Abstract
The basolateral amygdala (BLA) and ventral subiculum (vSub) of the hippocampus convey emotion and context information, respectively, to the nucleus accumbens (NAc). Using in vivo extracellular recordings from NAc neurons, we examined how acute and repeated restraint stress alters the plasticity of the vSub and BLA afferent pathways. High frequency (HFS) and low frequency (LFS) stimulation was applied to the vSub to assess the impact on NAc responses to vSub and BLA inputs. In addition, iontophoretic application of the D2-antagonist sulpiride was used to explore the role of dopamine in the NAc in mediating the effects of stress on plasticity. Acute and repeated restraint caused disparate effects on BLA- and vSub-evoked responses in the NAc. Following repeated restraint, but not after acute restraint, HFS of the vSub failed to potentiate the vSub-NAc pathway while instead promoting a long lasting reduction of the BLA-NAc pathway, and these effects were independent of D2-receptor activity. In contrast, LFS to the vSub pathway after acute restraint resulted in potentiation in the vSub-NAc pathway while BLA-evoked responses were unchanged. When sulpiride was applied prior to LFS of the vSub after acute stress, there was a pronounced decrease in vSub-evoked responses similar to control animals.
This work provides new insight into the impact of acute and repeated stress on the integration of context and emotion inputs in the nucleus accumbens. These data support a model of stress whereby the hippocampus is inappropriately activated and dominates the information processing within this circuit via a dopaminergic mechanism after acute bouts of stress.
Keywords: acute stress, repeated stress, nucleus accumbens, hippocampus, amygdala, dopamine
Introduction
Despite the link between stress and the development or exacerbation of psychiatric illness, few studies have compared the neurophysiological effects of acute and repeated stressors. Animal models of anxiety disorders, including repeated exposure to stressors such as restraint, exhibit altered associative learning, decreased responsivity to rewarding stimuli, increased aggression, and impaired attentional set-shifting (Papp et al., 1991; Moreau et al., 1992; Rademacher and Hillard, 2007; Bondi et al., 2008; Wood et al., 2008). Most of these behaviors are attributed to modifications in hippocampal, prefrontal cortical, and basolateral amygdala (BLA) functioning resulting from chronic exposure to stress. Furthermore, there are likely long-lasting changes to the associated neural circuits (Ressler and Mayberg, 2007). Repeated, but not acute, restraint stress can alter normal plasticity, morphology, and neurochemistry in both BLA and hippocampus (Conrad et al., 1999; Copeland et al., 2005; Donohue et al., 2006; Garcia et al., 2008). Chronic stress results in atrophy of apical dendrites, along with a reduced number of synapses, in the CA3 region of the hippocampus (Conrad et al., 1999; Fuchs et al., 2006). In contrast, chronic stress elicits dendritic hypertrophy in the amygdala (Vyas et al., 2002). However, it remains unclear how the integration of inputs from the BLA and the ventral subiculum of the hippocampus (vSub) in a region of convergence such as the nucleus accumbens (NAc) (O’Donnell and Grace, 1995) might be altered by stress to cause the aberrant behavioral responses that are typical of mood disorders.
Stress also impacts the dopamine (DA) system. Acute stressors typically only transiently increase DA release in the NAc (Stevenson and Gratton, 2003). In contrast, restraint stress, via activation of the vSub, causes an increase in the population activity of DA neurons in the ventral tegmental area (Valenti and Grace, 2008; Valenti et al., 2012). Consequently, there is elevated tissue HVA levels and increased DA transporter activity follow chronic stress in mice (Copeland et al., 2005).
Consistent with the opposing effects of chronic stress on DA and morphology of the hippocampus and BLA, there are contrasting effects on synaptic plasticity. Repeated restraint stress attenuates long-term potentiation (LTP) in multiple hippocampal subregions, including dentate gyrus, CA1, and CA3 (Pavlides et al., 2002). In contrast, despite elevating blood corticosterone levels, acute restraint does not alter the induction of LTP in the hippocampus (Pavlides et al., 2002). Stress paradigms that reduce LTP in the hippocampus will enhance LTP in the BLA (Rodriguez Manzanares et al., 2005; Vouimba et al., 2006). In addition, stress can diminish the activity of GABAergic interneurons in the BLA, suggesting an increased responsiveness to emotional stimuli (Braga et al., 2002; Rodriguez Manzanares et al., 2005). Previous work from our laboratory demonstrated that inactivation of the vSub potently attenuates the BLA drive of the NAc, supporting a strong modulatory influence of the vSub on BLA-evoked responses in the NAc. (Gill and Grace, 2011). In addition, dopamine in the NAc, acting at D2 receptors, is known to modulate the gating of convergent glutamatergic input from the BLA, PFC, and vSub to the NAc (Floresco et al., 2001a; Belujon and Grace, 2008; Gill and Grace, 2011). The present study utilized high- and low-frequency stimulation of the vSub to test the disparate impact of acute and repeated restraint stress on vSub and BLA-evoked plasticity by individual neurons in the NAc. In addition, iontophoretically applied sulpiride was used to determine the involvement of dopamine D2 receptors within the NAc in mediating the effects of acute and repeated restraint stress on plasticity.
Methods
Animals
All experiments were conducted according to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Adult male Sprague-Dawley rats (Hilltop) weighing 300–400g were used. Animals were pair-housed in a temperature (22 °C) and humidity (47%) controlled environment with a 12-hour light/dark cycle (lights on at 7 a.m.) with ad libitum access to both food and water. Testing was performed at least one week after animals’ arrived.
Restraint Stress
Animals undergoing acute or repeated restraint stress were removed from their home cages and transported to an adjacent behavioral testing room in opaque individual transport tubs. Animals were then placed in clear Plexiglas restraint tubes (Harvard Apparatus) and a nose guard was adjusted within the tube so that voluntary movement was limited. Animals were monitored during the restraint period and were removed from the tube in the event of restricted respiration. Acute and repeated (10 sessions, 24hr interval) restraint consisted of 2 and 1hr sessions in the restraint tube, respectively. The same restraint protocols have been used previously in our laboratory to show the impact of acute and repeated restraint stress on dopamine activity in the ventral tegmental area(Valenti and Grace, 2008). Control animals were handled briefly daily prior to electrophysiological recordings.
Extracellular Single-Unit Recordings and Afferent Stimulation
Animals were anesthetized with an initial dose of chloral hydrate (Sigma, 400mg.kg, i.p.) and were supplemented periodically (i.v.) to maintain a suppression of the hindlimb withdrawal reflex. After being placed in a stereotaxic frame (Kopf, Tujunga, CA), rats were implanted with a catheter in the lateral tail vein to allow for intravenous injection of anesthesia. Body temperature was maintained at 37°C with a temperature-controlled heating pad (Fintronics). In vivo extracellular recordings were conducted using single glass microelectrodes (WPI; impedance 6–8MΩ) filled with a 2% Chicago Sky Blue (Sigma) solution in 2M NaCl. Electrodes were placed in the nucleus accumbens (AP, +1.25 mm; ML, +1.00 mm from bregma and −6.0 to −7.0 mm ventral of brain surface) using a hydraulic microdrive (Kopf). Spontaneous and evoked neural activity was monitored in each track until an individual neuron was encountered that exhibited monosynaptic responses to both BLA and vSub stimulation.
Afferent stimulation of the BLA and vSub was accomplished via implanted concentric bipolar electrodes (NEX-100X; Rhodes Medical Instruments). A dual output stimulator (S8800; Grass Technologies) was used to generate single current pulses (duration, 0.20msec; intensity 300μA; 0.5Hz) while the recording microelectrode was advanced slowly into the NAc.
High and Low Frequency Stimulation
For the baseline recording period, the current intensity was adjusted to obtain approximately 50% evoked spike probability for both vSub and BLA stimulation (0.5Hz; 100 μsec latency between BLA and vSub simulation). For both high- and low-frequency stimulation of the vSub pathway, a supramaximal current intensity was used (minimum current intensity to attain 100% spike probability; <1mA). High frequency stimulation consisted of 20Hz, 0.2 msec pulses, 10 sec duration. Low frequency stimulation consisted of 5Hz, 0.2 msec pulses, 100 sec duration. Following HFS and LFS, the current intensity was reduced to the same values utilized during the baseline period.
Sulpiride Iontophoresis
5-barrel glass ionotophoretic electrodes (ASI instruments) were used in a subset of recordings from acute and repeated stress rats to examine the effects of local application of sulpiride on NAc responses. At the beginning of each track through the NAc, a retaining current (−5μA) was applied to the sulpiride barrels. In addition, a balancing current was applied to a barrel containing 3M NaCl. Once a neuron exhibiting monosynaptic responses to vSub and BLA stimulation was encountered, evoked responses were recorded with the retention current on. Following this baseline period (10 min) or 30 min after LFS or HFS, sulpiride (10mM, pH 4.5) was iontophoretically applied (+20μA). The parameters employed for sulpiride iontophoresis were selected based on previous reports of alterations in the behavioral-correlated firing activity of striatal neurons (Inase et al., 1997; Watanabe and Kimura, 1998).
Histology
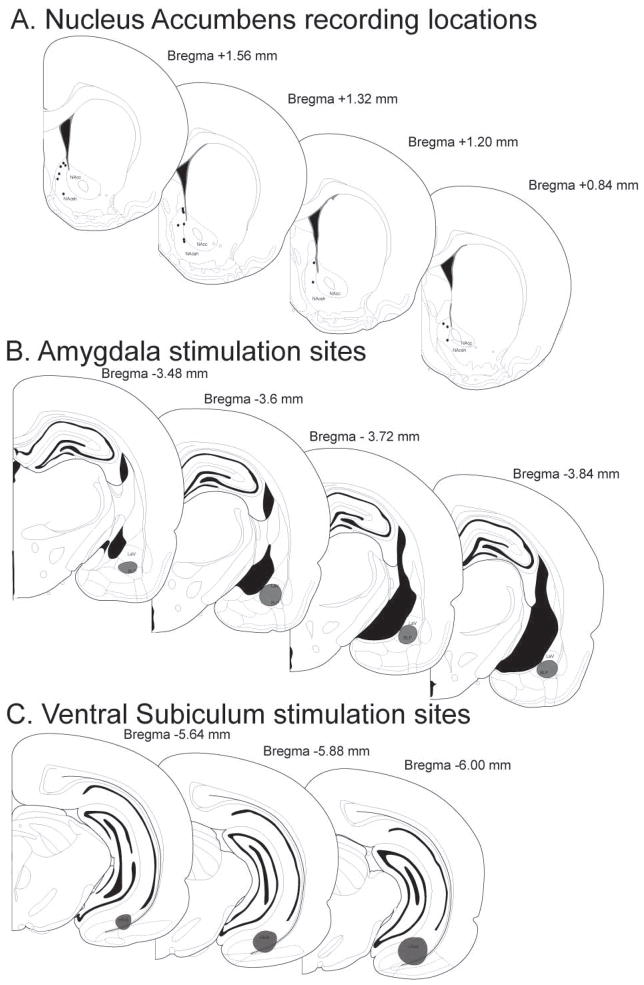
At the completion of the electrophysiological experiments, the recording location was marked via electrophoretic ejection of Chicago sky blue dye from the tip of the recording electrode (−20μA constant current, 30 min). Small lesions were made at the tip of each stimulation electrode (250μA, 10 sec current pulse) and visualized by adding potassium ferrocyanide during post-fixation. Rats were then euthanized with an overdose of chloral hydrate (additional 400 mg.kg, i.v.) and decapitated. The brain was removed and fixed for at least 48 hr. in 8% w/v paraformaldehyde (in PBS) and cryoprotected in 25% w/v sucrose (in PBS). Brains were sectioned (60μm coronal sections), mounted onto gelatin-chrom alum-coated slides, and stained with a mixture of cresyl violet and neutral red for histochemical verification of electrode sites. All histology was performed with reference to a stereotaxic atlas (Paxinos and Watson, 1996). Illustrated in Fig 1 is a representation of the localization of recording sites within the NAc and stimulation sites in the BLA and vHPC.
Figure 1.
Histological verification of electrophysiological recording and stimulation sites. (A) Schematic of coronal sections of the rat brain (Paxinos and Watson, 1996) with representative (for clarity, around 50%) recording sites in the NAc shell (s-NAc). (B) Stimulation location in BLA. (C) Stimulation location in vSub. (BLA = basolateral amygdala, vSub = ventral subiculum of the hippocampus, c-NAc = NAc core).
Analysis
Electrophysiological analysis of NAc neuronal activity was performed with LabChart software (AD Instruments). All data are represented as the mean ± SEM unless otherwise stated. All statistics were calculated using the SigmaStat software program (Systat Software, San Jose, CA). Repeated measures one-way ANOVA’s were used to assess the change in spike probability over time (comparing the last 5 min of the baseline period and each subsequent 5 min bin) following either HFS or LFS with stress as the between-subjects factor. In addition, two-way repeated measures ANOVAs were used to test the combined effect of sulpiride and stress on post-HFS and LFS NAc responses. Bonferroni-corrected post-hoc analysis for multiple comparisons was used for significant main effects.
Results
Stimulation electrodes were placed in the lateral and basolateral subregions of the amygdala as well as the ventral subiculum of the hippocampus (Fig. 1). Electrophysiological recordings were restricted to the caudal shell subregion of the NAc (Fig. 1) based on previous examination of the gating of vSub and BLA input in this area and the subsequent modulation by dopamine (Gill and Grace, 2011). In addition, this caudal subregion of the NAc (<+1.4mm relative to bregma) is known to modulate fear and defensive behaviors (Reynolds and Berridge, 2001, 2002; Faure et al., 2008); two behavioral responses that are likely to be sensitive to stress.
A total of 61 neurons were recorded in 61 rats, one neuron per rat. Medium spiny neurons receive convergent monosynaptic input from both the BLA and vSub (O’Donnell and Grace, 1995; French and Totterdell, 2003). All neurons reported in the present study were excited by stimulation of both the BLA and vSub. The average monosynaptic response latencies to BLA and vSub stimulation were 14.13 ± 0.7ms and 17.38 ± 0.76ms, respectively, which is consistent with previous reports (O’Donnell and Grace, 1995; Mulder et al., 1998; Gill and Grace, 2011). There were no differences between the control, acute, or repeated stress animals in the amount of current required to attain 50% spike probability in BLA (757 ± 72.41μA, 642 ± 70.95 μA, and 680.00 ± 59.71 μA, respectively; One-way ANOVA, F = 0.76, p>.05.) or vSub-evoked responses (527 ± 65.86, 564.80 ± 51.18, and 477.33 ± 65.70, respectively; One-way ANOVA, F = 0.45, p >.05).
High-frequency stimulation of the vSub differentially alters the plasticity in NAc of animals exposed to acute vs. repeated restraint stress
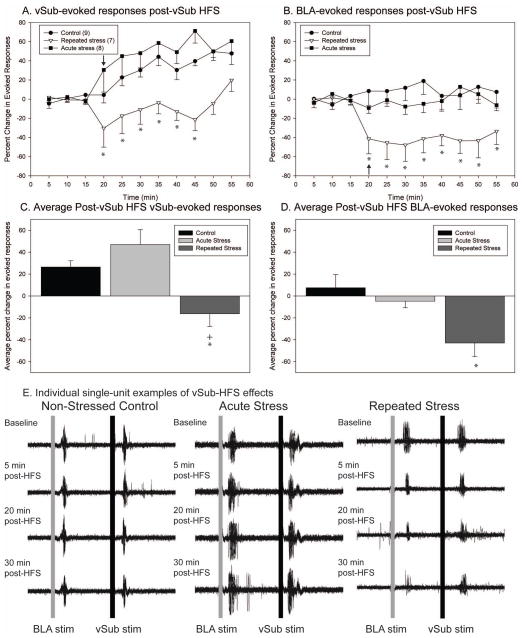
Given the reported activation of BLA and vSub in response to stress, we tested whether restraint stress would alter the integration of input from these regions in the NAc. Changes in spike probabilities were interpreted as alterations in the influence that hippocampal and amygdala inputs, and presumably context- and emotion-related information arising from the vSub and BLA, exert over NAc neurons (Floresco et al., 2001). After recording stable baseline activity (≤10% variation) and evoked response probability for 15 min, high frequency stimulation (HFS, 20 Hz, 10 sec) was applied to the vSub. Changes in synaptic plasticity, or in the spike probability following HFS, were assessed by separate one-way repeated measures (RM) ANOVAs for the control, acute, and repeated stress groups. In addition, vSub and BLA-evoked responses were also considered separately. In both non-stressed control (n=9) and acute stressed rats (n=8), HFS of the vSub resulted in the expected potentiation of vSub-evoked responses compared to baseline evoked spike probabilities (Fig 2a; One-way RM ANOVAs, control F = 2.52, p = .02 and acute stress F = 5.96, p < .001). There was no potentiation of vSub-evoked responses following repeated stress (n=7; one-way RM ANOVA, F = 1.53, p > .05). Within bin (5 min) Bonferroni post-hoc comparisons between the three groups revealed that vSub-evoked responses were different between the repeatedly stressed and the acute stressed and non-stressed control rats up to 35 minutes post-HFS.
Figure 2.
Following repeated stress, high-frequency stimulation of the vSub produces the opposite effect on synaptic plasticity as that observed in controls or following acute stress. (A) High-frequency stimulation of the vSub results in a potentiation of vSub-evoked responses in the nucleus accumbens of control animals and as well in animals exposed to acute restraint stress. In repeatedly stressed animals, high-frequency stimulation of the vSub produces a long duration decrease in vSub-evoked responses. (B) In animals exposed to repeated restraint stress, there is a depression in the non-tetanized BLA pathway following high-frequency stimulation of the vSub. (C) The average vSub-evoked response post-HFS of the vSub pathway is significantly reduced following repeated stress. (D) The average BLA-evoked response post-HFS of the vSub pathway is significantly reduced following repeated stress. (E) Representative peristimulus time histograms (overlay of 30 traces) illustrating reduction in vSub and BLA-evoked responses in animal exposed to repeated restraint stress. (arrow indicates time of application of high-frequency stimulation to vSub; *Denotes p<0.05 when compared to non-stressed control animals, + denotes p<0.05 when compared to acute stress animals)
The impact of HFS of the vSub pathway was also examined on the non-tetanized BLA-evoked responses. In both non-stressed control rats and acutely stressed rats, HFS of the vSub did not significantly change BLA-evoked responses from baseline (Fig 2b; One-way RM ANOVAs, control F = .55, p > .05 and acute F = .63, p > .05, respectively). In contrast, in repeatedly stressed rats HFS of the vSub led to a significant decrease in BLA-evoked responses (F = 3.92, p < .001). Within bin (5 min) Bonferroni post-hoc comparisons between the three groups revealed that BLA-evoked responses were significantly reduced compared to non-stressed and acute stress animals throughout the post-HFS recording period (40 min).
Representative traces illustrating vSub-evoked responses from individual control, acute stressed, and repeatedly stress animals demonstrate the disparate effect of HFS of the vSub (Fig. 2E). The average change in evoked probability post-HFS was calculated and compared between the non-stressed controls and acute and repeatedly stressed animals for both vSub and BLA-evoked responses (Fig. 2c and d). Both non-stressed controls and acute stressed rats exhibited significantly larger potentiation in the vSub-evoked responses than repeatedly stressed rats (One-way ANOVA, F = 9.90, p <.001). In addition, BLA-evoked responses were significantly reduced in repeated stressed animals compared to non-stressed control rats (One-way ANOVA, F = 5.92, p = .009). Therefore, with continued exposure to stress, there is a reduction in the efficacy of both context-related input from the vSub as well as emotion-related input from the BLA.
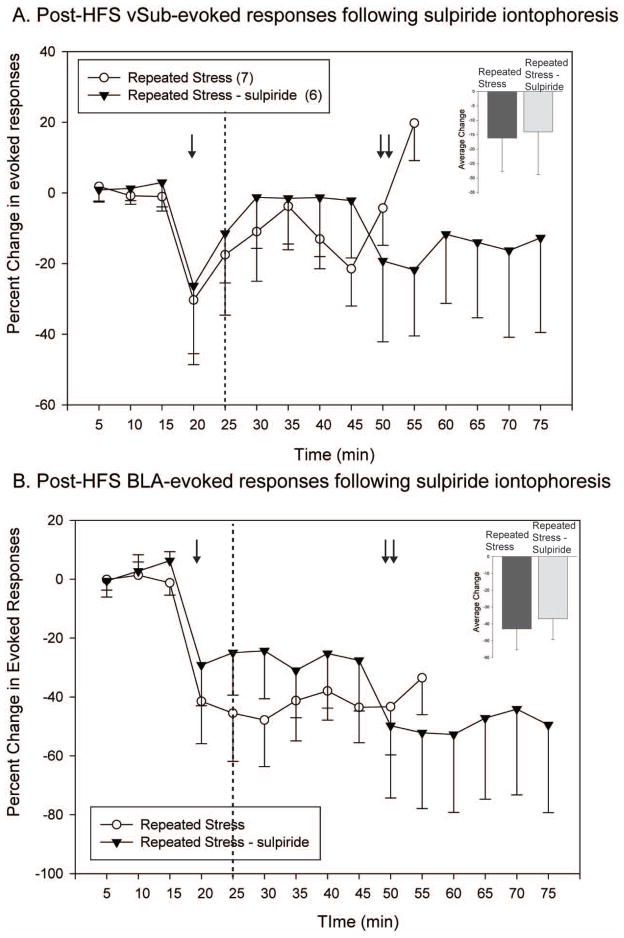
Sulpiride iontophoresis during high-frequency stimulation of the vSub in repeatedly stressed animals fails to alter repeated stress-induced changes in NAc plasticity
The effect of iontophoretic application of sulpiride during HFS of the vSub in repeated stressed animals was tested (Fig. 3). Sulpiride did not alter the blunted vSub-evoked responses following HFS of the vSub in repeatedly stressed animals (n = 6, One-way RM ANOVA, F = 1.02, p >.05). BLA-evoked responses were unaffected by the application of sulpiride in that HFS of the vSub continued to lead to a pronounced decrease of BLA-evoked responses in repeatedly stressed rats (One-way RM ANOVA, F = 4.87, p <.001). The depression of BLA-evoked responses persisted and appeared more pronounced even after the cessation of iontophoretic application of sulpiride 30 min post-HFS. Consequently, the effects of repeated stress on NAc plasticity are unlikely to be entirely mediated by dopamine D2 receptors within the NAc.
Figure 3.
The effect of repeated stress on high frequency stimulation-induced plasticity in the nucleus accumbens is partially dopamine-mediated. (A) The application of sulpiride (single arrow) to the nucleus accumbens neuron recorded via iontophoresis prior to high-frequency stimulation causes a heterogeneous response in repeatedly stressed animals, with some neurons exhibiting a potentiation and some neurons demonstrating a transient decrease. (B) During sulpiride administration, there is no change in the depression observed in the non-tetanized BLA-pathway following HFS of the vSub in repeatedly stressed animals. Interestingly, when sulpiride current is removed (double arrow), there is a further decrease in BLA-evoked responses. (single arrow indicates commencement of application of sulpiride via iontophoresis; dashed line indicates application of high-frequency stimulation to the vSub; double arrow indicates termination of iontophoresis current)
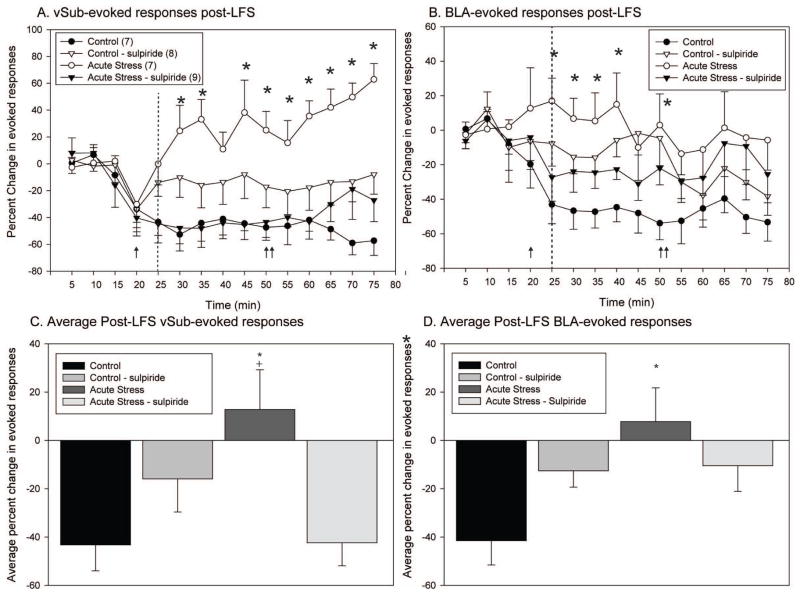
Low-frequency stimulation of the vSub following acute stress unmasks a dopamine-dependent potentiation of BLA- and vSub-evoked responses
Given the intact potentiation of the vSub pathway following HFS in acute stress animals, the impact of low-frequency stimulation (5 Hz, 500 pulses) on the same pathway was tested. In addition, in a subset of neurons the D2 receptor antagonist sulpiride was applied iontophoretically to establish the role of D2 receptors within the NAc in mediating the stress-induced changes in plasticity. Changes in synaptic plasticity, or in the spike probability following LFS, were assessed by separate one-way repeated measures (RM) ANOVAs for the control, acute, and repeated stress groups. In addition, vSub and BLA-evoked responses were also considered separately. Acute stressed animals (n=7) and control animals (n=7) demonstrated different BLA and vSub evoked responses in the NAc following LFS of the vSub pathwayIn control animals, LFS applied to the vSub caused a long-lasting reduction in both vSub and BLA-evoked responses (n = 7; Fig. 4a and b; one way RM ANOVAs, vSub F = 2.75, p = .004 and BLA F = 4.99 and p <.001, respectively). In contrast to the pattern of plasticity observed in non-stressed control rats, LFS of the vSub in acute stress rats (n=9) resulted in a potentiation of the vSub –evoked responses (One-way RM ANOVA, vSub F = 3.74, p <.001) with no impact on BLA-evoked responses (One-way RM ANOVA, BLA F = .58, p >.05).
Figure 4.
In rats exposed to acute stress, low-frequency stimulation of the vSub results in long-term potentiation, compared to the long-term depression observed in control rats. Moreover, this acute stress-induced alteration in plasticity is reversed by dopamine antagonism. (A) In control animals, low-frequency stimulation of the vSub results in a decrease in vSub-evoked responses. In contrast, in animals exposed to acute restraint stress, low-frequency stimulation of the vSub results in a potentiation of vSub-evoked responses that is reversed when sulpiride is applied iontophoretically to the nucleus accumbens neuron recorded prior to low-frequency stimulation. (B) In control animals, there is a depression in the non-tetanized BLA pathway following LFS of the vSub. There is no decrease in BLA-evoked responses in acute stress rats following LFS of the vSub. However, iontophoretic application of sulpiride to the nucleus accumbens prior to LFS partially reduces BLA-evoked responses in acute stressed rats. (C) The average vSub-evoked response post-LFS of the vSub pathway is significantly greater in acute stressed rats in comparison to non-stressed controls. In addition, this increase in vSub-evoked responses is reversed following ionotopohretic application of sulpiride in acute stress animals. (D) The average BLA-evoked response post-LFS of the vSub pathway is significantly enhanced following acute stress. The enhancement of BLA-evoked responses in acute stressed animals is reversed following iontophoretic application of sulpiride (arrow indicates commencement of application of sulpiride via iontophoresis; dashed line indicates application of low-frequency stimulation to the vSub; double arrow indicates indicates removal of iontophoresis current; *Denotes p<0.05 when compared to non-stressed control animals, + denotes p<0.05 when compared to acute stress control iontophoresis)
There were also opposing effects of sulpiride iontophoresis during LFS in acute stress and non-stressed animals. In non-stressed control rats, when sulpiride was applied iontophoretically during LFS, LFS of the vSub produced no change in vSub-evoked responses and a smaller decrease in BLA-evoked responses (One-way RM ANOVAs, vSub F = 1.20, p >.05 and BLA F = 2.23, p = .02, respectively). In acute stress rats, when sulpiride was applied iontophoretically during LFS, there was a reversal of the vSub-induced plasticity, changing the facilitation into a decrease in vSub-evoked responses (One-way ANOVA, vSub F = 2.61, p = .005). In particular, in the presence of sulpiride, LFS produced an effect on vSub-evoked responses in the acute stressed rats that was similar to that observed in controls. However, even after iontophoretic application of sulpiride during LFS, there was still no change in BLA-evoked responses in acute stress rats (One-way ANONVA, BLA F = .85, p >.05). Within bin (5 min) Bonferroni post-hoc comparisons revealed that the pattern of vSub evoked responses observed in acute stress animals was significantly different from all other groups (acute stress treated with sulpiride, non-stressed controls, non-stressed controls treated with sulpiride) throughout the post-LFS recording period (up to 1 hour). It is important to note that the difference between the groups persisted even when the sulpiride current was removed 30 min post-LFS. Within bin comparisons of the BLA-evoked responses revealed differences between acute stress rats and non-stressed controls at the 25, 30, 35, 40, and 50 min time points.
The average change in evoked probability post-LFS, with and without sulpiride applied iontophoretically, was calculated and compared between the non-stressed controls and acute stress animals, with separate comparisons for vSub and BLA-evoked responses (Fig. 4c and d). Acute stress animals exhibited significantly larger post-LFS vSub-evoked spike probabilities than non-stressed controls, non-stressed controls treated with sulpiride, and acute stress rats treated with sulpiride (One-way ANOVA, vSub F = 4.75, p <.01). The application of sulpiride in non-stressed controls did not significantly alter the average post-LFS vSub-evoked responses. Acute stress also reversed the significant reduction in BLA-evoked responses that was observed in non-stressed control rats following LFS of the vSub and this effect was blocked by sulpiride (One-way ANOVA, BLA F = 4.08, p = .02). Therefore, the acute stress-induced alterations in the gating of vSub and BLA input to NAc was dopamine-dependent.
Discussion
This study illustrates that vSub-induced plasticity in the NAc, as well as the gating of BLA responses following vSub activation, is differentially sensitive to acute and repeated stress. We have shown previously that input from the vSub is a potent modulator of NAc responses to BLA stimulation and likely modulates behavioral responses to aversive stimuli (Gill and Grace, 2011). HFS and LFS stimulation were utilized in an attempt to potentiate and depotentiate input to the NAc, respectively, perhaps reflecting changing influence of contextual input arising from the vSub. In the current study, acute stress led to an LFS-induced potentiation of vSub input to the NAc that was mediated by dopamine D2 receptors. In contrast, repeated restraint reduced the efficacy of vSub- and BLA-evoked responses after HFS of the vSub in a DA-independent manner. Given the role of the NAc in mediating aversive and appetitive responses (Reynolds and Berridge, 2008), stress-induced alterations in the modulation of BLA and vSub responses in the NAc could selectively alter the context-dependent gating of behaviors.
Stress induced alterations in hippocampal and amygdalar plasticity
Morphological changes, such as alterations in dendritic spine density, often coincide with changes in synaptic efficacy. Stress produces differential morphological effects on the HPC and BLA, with dendritic atrophy in hippocampal area CA3, but a growth of dendrites and spines in the BLA (Lakshminarasimhan and Chattarji, 2012). Moreover, long-term potentiation is enhanced in the BLA following acute stress, while LTP in the HPC is blocked following repeated stress (Hugues et al., 2003; Sarabdjitsingh et al., 2012). This would suggest that the output from BLA and vSub following acute and repeated stress would likewise be altered and impact plasticity in downstream structures.
In the current study, acute stress did not alter the enhanced activation of the NAc by the vSub following HFS of the vSub, nor did it affect BLA-evoked responses in the NAc. However, whereas LFS typically results in a synaptic depression in afferent structures (Yang et al., 2006), following acute stress LFS of the vSub instead caused a potentiation of vSub responses as well as blocked the decrease in BLA-evoked responses observed in control animals. Thus, following a single bout of aversive, stressful stimuli, the relative influence of hippocampal-mediated contextual input is more effective at modulating NAc responses than is the BLA.
Following repeated restraint stress, unlike what was observed following acute stress, the pattern of vSub-induced plasticity is altered. Specifically, both hippocampal-evoked contextual and amygdala-related emotional inputs to NAc are blunted following HFS of the vSub, perhaps reflecting the impact of uncontrollable aversive experiences. In such situations, vSub activity would be ineffective in promoting a behavioral response via its connectivity to NAc and perhaps be disconnected from emotional stimuli.
Stress-induced alterations in DA-dependent gating in NAc
Both stress and glutamatergic afferent activation increase DA release, potentially via HPC or BLA, in the NAc. In vivo microdialysis studies have shown that NMDA-induced activation of the ventral hippocampus acutely increases the release of DA in the shell subregion of the NAc (Legault and Wise, 1999; Peleg-Raibstein and Feldon, 2006). HFS of the BLA also causes a long-lasting increase in DA efflux in the NAc via local mechanisms (Howland et al., 2002). Moreover, DA release in the NAc modulates the response to afferent input from BLA and vSub. Accordingly, systemic or local infusions into the NAc of the D1 receptor antagonist, SCH23390, blocks the HFS induced potentiation of both BLA-evoked and vSub-evoked NAc activity (Floresco et al., 2001b, a; Goto and Grace, 2005). In addition, VTA stimulation can attenuate both BLA and hippocampal-evoked responses in NAc (Yang and Mogenson, 1984; DeFrance et al., 1985; Mogenson et al., 1988). Thus, stress-induced changes in NAc DA impacts the gating of BLA and vSub input. Acute and repeated restraint differentially alters the release of DA in the NAc. We showed previously that the spontaneous activity of DA neurons in the VTA is increased following acute and repeated restraint stress (Valenti and Grace, 2008; Valenti et al., 2012). Other studies have shown that NAc DA release is increased following exposure to various aversive stimuli, including foot shock, tail pinch, and restraint (Horvitz, 2002). We show in the present study that iontophoretic application of a D2 antagonist in the NAc is effective in reversing the stress-induced alterations in plasticity after acute, but not repeated stress. The finding that DA has less of a modulatory effect on BLA and vSub plasticity after repeated stressors could be due to compensatory alterations in DA receptor expression. There is evidence that the distinction between high and low reactivity to stress is based partially on increased D2-receptor expression in the NAc (Knapman et al., 2010b; Knapman et al., 2010a). Consequently, stress-induced increases in D2 receptor activation in the NAc could impact behavioral responses to future stressors by increasing the likelihood of aversive responses (Faure et al., 2008). It has been shown previously that appetitive behaviors are regulated by the anterior or rostral NAc, while the posterior or caudal NAc is important for mediating behavioral responses to fearful or aversive stimuli (Basso and Kelley, 1999; Reynolds and Berridge, 2001, 2002; Kerfoot et al., 2007; Reynolds and Berridge, 2008). Indeed, stress can cause an expansion of the caudal fear-generating zone of the NAc at the expense of appetitve behaviors (Reynolds and Berridge, 2008). It is likely that the stress-induced shift increase fearful behavior is mediated by D1/D2 receptors activation in the caudal NAc (Faure et al., 2008).
Impact of acute and repeated stress on behavior
Studies have shown that exposure to both acute and repeated stress can promote long-lasting behavioral consequences that can be attributed to alterations in amygdalar and hippocampal output. Interestingly, despite having disparate effects on vSub-induced plasticity in the NAc, during the standard fear conditioning paradigms when passive fear responses are observed, acute and repeated stress have similar behavioral consequences. Indeed, previous restraint stress that reduces GABAergic function in the BLA and augments LTP of BLA targets also results in enhanced fear conditioning (Rodriguez Manzanares et al., 2005). Contextual fear learning is similarly enhanced following a single session of foot shock (Rau et al., 2009). One possibility is that sustained HPC and BLA activation underlies the association of novel contexts with previous aversive experiences, leading to a similar fearful response. Alternatively, effectively reducing HPC and BLA-evoked responses in the NAc could facilitate a reduction in the expression of fear behaviors. Indeed, LFS of the HPC facilitates the extinction of conditioned fear (Cleren et al., 2013). Based on the data from the current study showing that after acute stress LFS is no longer able to reduce HPC-evoked activity in the NAc, persistent fear responses might ensue during extinction as a result of this heightened HPC-output. Consistent with this idea, it has been shown that exposure to acute stress can induce the reemergence of previously extinguished fear (Deschaux et al., 2013). Consequently, acute stress promotes the generation of new fear responses and rapid reactivation of previously learned fear responses. When the BLA and HPC circuits are activated repeatedly by stress, passive fear responses are enhanced to a similar degree after acute stress (Rodriguez Manzanares et al., 2005; Daviu et al., 2010; McGuire et al., 2010). In addition, repeated stress enhances both cued and contextual fear learning (Conrad et al., 1999; Atchley et al., 2012). It can be concluded that short duration, acute stressors, as well as repeated stressors can enhance learning during tasks with an emotional component. However, it is important to note that there are few studies that directly compare the impact of acute versus repeated stressors on performance.
There is also evidence that acute and repeated stress can alter the use of spatial context information and impair performance during spatial navigation tasks that lack an inherent emotional component (Diamond and Rose, 1994; Park et al., 2008). The impact of stress on the flexible use of spatial context information is likely the result of alterations in normal hippocampal function. Pharmacological interventions during repeated stress exposure that prevent hippocampal atrophy resulting from repeated stress also ameliorate the stress-associated cognitive impairments (Conrad et al., 1999; McEwen, 1999). It will be especially important to identify potential therapeutic interventions that are effective in restoring normal hippocampal function after, as opposed to during, the stress experience. Crucial future experiments should directly assess the impact of acute and repeated stress on behavior and the role of alterations in hippocampal processing on these changes.
Conclusion
Acute and repeated stress has disparate effects on the modulation of context and emotion inputs to the NAc. By differentially changing the activation resulting from vSub and BLA inputs to the NAc, acute and repeated stress likely impact the behavioral responses, in particular consummatory and fear-related behaviors, to subsequent novel contextual stimuli.
Acknowledgments
This work was supported by United States Public Health Service Grants MH57440 (A.A.G.) and T32-MH18273-23 (K.M.G). We thank Niki MacMurdo for her assistance with the histology; Joe Snatchko for his aid in the behavioral testing, and Pauline Belujon for critical reading and helpful discussions.
Footnotes
Financial Disclosures:
Statement of Interest: Dr. Grace has received financial support from Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, and Asubio. Dr. Gill has no biomedical financial interests or potential conflicts of interest to report.
References
- Atchley D, Hankosky ER, Gasparotto K, Rosenkranz JA. Pharmacological enhancement of calcium-activated potassium channel function reduces the effects of repeated stress on fear memory. Behav Brain Res. 2012;232:37–43. doi: 10.1016/j.bbr.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Post RM, Li H. Lamotrigine reduces spontaneous and evoked GABAA receptor-mediated synaptic transmission in the basolateral amygdala: implications for its effects in seizure and affective disorders. Neuropharmacology. 2002;42:522–529. doi: 10.1016/s0028-3908(01)00198-8. [DOI] [PubMed] [Google Scholar]
- Cleren C, Tallarida I, Guiniec EL, Janin F, Nachon O, Canini F, Spennato G, Moreau JL, Garcia R. Low-frequency stimulation of the ventral hippocampus facilitates extinction of contextual fear. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarin?os AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Copeland BJ, Neff NH, Hadjiconstantinou M. Enhanced dopamine uptake in the striatum following repeated restraint stress. Synapse. 2005;57:167–174. doi: 10.1002/syn.20169. [DOI] [PubMed] [Google Scholar]
- Daviu N, Fuentes S, Nadal R, Armario A. A single footshock causes long-lasting hypoactivity in unknown environments that is dependent on the development of contextual fear conditioning. Neurobiol Learn Mem. 2010;94:183–190. doi: 10.1016/j.nlm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- DeFrance JF, Sikes RW, Chronister RB. Dopamine action in the nucleus accumbens. J Neurophysiol. 1985;54:1568–1577. doi: 10.1152/jn.1985.54.6.1568. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Zheng X, Lavigne J, Nachon O, Cleren C, Moreau JL, Garcia R. Post-extinction fluoxetine treatment prevents stress-induced reemergence of extinguished fear. Psychopharmacology (Berl) 2013;225:209–216. doi: 10.1007/s00213-012-2806-x. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann N Y Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- Donohue HS, Gabbott PLA, Davies HA, Rodri?guez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, Stewart MG. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: A stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001a;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. Journal of Neuroscience. 2001b;21:6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci. 2006;11:2746–2758. doi: 10.2741/2004. [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiology of Learning and Memory. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Gill KM, Grace AA. Heterogeneous processing of amygdala and hippocampal inputs in the rostral and caudal subregions of the nucleus accumbens. Int J Neuropsychopharmacol. 2011;14:1301–1314. doi: 10.1017/S1461145710001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. Journal of Neuroscience. 2002;22:1137–1145. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Kessal K, Hunt MJ, Garcia R. A conditioned stressful environment causes short-term metaplastic-like changes in the rat nucleus accumbens. J Neurophysiol. 2003;90:3224–3231. doi: 10.1152/jn.00895.2002. [DOI] [PubMed] [Google Scholar]
- Inase M, Li BM, Tanji J. Dopaminergic modulation of neuronal activity in the monkey putamen through D1 and D2 receptors during a delayed Go/Nogo task. Exp Brain Res. 1997;117:207–218. doi: 10.1007/s002210050217. [DOI] [PubMed] [Google Scholar]
- Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learn Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapman A, Heinzmann JM, Holsboer F, Landgraf R, Touma C. Modeling psychotic and cognitive symptoms of affective disorders: Disrupted latent inhibition and reversal learning deficits in highly stress reactive mice. Neurobiol Learn Mem. 2010a;94:145–152. doi: 10.1016/j.nlm.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Knapman A, Heinzmann JM, Hellweg R, Holsboer F, Landgraf R, Touma C. Increased stress reactivity is associated with cognitive deficits and decreased hippocampal brain-derived neurotrophic factor in a mouse model of affective disorders. J Psychiatr Res. 2010b;44:566–575. doi: 10.1016/j.jpsychires.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7:e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McGuire J, Herman JP, Horn PS, Sallee FR, Sah R. Enhanced fear recall and emotional arousal in rats recovering from chronic variable stress. Physiol Behav. 2010;101:474–482. doi: 10.1016/j.physbeh.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR, Yim CY. Influence of dopamine on limbic inputs to the nucleus accumbens. Ann N Y Acad Sci. 1988;537:86–100. doi: 10.1111/j.1749-6632.1988.tb42098.x. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol. 1992;2:43–49. doi: 10.1016/0924-977x(92)90035-7. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci. 1998;18:5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15:271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, editors. The rat brain in stereotaxic coordinates. San Diego: Academic; 1996. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Hillard CJ. Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:633–641. doi: 10.1016/j.pnpbp.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI, 2nd, Fanselow MS, Homanics GE, Sonner JM. Gamma-aminobutyric acid type A receptor alpha 4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg. 2009;109:1816–1822. doi: 10.1213/ANE.0b013e3181bf6ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nature Neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking” / “disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Stevens S, Boeh H. Stress enhancement of fear learning in mice is dependent upon stressor type: Effects of sex and ovarian hormones. Neurobiol Learn Mem. 2010;94:254–262. doi: 10.1016/j.nlm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Kofink D, Karst H, de Kloet ER, Joels M. Stress-induced enhancement of mouse amygdalar synaptic plasticity depends on glucocorticoid and ss-adrenergic activity. PLoS One. 2012;7:e42143. doi: 10.1371/journal.pone.0042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CW, Gratton A. Basolateral amygdala modulation of the nucleus accumbens dopamine response to stress: Role of the medial prefrontal cortex. European Journal of Neuroscience. 2003;17:1287–1295. doi: 10.1046/j.1460-9568.2003.02560.x. [DOI] [PubMed] [Google Scholar]
- Valenti O, Grace A. Acute and repeated restraint stress induce a pronounced and sustained activation of VTA DA neuron population activity; XXVI CINP Congress; Munich. 2008. [Google Scholar]
- Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci. 2012;35:1312–1321. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kimura M. Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J Neurophysiol. 1998;79:2568–2580. doi: 10.1152/jn.1998.79.5.2568. [DOI] [PubMed] [Google Scholar]
- Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic Immobilization Stress Alters Aspects of Emotionality and Associative Learning in the Rat. Behavioral Neuroscience. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res. 1984;324:69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- Yang LX, Jin CL, Zhu-Ge ZB, Wang S, Wei EQ, Bruce IC, Chen Z. Unilateral low-frequency stimulation of central piriform cortex delays seizure development induced by amygdaloid kindling in rats. Neuroscience. 2006;138:1089–1096. doi: 10.1016/j.neuroscience.2005.12.006. [DOI] [PubMed] [Google Scholar]