to a guanine plus cytosine content of 56 to 58% and a molecular weight of 130 × 106 daltons, determined by velocity sedimentation. These data, together with electron micrographs of sections of cells infected with material from either band suggest that two types of infectious frog virus particles exists, rather than a second virus in the frog virus stocks. The composition of frog virus was determined. It was found that highly purified preparations of frog virus were composed of 55.8% protein, 30.1% deoxyribonucleic acid, and 14.2% lipid. The kinetics of adsorption and uncoating of FV-3 was studied with radioactive virus. Uncoating is comparatively rapid and in contrast to poxvirus is unaffected by inhibitors of protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellett A. J. The iridescent virus group. Adv Virus Res. 1968;13:225–246. doi: 10.1016/s0065-3527(08)60254-7. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., LEE A. J. DISCUSSION AND PRELIMINARY REPORTS. THE NUCLEIC ACID OF HUMAN CYTOMEGALOVIRUS. Virology. 1964 May;23:105–107. doi: 10.1016/s0042-6822(64)80014-3. [DOI] [PubMed] [Google Scholar]
- Davern C. I. Isolation of the DNA of the E. coli chromosome in one piece. Proc Natl Acad Sci U S A. 1966 Apr;55(4):792–797. doi: 10.1073/pnas.55.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs A. J., Harrison B. D., Watson D. H., Wildy P. What's in a virus name? Nature. 1966 Jan 29;209(5022):450–454. doi: 10.1038/209450a0. [DOI] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. I. The isolation and properties of virus from normal and tumor tissue. Virology. 1966 May;29(1):133–148. doi: 10.1016/0042-6822(66)90203-0. [DOI] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. I. The isolation and properties of virus from normal and tumor tissue. Virology. 1966 May;29(1):133–148. doi: 10.1016/0042-6822(66)90203-0. [DOI] [PubMed] [Google Scholar]
- Hochberg E., Becker Y. Adsorption, penetration and uncoating of herpes simplex virus. J Gen Virol. 1968 Mar;2(2):231–241. doi: 10.1099/0022-1317-2-2-231. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. II. THE MOLECULAR BASIS OF THE UNCOATING PROCESS. J Mol Biol. 1964 Feb;8:277–288. doi: 10.1016/s0022-2836(64)80137-6. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Relationship between protein synthesis and viral deoxyribonucleic acid synthesis. J Virol. 1967 Feb;1(1):110–114. doi: 10.1128/jvi.1.1.110-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McAuslan B. R., Smith W. R. Deoxyribonucleic acid synthesis in FV-3-infected mammalian cells. J Virol. 1968 Oct;2(10):1006–1015. doi: 10.1128/jvi.2.10.1006-1015.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Spear P. G., Roizman B. Some biophysical properties of frog viruses and their DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1155–1157. doi: 10.1073/pnas.56.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. PROPERTIES OF THE NUCLEIC ACIDS FROM SOME HERPES GROUP VIRUSES. Virology. 1964 Feb;22:288–292. doi: 10.1016/0042-6822(64)90017-0. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Wohlhieter J. A., Falkow S., Citarella R. V. Purification of episomal DNA with cellulose nitrate membrane filters. Biochim Biophys Acta. 1966 Dec 21;129(3):475–481. doi: 10.1016/0005-2787(66)90062-1. [DOI] [PubMed] [Google Scholar]