Abstract
Mutations affecting the SLAM-associated protein (SAP) are responsible for the X-linked lympho-proliferative syndrome (XLP), a severe primary immunodeficiency syndrome with disease manifestations that include fatal mononucleosis, B cell lymphoma and dysgammaglobulinemia. It is well accepted that insufficient help by SAP−/− CD4+ T cells, in particular during the germinal center reaction, is a component of dysgammaglobulinemia in XLP patients and SAP−/− animals. It is however not well understood whether in XLP patients and SAP−/− mice B cell functions are affected, even though B cells themselves do not express SAP. Here we report that B cell intrinsic responses to haptenated protein antigens are impaired in SAP−/− mice and in Rag−/− mice into which B cells derived from SAP−/− mice together with wt CD4+ T cells had been transferred. This impaired B cells functions are in part depending on the genetic background of the SAP−/− mouse, which affects B cell homeostasis. Surprisingly, stimulation with an agonistic anti-CD40 causes strong in vivo and in vitro B cell responses in SAP−/− mice. Taken together, the data demonstrate that genetic factors play an important role in the SAP-related B cell functions. The finding that anti-CD40 can in part restore impaired B cell responses in SAP−/− mice, suggests potentially novel therapeutic interventions in subsets of XLP patients.
Keywords: SAP, XLP, SLAM-family receptors
1. INTRODUCTION
X-linked lympho-proliferative disease (XLP) develops due to the lack of a functional SLAM-Associated Protein (SAP) caused by mutations in the Sh2d1a gene [1-5]. Whilst more than half of the patients develop EBV-induced fatal mononucleosis, other disease manifestations are B cell lymphomas or severe dysgammaglobulinemia [6-9]. Whether the different disease manifestations among male members of one XLP family are dependent upon genetic modifiers or environmental components is not well understood [7]. Whereas it is assumed that EBV plays a role in the pathogenesis of B cell lymphomas in XLP patients, the dysgammaglobulinemia can develop either in the presence or absence of EBV. Because SAP−/− mice cannot be infected by EBV, they have become useful tools for dissect the role of SAP in antibody responses. Similarly to humans, mice with SAP deficiency (SAP−/−) do not develop adequate germinal centers in response to viral infection or immunization with T cell-dependent antigens [9-15].
The single SH2-domain adapter SAP modulates signal transduction networks initiated by the engagement of several of the SLAM-Family (SLAMF) cell surface receptors in T-, NK-, and NKT cells [1,3-5]. The three-pronged interaction of SAP with specific Tyrosine –containing motifs (ITSM) in the cytoplasmic tail of six of the nine members of this family of adhesion molecules, i.e. SLAMF1, 3, 4, 5, 6 and 7, are understood in considerable detail [16-22]. SLAMF1, 3, 5, 6 and 7 are co-expressed on the surface of both T and B cells and these adhesion molecules can partake in the immune synapse. Hence SAP is thought to modulate a signal transduction network that in turn regulate T / B cell dependent immune responses [1,3].
As T cell-dependent humoral immunity develops as a result of precisely orchestrated serial interactions of myeloid- and lymphoid cell populations, SLAMF receptors play a diverse role in these processes [12-14,23]. Correct and sustained antibody responses are highly dependent on T cell expression of SAP, which appears to be requisite for germinal center development and humoral memory. Furthermore, the role of NKT cells in the germinal center reaction is also directly and indirectly regulated by SAP [24-26].
Whether and how the absence of SAP in XLP patients or SAP-deficient mice affects B cell intrinsic functions is not well understood, particularly because B cells themselves do not express the adapter [27-29]. To address this question, we evaluated B cell responses in SAP−/− B6 and SAP−/− BALB/c mice employing several immunizing or activating conditions. Surprisingly, we found that SAP deficiency can modulate several B cell responses and that this process is influenced by genetic and environmental factors.
2. Materials and methods
2.1 Mice
Wild-type (wt) C57BL/6 (B6) and BALB/c mice, as well as Rag−/− mice (in B6 and BALB/c backgrounds) were obtained from the Jackson Laboratory. SAP−/− mice were described previously and backcrossed to the B6 and BALB/c backgrounds for at least seven generations [31]. Animal studies were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
2.2 Immunizations
Animals were immunized intraperitoneally (i.p.) with 50 μg NP-KLH (Biosearch Technologies) in CFA (1:1), Alum (1:2) or without any adjuvant in PBS in 200 μl volume.
2.3 ELISA
For measuring antigen-specific antibodies: High binding plates (Costar) were coated overnight at 4°C with [NP-BSA] (50 μg/ml; Biosearch Technologies Inc). HRPO-conjugated sheep anti-mouse IgG (Amersham) or goat anti-mouse IgM antibody (Serotec) was used for detection.
For measuring total antibodies: Biotinylated goat anti-mouse IgM, IgG1 or IgE antibodies (Southern Biotech) were bound to streptavidin pre-coated plates (R&D systems) for capturing respective antibodies in serum- or supernatant samples, then HRPO-conjugated goat anti-mouse IgM, IgG1 or IgE antibodies (Southern Biotech) were applied for detection.
2.4 Flow cytometry
Single cell suspensions of splenocytes were stained with the following antibodies and reagents after blocking non-specific binding with anti-CD16/32 (93) and 15% rabbit-serum: αCD11c (N418), αCD19 (1D3), αCD21 (eBio4E3), αCD23 (B3B4), αCD93 (AA4.1), αCD138 (281-2), αB220 (RA3-6B2), αFas (Jo2), αT- and B cell activation antigen (GL-7), αGR-1 (RB6-8C5), αF4/80 (BM8) antibodies were purchased from eBioscience, BD Pharmingen or BioLegend. Data was acquired with LSRII cytometer (BD Pharmingen) and analyzed using FlowJo software (Treestar). Dead cells were excluded by DAPI uptake.
2.5 Cell transfers
Naïve (CD62Lhi) CD4+ T cells and naïve (CD43-) B cells were isolated from the spleen by negative selection using magnetic cell separation kits (Miltenyi), then mixtures of 3×106 T cells and 8×106 B cells in 300μL PBS were injected i.v. into Rag−/− hosts. In other experiments wt and SAP−/− B cells were loaded with CMRA and CFSE (Invitrogen) cell trackers, respectively, according to the manufacturer's protocols. After mixing in 1:1 ratio, the cells were rested in complete media at 37°C for 1h, washed, then 107 B cells were co-injected i.v. to Rag−/− recipients.
2.6 In vitro B cell assays
Naïve B cells were isolated from the spleen by negative selection using a magnetic cell separation kit (Miltenyi). Cells were stimulated in 48-well plates with agonistic anti-CD40 antibody (FGK4.5; the clone is a gift of Dr A. Rolink [32] and the IgG was purified by BioXell) plus recombinant mouse IL-4 (BioLegend). Proliferating (tetraploid) and apoptotic (subdiploid) cells were distinguished by propidium-iodide (Invitrogen) staining in hypotonic solution. IgG1 and IgE antibody secretion was determined by ELISA.
3. RESULTS
3.1 SAP deficiency impairs T-dependent humoral responses in both BALB/c and B6 mice
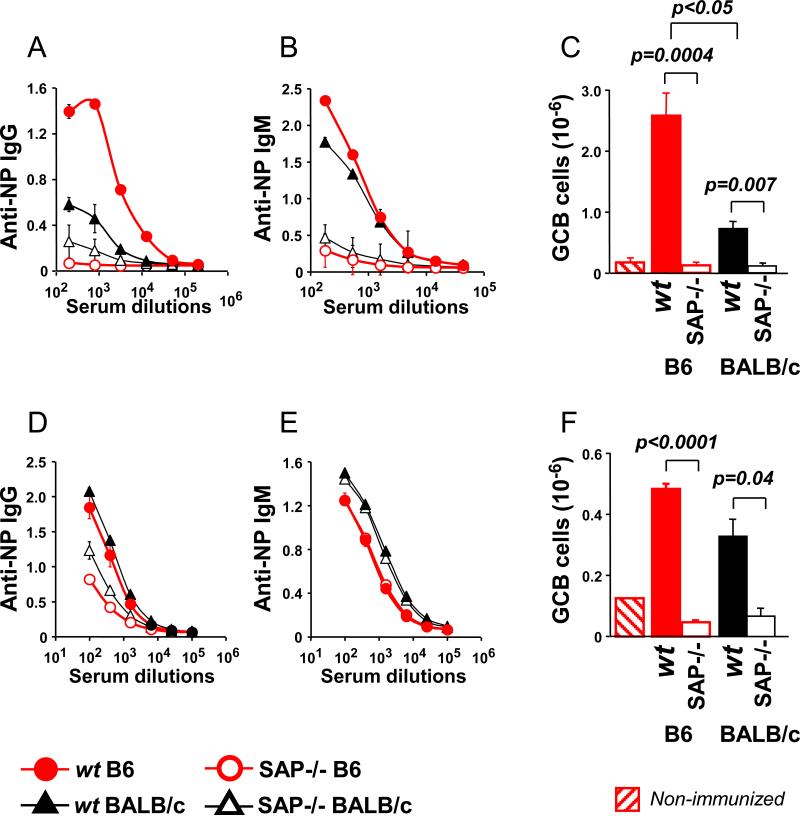
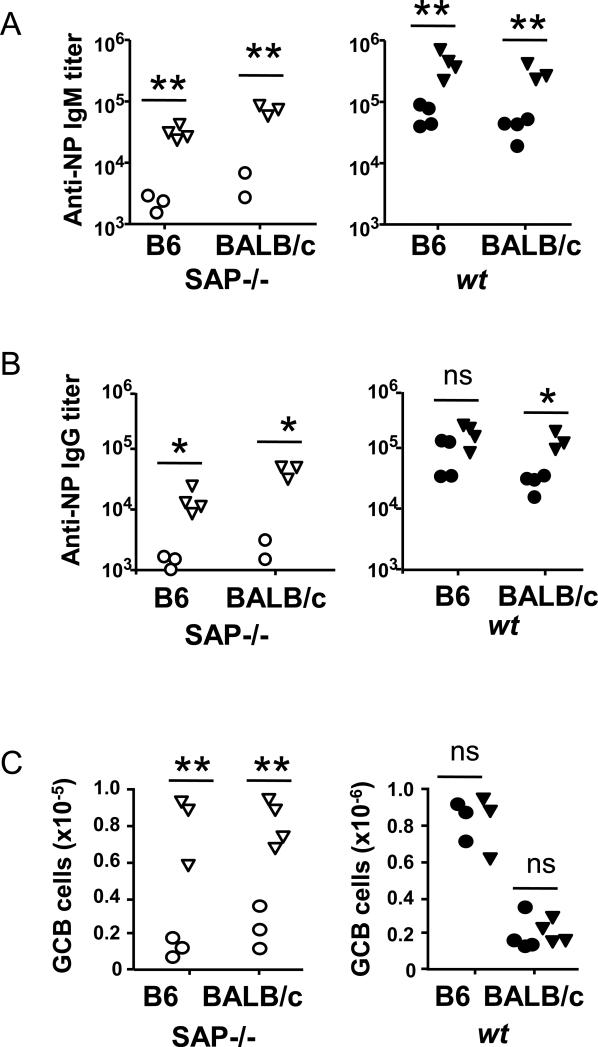
Upon immunization with the T cell-dependent antigen NP-KLH [CFA as adjuvant], hapten specific IgG and IgM levels in the serum of SAP−/− B6 or SAP−/− BALB/c mice was dramatically lower than in wt B6 or BALB/c mice (Fig. 1A and B). The ability to form germinal centers was also affected by the absence of SAP, regardless of the genetic background of the mouse (Fig. 1C). Surprisingly, anti-NP IgG , but not anti-NP IgM, responses were invariably higher in wt B6 mice than in wt BALB/c mice, (Fig. 1A-C). This strain difference was reflected in a higher number of germinal center B cells in B6 compared to BALB/c mice. When Alum was used as adjuvant, SAP−/− mice were again unable to form germinal centers and sufficient NP-specific IgG antibodies (Fig. 1D, F), but generated comparable levels of anti-NP IgM antibodies compared to their wt counterparts (Fig. 1E). Here the strain differences were less pronounced.
Figure 1.
Anti-NP responses in SAP−/− and wt mice are in part determined by the genetic background. SAP−/− or wt B6 and BALB/c mice were immunized with NP-KLH using either CFA (A-C) or Alum (D-F) as adjuvant. Immune responses were followed by anti-NP IgG (A and D) and anti-NP IgM (B and E) titers in the serum, as well as by flow cytometry of the splenic GCB (B220+GL7+Fas+) cells (C and F).
Data are representative of either 2 or 3 independent experiments with 3 to 5 animals per group.
Taken together, the data show that both in wt mice as well in SAP−/− mice, background strain differences could affect NP-specific T cell-dependent B cell responses.
3.2 SAP deficiency affects B cell responsiveness and homeostasis in a genetic background-dependent fashion
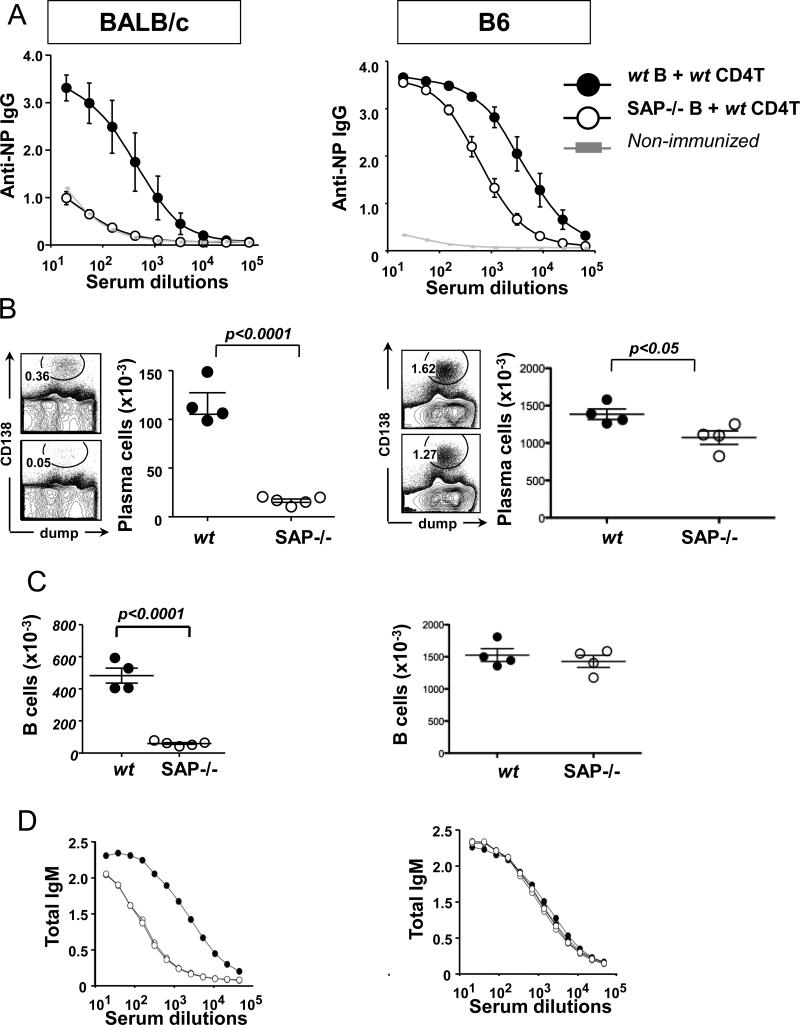
Next, we assessed whether the impaired humoral responses in SAP−/− mice were exclusively caused by SAP deficiency of T cells or whether B cells contributed to the phenotype. To this end, wt CD4+ T cells together with SAP−/− or wt B cells were co-transferred into either BALB/c or B6 Rag−/− mice. Twenty-four hours post-transfer the recipient Rag−/− mice were immunized with NP-KLH in CFA (Fig. 2). Surprisingly, even in the presence of SAP sufficient CD4+ T helper cells, the BALB/c recipients into which SAP−/− B cells had been transferred had dramatically lower NP-specific antibody titers and numbers of plasma cells than Rag−/− mice, which had received wt B cells (p<0.0001) (Fig. 2A-B). This “B cell effect” was also found on SAP−/− B6 mice, albeit to a slightly lesser extent (p<0.05; Fig. 2A-B: right vs. left panels). Further analyses revealed, that the number of splenic B cells was severely reduced in recipients of SAP−/− BALB/c B cells, but not in recipients of SAP−/− B6 B cells (Fig. 2C). These latter results were in agreement with the total IgM titers in the serum (Fig. 2D), suggesting a reduced ability of SAP−/− BALB/c B cells to survive in the Rag−/− recipients.
Figure 2.
Rag−/− hosts (BALB/c: left column or B6: right column) were adoptively transferred with SAP−/− or wt B cells together with wt CD4+ T cells from donors with respective genetic backgrounds, then immunized with NP-KLH in CFA 24 hours later.
(A) NP-specific serum IgG titers
(B) CD138+Dump- plasma cells in the spleen [Dump: CD11c, F4/80, Gr-1]
(C) Total splenic B cell numbers (B220+CD19+Dump-)
(D) Total serum IgM titers
Data are representative of at least two independent experiments with 4-5 recipient animals per group.
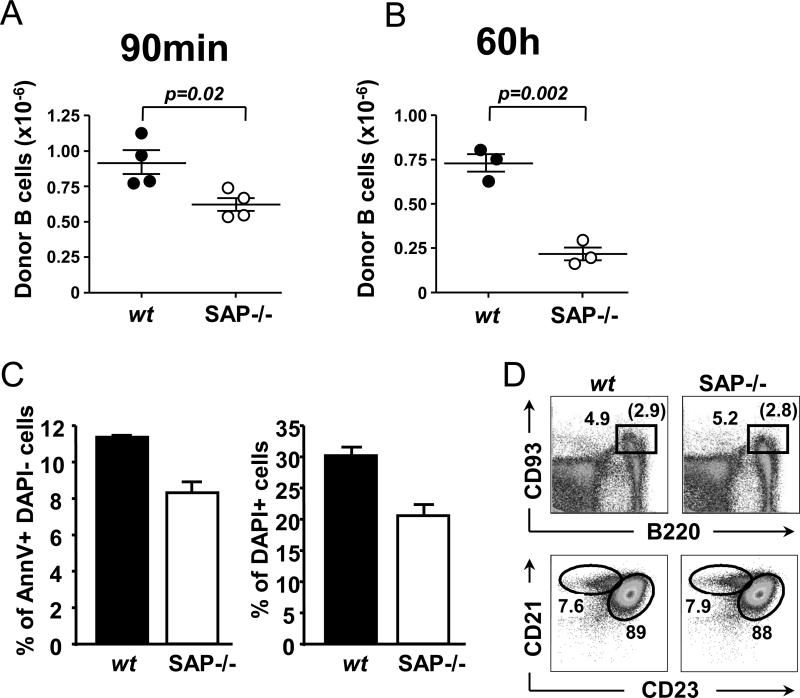
To discern whether the rapid decline of SAP−/− BALB/c B cells in Rag−/− recipients was B cell intrinsic or caused by interactions with the wt CD4+ T cells, we co-injected cell tracker marked wt BALB/c and SAP−/− BALB/c B cells, loaded with CMRA orange and CFSE, respectively, into Rag−/− mice. Whilst 90 minutes after the co-transfer of wt and mutant B cells already significantly less SAP−/− B cells than wt B cells were recovered from the spleen of the recipient mice this difference was more pronounced 60 hours after the co-transfer (Fig. 3A and B).
Figure 3.
Rag−/− BALB/c hosts were transferred with a 1:1 mixture of wt and SAP−/− B cells loaded with cell trackers CMRA and CFSE, respectively. B cells numbers of recipient spleens were analyzed 90 minutes (A) or 60 hours (B) after cell transfers. Data is representative of two independent experiments. A portion of the donor B cell mixture was in vitro cultured in complete media at 37°C for 6 hours, then viability was tested by AnnexinV staining and DAPI uptake (C). Flow cytometric analysis of splenic B cell populations in wt and SAP−/− BALB/c mice (D); upper panel: gated on DAPI- lymphocytes, lower panel: gated on DAPI-B220+CD93- lymphocytes. Data is representative of multiple independent staining experiments of at least 2-3 animals per group in each time.
The possibility that the outcomes of this experiment were influenced by the purification procedure and/or the cell tracking dyes, was excluded because mutant and wt B cells were equally viable after in vitro culturing of B cells for 6 hours, (Fig. 3C). This was consistent with ex vivo analyses which showed that SAP−/− BALB/c mice have a normal splenic B cell compartment (Fig. 3D and data not shown).
We conclude that upon co-transfer of SAP−/− BALB/c or SAP−/− B6 B cells with wt CD4+ T cells into Rag−/− mice B cell responses are impaired in their response to antigen in the presence CFA. SAP−/− BALB/c, but not SAP−/− B6 B lymphocytes display an altered homeostasis.
3.3 Altered in vitro and in vivo responses by SAP deficient B cells to agonistic anti-CD40
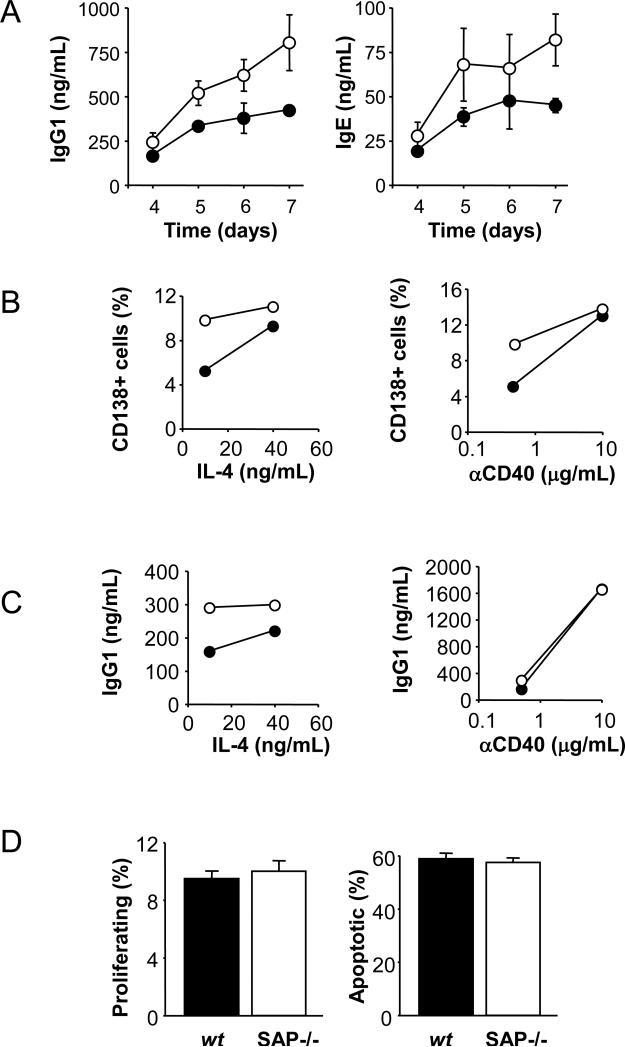
To further evaluate the concept that key functions of B cells derived from SAP−/− mice are altered, purified wt and SAP−/− splenic B cells were stimulated in vitro with suboptimal doses of agonistic anti-CD40 in the presence of recombinant mouse IL-4. Surprisingly, SAP-deficient B cells produced more IgG1 and IgE antibodies in these in vitro experiments (Fig. 4A). Concomitantly, SAP−/− B cell cultures contained higher percentages of CD138+ plasma cells than cultures of wt cells (Fig. 4B). Whereas varying the amounts of IL-4 in the culture abated the responses, increasing the amount of anti-CD40 eliminated the difference between SAP−/− and wt B cell-responsiveness (Fig. 4C). As shown in Fig. 4D, after 3 days in culture with these stimuli, proliferation and activation induced cell death of wt and SAP−/− B cells were identical.
Figure 4.
In vitro stimulations by anti-CD40 and IL-4 of SAP−/− BALB/c (white marks) and wt (black marks) purified splenic B cells.
(A) Secreted IgG1 (left) and IgE (right) antibodies were measured by ELISA from the supernatant of B cell cultures stimulated by 0.5μg/mL anti-CD40 plus 10ng/mL IL-4 for the indicated time.
- - 0.5μg/mL anti-CD40 plus 10 or 40ng/mL IL-4 (left)
- - 0.5 or 10μg/mL anti-CD40 plus 10ng/mL IL-4 (right)
(D) Percentages of tetraploid (left) and subdiploid (right) cells after 3 days of stimulations by 0.5μg/mL anti-CD40 plus 10ng/mL IL-4.
Taken together, the data suggest that SAP−/− BALB/c B cells could be hyper responsive to agonistic anti-CD40.
To evaluate how and whether SAP−/− B cells responded in vivo to anti-CD40, 100μg of the purified agonistic anti-CD40 monoclonal antibody was injected into SAP−/− and wt mice 24hrs after administering NP-KLH. Thirteen days post-immunization antigen-specific IgM and IgG levels in the serum of SAP−/− mice, which had received anti-CD40 were dramatically higher than in control mice injected with PBS (Fig. 5A and B). Anti-NP IgM and IgG serum levels in wt BALB/c mice were also affected by triggering CD40, and to a lesser extent in wt B6 mice (Fig. 5A,B). Most importantly, anti-CD40 caused an increase in the number of germinal center B cells in SAP−/− B6 and SAP−/− BALB/c mice, whereas the agonistic antibody did not affect GC B cell numbers in the wt strains (Fig. 5C).
Figure 5.
The indicated strains of wt and SAP−/− animals were i.p. immunized with NP-KLH on day 0, then i.v. injected with 100μg agonistic anti-CD40 antibody FGK4.5 (triangles) or PBS (circles) 24 hours later. NP-specific serum IgM (A) and IgG (B) titers were determined at day 5. The number of splenic B220+GL7+Fas+ GCB cells (C) were measured at day 13. One experiment with 3-4 animals in each group.
* p<0.01, ** p<0.001, (ns) non-significant.
Taken together, the outcomes of both in vitro and in vivo experiments demonstrate that agonistic anti-CD40 re-activates part of the humoral immune responses that are impacted by SAP-deficiency. This effect is more pronounced in SAP−/− BALB/c than in SAP−/− B6 mice, possibly reflecting the higher responses in B6 mice as compared to BALB/c mice.
4. DISCUSSION
The outcomes of previous studies indicated that while T-independent humoral responses are unaffected by the absence of SAP [10-11], T-dependent antibody responses are profoundly affected in SAP−/− mice [9-15]. For B cells to enter the germinal center reaction and to subsequently differentiate into memory or long lived plasma cells, stimulation by sustained conjugations with SAP+ T follicular helper (Tfh) cells is requisite [12]. Similarly, SAP+ NKT cells contribute to the last stages of the germinal center reaction [24]. The most plausible explanations are that SAP-deficient Tfh and NKT cells are unable to efficiently engage with B cells either because of an impaired “inside-out” signaling of several SLAM-Family receptors [30]. The latter could for instance be caused by the recruitment of the inhibitory tyrosine phosphatase SHP-1 by key SLAMF receptors, e.g. SLAMF6 [15], which in turn affects TCR/CD3 signal transduction in the immune synapse [30]. Beyond defects in T cell help, alterations in the B cell compartment could also be responsible for insufficient humoral reactions of SAP−/− animals, as has been reported previously by our laboratory [10].
In this study we provide evidence in support of the concept that the different XLP disease manifestations among male members of one family with the same SAP mutation are caused by both genetic modifiers and environmental factors. Through the use of SAP−/− B6 and SAP−/− BALB/c mice we find that the severity of dysgammaglobulinemia is dependent upon the genetic background of the mice. This in part reflects wt strain dependent differences in antibody responses. Interestingly, at the endpoint of the co-transfer experiments into Rag−/− recipients the number of SAP−/− B6 B cells is the identical to the number of wt B cells. However, the production of anti-NP antibodies is considerably reduced as compared to wt B cells. The same observation was made upon co-transferring SAP−/− BALB/c B cells with wt CD4+ T cells. Studies by other investigators did not indicate any difference in the responsiveness of SAP−/− B6 and wt B cells when co-transferred with wt T cells into Rag−/− recipients [9,11]. This is likely to be caused by differences in the nature of the antigen/pathogen or adjuvant. For instance, whereas Slamf5−/− are impaired in their ability to form germinal centers in response to protein antigens, antibody responses to acute LCMV and vaccinia virus infections are identical to those in wt mice [13,15].
As signaling through CD40 is one of the most important factors of T cell-mediated co-stimulation to B cells, we evaluated whether an agonistic anti-CD40 antibody could activate SAP−/− B cells both in vitro and in vivo. SAP−/− BALB/c B cells behaved differently from their wt counterparts, showing a hyper-active phenotype, that we did not detect in the B6 genetic background.
For IgM antibody responses to T-dependent antigens, the scenario is however more complex. As our data demonstrate, primary IgM antibody secretion can be intact in SAP−/− animals, depending on the applied adjuvant. Moreover, by the administration of an agonistic anti-CD40 antibody, defective early IgG responses in SAP−/− mice can be partially rescued. Although anti-CD40 elicited a significant increase in the frequency of germinal centers in SAP−/− mice, their numbers are still an order of magnitude lower than in wt animals. We also need to take into consideration that the transition of B cells into GCB cells requires sequential interactions with T cells, and therefore multiple anti-CD40 injections might be necessary (at the right time) to fully recapitulate all CD40L triggered stimuli that wt Tfh cells are capable of providing. Also, other Tfh cell-promoted co-stimulating factors - including signaling through the SLAM-family receptors - that can further shape the magnitude and the quality of late primary and secondary B cell responses, should be supplemented.
In sum, the data suggest that like in XLP patients, in SAP−/− mice the type of infection and the genetic background plays an important role in determining to what degree humoral protection against pathogens will develop. In addition, SAP mutations can also facilitate the development of a hyper active B cell phenotype in a genetic background dependent manner, which might contribute to B cell tumor progression in a sub group of XLP patients.
Highlights.
Anti-CD40 in part re-activates humoral responses that are impacted by SAP deficiency
SAP deficiency affects B cell responsiveness in a genetic background-dependent manner
Environmental factors affect the severity of humoral deficiencies of SAP−/− mice
Acknowledgements
We thank Boaz Van Driel and Drs. Ninghai Wang, Guoxing Wang, Gongxian Liao, Chunyan Ma, Katalin Kis-Toth, Jing Hua for thoughtful discussions. Special thanks to Michael O'Keeffe for technical and conceptual support. This study was funded by a grant from the NIH (PO1 AI 076210 to CT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
References
- 1.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–9. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 2.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–35. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 3.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, Terhorst C. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 4.Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin Immunopathol. 2010;32:157–71. doi: 10.1007/s00281-009-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 6.Morra M, Howie D, Grande MS, Sayos J, Wang N, Wu C, Engel P, Terhorst C. X-linked lymphoproliferative disease: a progressive immunodeficiency. Annu Rev Immunol. 2001;19:657–82. doi: 10.1146/annurev.immunol.19.1.657. [DOI] [PubMed] [Google Scholar]
- 7.Morra M, Silander O, Calpe S, Choi M, Oettgen H, Myers L, Etzioni A, Buckley R, Terhorst C. Alterations of the X-linked lymphoproliferative disease gene SH2D1A in common variable immunodeficiency syndrome. Blood. 2001;98:1321–5. doi: 10.1182/blood.v98.5.1321. [DOI] [PubMed] [Google Scholar]
- 8.Parolini O, Kagerbauer B, Simonitsch-Klupp I, Ambros P, Jaeger U, Mann G, Haas OA, Morra M, Gadner H, Terhorst C, Knapp W, Holter W. Analysis of SH2D1A mutations in patients with severe Epstein-Barr virus infections, Burkitt's lymphoma, and Hodgkin's lymphoma. Ann Hematol. 2002;81:441–7. doi: 10.1007/s00277-002-0490-3. [DOI] [PubMed] [Google Scholar]
- 9.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–7. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 10.Morra M, Barrington RA, Abadia-Molina AC, Okamoto S, Julien A, Gullo C, Kalsy A, Edwards MJ, Chen G, Spolski R, Leonard WJ, Huber BT, Borrow P, Biron CA, Satoskar AR, Carroll MC, Terhorst C. Defective B cell responses in the absence of SH2D1A. Proc Natl Acad Sci U S A. 2005;102:4819–23. doi: 10.1073/pnas.0408681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–65. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled TB cell interactions underlie germinal centre formation. Nature. 2008;455:764–9. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–65. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi H. From SAP-less T cells to helpless B cells and back: dynamic T-B cell interactions underlie germinal center development and function. Immunol Rev. 2012;247:24–35. doi: 10.1111/j.1600-065X.2012.01119.x. [DOI] [PubMed] [Google Scholar]
- 15.Kageyama R, Cannons JL, Zhao F, Yusuf I, Lao C, Locci M, Schwartzberg PL, Crotty S. The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development. Immunity. 2012;36:986–1002. doi: 10.1016/j.immuni.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poy F, Yaffe MB, Sayos J, Saxena K, Morra M, Sumegi J, Cantley LC, Terhorst C, Eck MJ. Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol Cell. 1999;4:555–61. doi: 10.1016/s1097-2765(00)80206-3. [DOI] [PubMed] [Google Scholar]
- 17.Morra M, Lu J, Poy F, Martin M, Sayos J, Calpe S, Gullo C, Howie D, Rietdijk S, Thompson A, Coyle AJ, Denny C, Yaffe MB, Engel P, Eck MJ, Terhorst C. Structural basis for the interaction of the free SH2 domain EAT-2 with SLAM receptors in hematopoietic cells. EMBO J. 2001;20:5840–52. doi: 10.1093/emboj/20.21.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao E, Ramagopal UA, Fedorov A, Fedorov E, Yan Q, Lary JW, Cole JL, Nathenson SG, Almo SC. NTB-A receptor crystal structure: insights into homophilic interactions in the signaling lymphocytic activation molecule receptor family. Immunity. 2006;25:559–70. doi: 10.1016/j.immuni.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Yan Q, Malashkevich VN, Fedorov A, Fedorov E, Cao E, Lary JW, Cole JL, Nathenson SG, Almo SC. Structure of CD84 provides insight into SLAM family function. Proc Natl Acad Sci U S A. 2007;104:10583–8. doi: 10.1073/pnas.0703893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–60. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 21.Hwang PM, Li C, Morra M, Lillywhite J, Muhandiram DR, Gertler F, Terhorst C, Kay LE, Pawson T, Forman-Kay JD, Li SC. A “three-pronged” binding mechanism for the SAP/SH2D1A SH2 domain: structural basis and relevance to the XLP syndrome. EMBO J. 2002;21:314–23. doi: 10.1093/emboj/21.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5:149–54. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 23.Berger SB, Romero X, Ma C, Wang G, Faubion WA, Liao G, Compeer E, Keszei M, Rameh L, Wang N, Boes M, Regueiro JR, Reinecker HC, Terhorst C. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol. 2010;11:920–7. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detre C, Keszei M, Garrido-Mesa N, Kis-Toth K, Castro W, Agyemang AF, Veerapen N, Besra GS, Carroll MC, Tsokos GC, Wang N, Leadbetter EA, Terhorst C. SAP expression in invariant NKT cells is required for cognate help to support B-cell responses. Blood. 2012;120:122–9. doi: 10.1182/blood-2011-11-395913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King IL, Fortier A, Tighe M, Dibble J, Watts GF, Veerapen N, Haberman AM, Besra GS, Mohrs M, Brenner MB, Leadbetter EA. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2011;13:44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, Nutt SL, Brink R, Godfrey DI, Batista FD, Vinuesa CG. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2011;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 27.Veillette A, Zhang S, Shi X, Dong Z, Davidson D, Zhong MC. SAP expression in T cells, not in B cells, is required for humoral immunity. Proc Natl Acad Sci U S A. 2008;105:1273–8. doi: 10.1073/pnas.0710698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Alem U, Li C, Forey N, Relouzat F, Fondanèche MC, Tavtigian SV, Wang ZQ, Latour S, Yin L. Impaired Ig class switch in mice deficient for the X-linked lymphoproliferative disease gene Sap. Blood. 2005;106:2069–75. doi: 10.1182/blood-2004-07-2731. [DOI] [PubMed] [Google Scholar]
- 29.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–7. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 30.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol. 2012;24:107–15. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, Sosa MR, Edwards MJ, Borrow P, Satoskar AR, Sharpe AH, Biron CA, Terhorst C. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–4. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 32.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–30. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]