Abstract
Alpha-Galactosylceramide (α-Galcer), a specific agonist for invariant natural killer T (iNKT) cells, is being evaluated in clinical trials for the treatment of viral hepatitis and liver cancer. However, the results from α-Galcer treatment are mixed, partially because of the variety of cytokines produced by activated iNKT cells that have an unknown synergistic effect on the progression of liver disease. It is well documented that injection of α-Galcer induces mild hepatitis with a rapid elevation in the levels of IL-4 and a delayed elevation in the levels of IFN-γ, and both of these cytokines are thought to mediate many functions of iNKT cells. Surprisingly, genetic deletion of both IL-4 and IFN-γ aggravated, rather than abolished, α-Galcer-induced iNKT hepatitis. Moreover, genetic ablation of IL-4, the IL-4 receptor, or its downstream signaling molecule STAT6 ameliorated α-Galcer-induced neutrophil infiltration, liver injury, and hepatitis. In contrast, genetic deletion of IFN-γ, the IFN-γ receptor, or its downstream signaling molecule STAT1 enhanced liver neutrophil accumulation, thereby exacerbating liver injury and hepatitis. Moreover, depletion of neutrophils eradicated α-Galcer-induced liver injury in wild-type, IL-4 knockout, and IFN-γ knockout mice.
Conclusions
Our results propose a model in which activated iNKT cells rapidly release IL-4, which promotes neutrophil survival and hepatitis but also sequentially produce IFN-γ, which acts in a negative feedback loop to ameliorate iNKT hepatitis by inducing neutrophil apoptosis. Thus, modification of iNKT production of IL-4 and IFN-γ may have the potential to improve the efficacy of α-Galcer in the treatment of liver disease.
Keywords: liver disease, STAT1, STAT6, α-Galcer
Introduction
Natural killer T (NKT) cells are a heterogeneous group of nonconventional T lymphocytes that recognize, through their T-cell receptors (TCRs), glycolipid antigens presented by the nonclassical MHC class I-like molecule CD1.1 CD1d-restricted NKT cells can be divided into two subsets: type I and type II NKT cells. Type I NKT (invariant NKT or iNKT) cells represent the predominant subset and exclusively express an invariant TCR-α chain, whereas type II NKT cells express more diverse TCRs.1 The naturally occurring glycolipid α-Galactosylceramide (α-Galcer), originally isolated from a marine sponge, was discovered in 1993 during a screen for novel cancer therapeutic agents2 and was later found to be a specific agonist for mouse and human iNKT cells.3 It is now well established that α-Galcer is a strong ligand capable of inducing iNKT activation and the rapid production of Th1 (IFN-γ) and Th2 (IL-4) cytokines as well as many other cytokines, such as IL-17 and TNF-α, thereby affecting a wide variety of functions in innate and adaptive immunity.1 Owing to its potent immunomodulatory properties, α-Galcer has been actively investigated in preclinical and clinical studies for the treatment of cancer, infections, and autoimmune and inflammatory diseases.4
The therapeutic potential of α-Galcer for the treatment of liver disease has received particular attention5 because of the enrichment of iNKT cells in the liver.6 Mouse and human liver lymphocytes contain 20–35% and 10–15% iNKT cells, respectively,6 whereas peripheral blood lymphocytes contain less than 5% iNKT cells. Accumulating evidence suggests that iNKT cells play complex and even opposing roles in controlling liver injury, regeneration, fibrosis, and liver tumor transformation in different animal models and in patients with different stages or types of liver diseases.6–8 This involvement is likely a result of the wide array of cytokines produced by iNKT cells. For example, iNKT cells not only can produce anti-fibrotic cytokine such as IFN-γ, to inhibit liver fibrosis,9 but also can produce IL-4, IL-13, hedgehog, and osteopontin to exacerbate liver fibrosis.10 The production of both type I (IFN-γ) and type II (IL-4) cytokines is a hallmark of iNKT activation, which mediates many important functions in the liver.6–8 The action of IFN-γ is mediated via the binding of IFN-γ receptor 1 (IFNGR1) and IFNGR2, whereas IL-4 exerts its effects via the binding of IL-4Rα and the gp140/γc chain or IL-4Rα and the IL-13Rα1 chain. These cytokines then activate signal transducer and activator of transcription 1 (STAT1) and STAT6, respectively, in hepatocytes, liver nonparenchymal cells, and immune cells and thereby play important roles in the pathogenesis of liver disease.11
Despite its complex and obscure immunomodulatory properties in the liver, α-Galcer is being evaluated in clinical trials for the treatment of viral hepatitis and liver cancer.5, 12–14 In these studies, patients tolerated α-Galcer treatment well, although the results of treatment in patients with liver diseases are inconsistent.5, 12–14 These inconclusive results are likely the result of α-Galcer inducing iNKT production of a wide variety of cytokines, the synergistic effects of which remain largely unknown for the control of hepatitis. These questions urgently need to be addressed prior to the application of α-Galcer in additional clinical trials for treating liver disease. Injection of α-Galcer into mice induces iNKT activation, with rapid production of IL-4 but delayed production of IFN-γ, which results in mild hepatitis and liver injury.15, 16 In the current study, we found that after α-Galcer injection, iNKT cells rapidly produce IL-4, which promotes liver neutrophil accumulation and hepatitis via a STAT6-dependent mechanism, whereas the subsequent production of IFN-γ acts in a negative feedback loop to control α-Galcer-induced hepatitis by inducing neutrophil apoptosis via a STAT1-dependent mechanism.
Materials and Methods
Mice
Eight- to ten-week-old male mice were used in this study. C57BL/6J, IFN-γ−/−, IFNGR−/−, IL-4−/−, and STAT6−/− mice on a C57B/6J background were purchased from the Jackson Laboratory (Bar Harbor, Maine). Balb/c and IL-4R−/− mice on a Balb/c background were also purchased from the Jackson Laboratory (Bar Harbor). STAT1−/− mice were originally purchased from Taconic (Hudson, NY) and backcrossed into a C57BL/6J background for at least 11 generations. IFN-γ−/− IL-4−/− dKO mice were generated as a result of several steps of cross-breeding between IFN-γ−/− and IL-4−/− mice. All animals were maintained in a specific pathogen-free facility and were cared for in accordance with National Institutes of Health guidelines, and this study was approved by the National Institute on Alcohol Abuse and Alcoholism animal care and use committee.
Murine iNKT cell-driven hepatitis
To induce murine iNKT cell-driven acute experimental hepatitis, a single intravenous (i.v.) injection of α-Galcer (3 µg in 300 µl vehicle) was administered to each mouse. Control mice received 300 µl of vehicle. α-Galcer (KRN7000) was purchased from Alexis Biochemicals Corporation (San Diego, CA) and dissolved in 0.5% polysorbate-20 [Tween-20] and diluted in sterile phosphate-buffered saline (PBS).
Statistical analysis
Data are expressed as the mean ± SEM for each group and were analyzed using GraphPad Prism software (version 5.0a; GraphPad Software, Inc, La Jolla, CA). To compare values obtained from 2 groups, Student t test was performed. To compare values obtained from 3 or more groups, single-factor analysis of variance (ANOVA) was used, followed by Tukey's post-hoc test. Statistical significance was designated at the P<0.05 level.
Additional Materials and Methods are included in supporting materials.
Results
Deletion of both IFN-γ and IL-4 exacerbates α-Galcer-induced liver injury and hepatitis
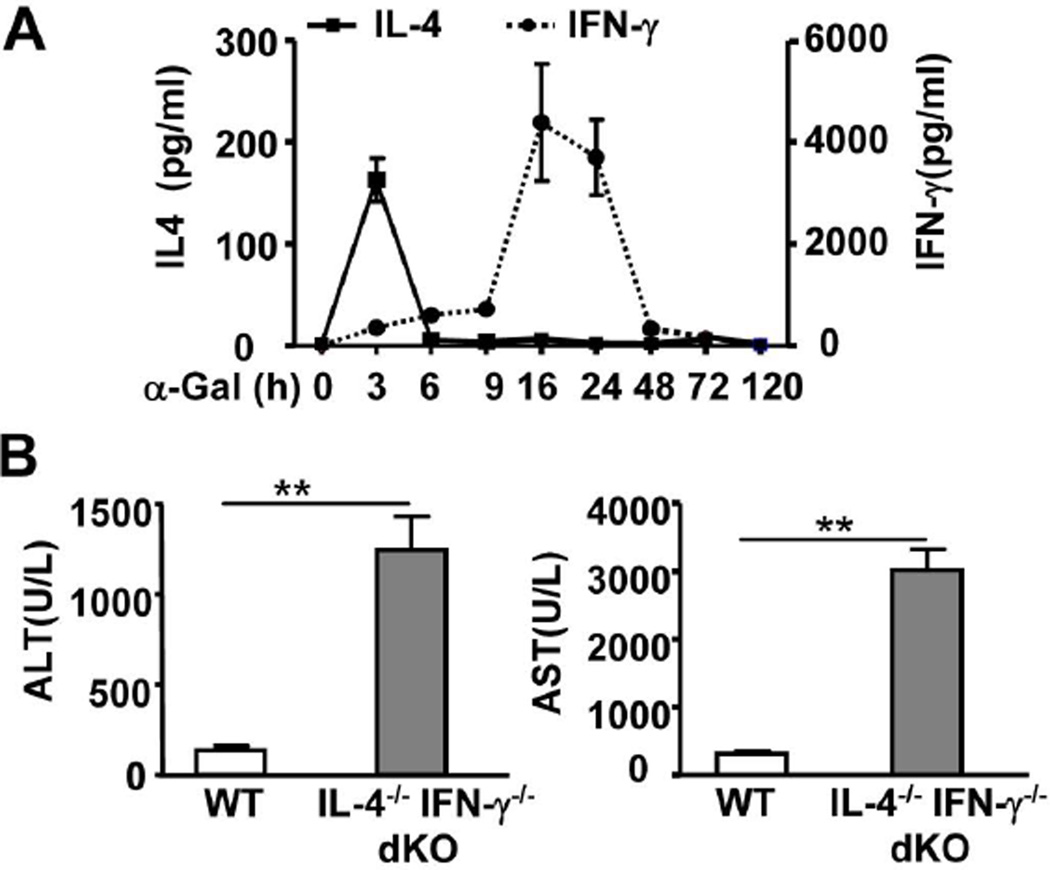
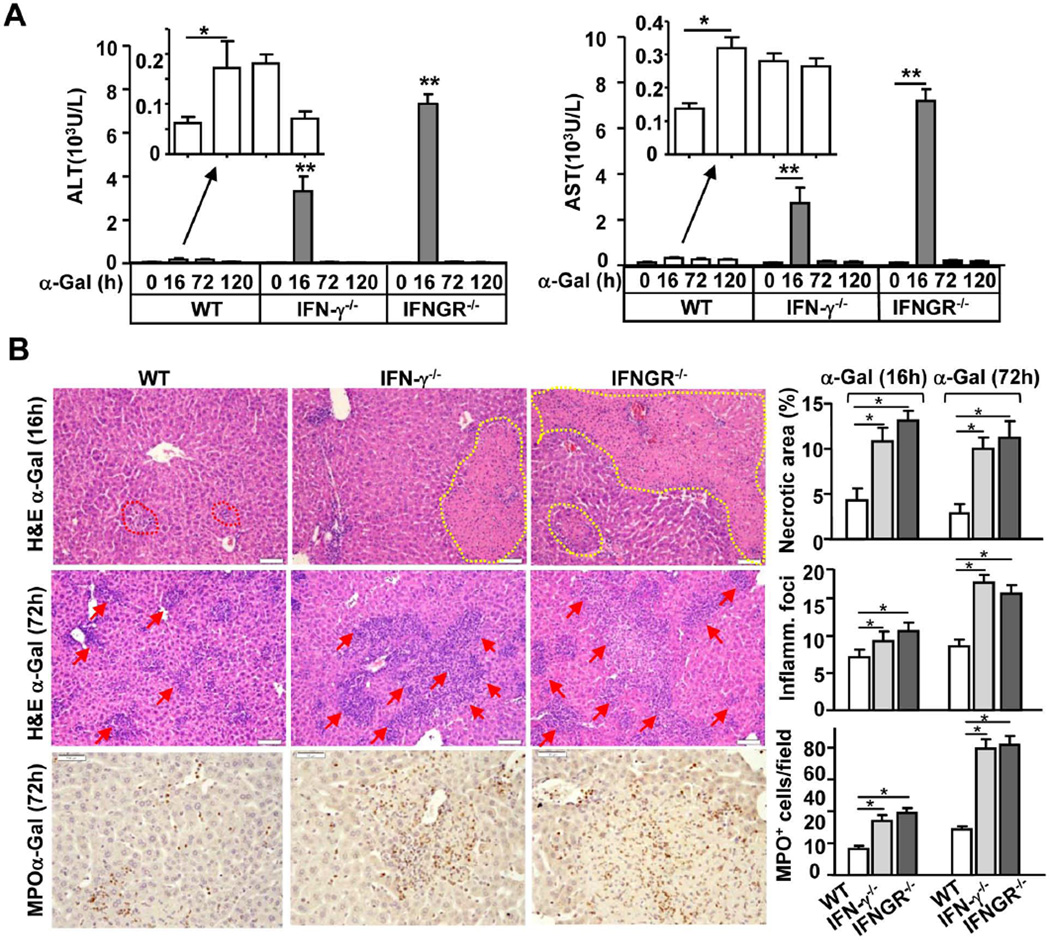
Injection of α-Galcer rapidly increased the serum levels of IL-4, with a peak effect at 3h post-injection, whereas the elevation of serum IFN-γ was delayed, with a peak effect at 16h post-injection (Fig. 1A). Administration of α-Galcer resulted in a mild elevation of serum aspartate aminotransferase (ALT) and alanine aminotransferase (AST) levels (Fig. 1A) and spotted necrosis (supporting Fig. 1) in wild-type (WT) C57BL/6 mice by 16h post-injection. However, to our surprise, α-Galcer induced 5- to 6- fold higher serum ALT and AST levels and a larger area of necrosis in IL-4−/− IFN-γ−/− double knockout (dKO) mice than those in WT mice at 16h after α-Galcer injection (Fig. 1 and supporting Fig. 1). In addition, administration of α-Galcer induced an accumulation of inflammatory foci in the livers of WT mice, with the peak effect occurring at 72h post-injection (Supporting Fig. 1), and the number of inflammatory foci was also much higher in dKO mice than that in WT mice (Supporting Fig. 1).
Fig. 1. IFN-γ−/− IL-4−/− dKO mice are more susceptible to α-Galcer-induced liver injury than WT mice.
(A) WT mice were treated with α-Galcer, and serum IL-4 and IFN-γ levels were measured. (B) WT and dKO mice were treated with α-Galcer. Sera were collected 16h post-injection for the measurement of ALT and AST. **P<0.01.
α-Galcer-induced hepatocellular damage is reduced in IL-4−/− or IL-4R−/− mice
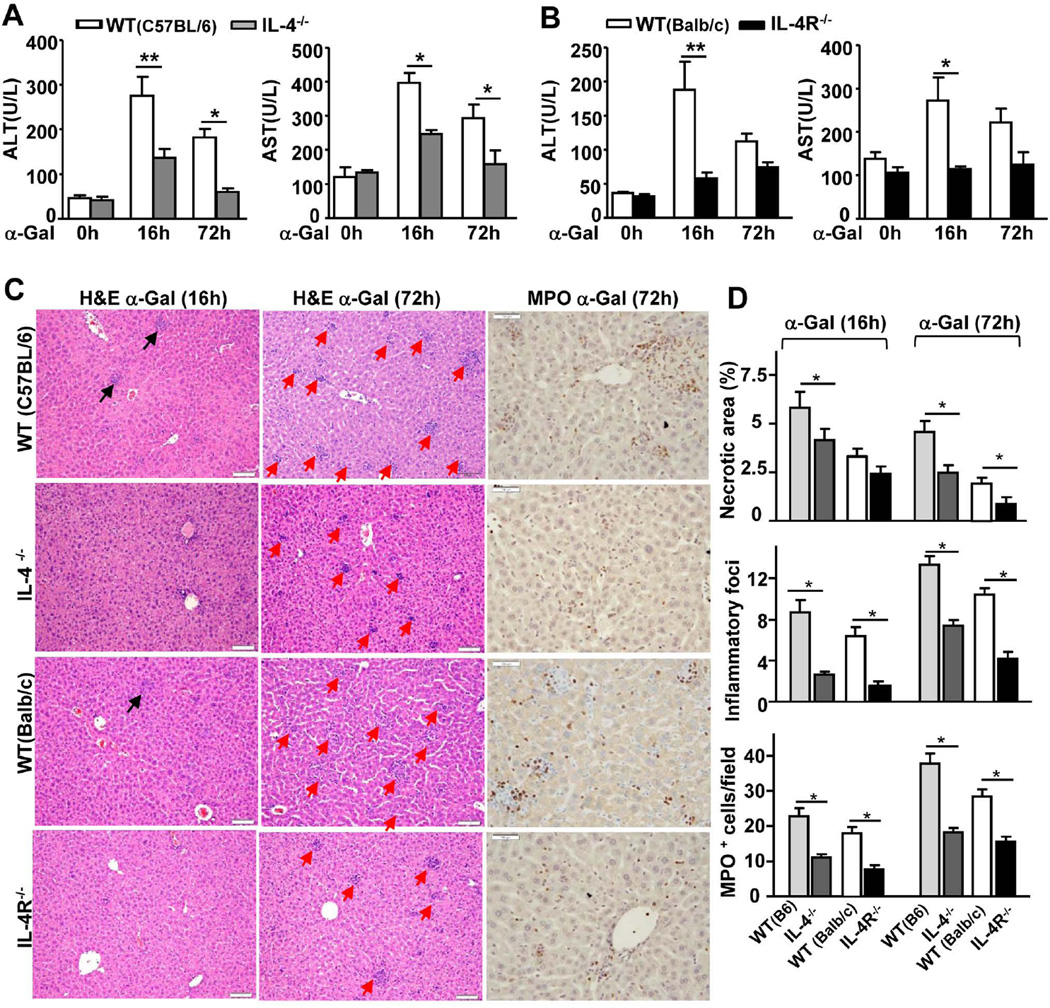
To determine the role of early production of IL-4 in α-Galcer-induced liver injury, we examined the effects in IL-4−/− and IL-4R−/− mice. As illustrated in Figs. 2A–B, α-Galcer-induced elevation of serum ALT and AST was lower in IL-4−/− and IL-4R−/− mice than in WT controls. Liver histology analyses further revealed that IL-4−/− and IL-4R−/− mice had reduced liver necrosis and fewer inflammatory foci than WT control mice after α-Galcer administration (Figs. 2C–D). The number of myeloperoxidase (MPO)-positive neutrophils was also lower in IL-4−/− and IL-4R−/− mice than in WT mice 72h after α-Galcer administration (Figs. 2C–D).
Fig. 2. IL-4−/− and IL-4R−/− mice are resistant to α-Galcer-induced liver injury and hepatitis compared with WT mice.
Mice were treated with a single dose of α-Galcer and euthanized at different time points post-injection. (A, B) Serum ALT and AST levels. (C) Representative H&E or anti-MPO antibody-stained liver sections from α-Galcer-treated mice (magnification,× 200). The red arrows indicate inflammatory foci. (D) The graphs represent the mean score or the number of MPO+ cells for each group of mice. *P<0.05, **P<0.01.
IL-4 is required for neutrophil accumulation post α-Galcer administration, which contributes to liver injury
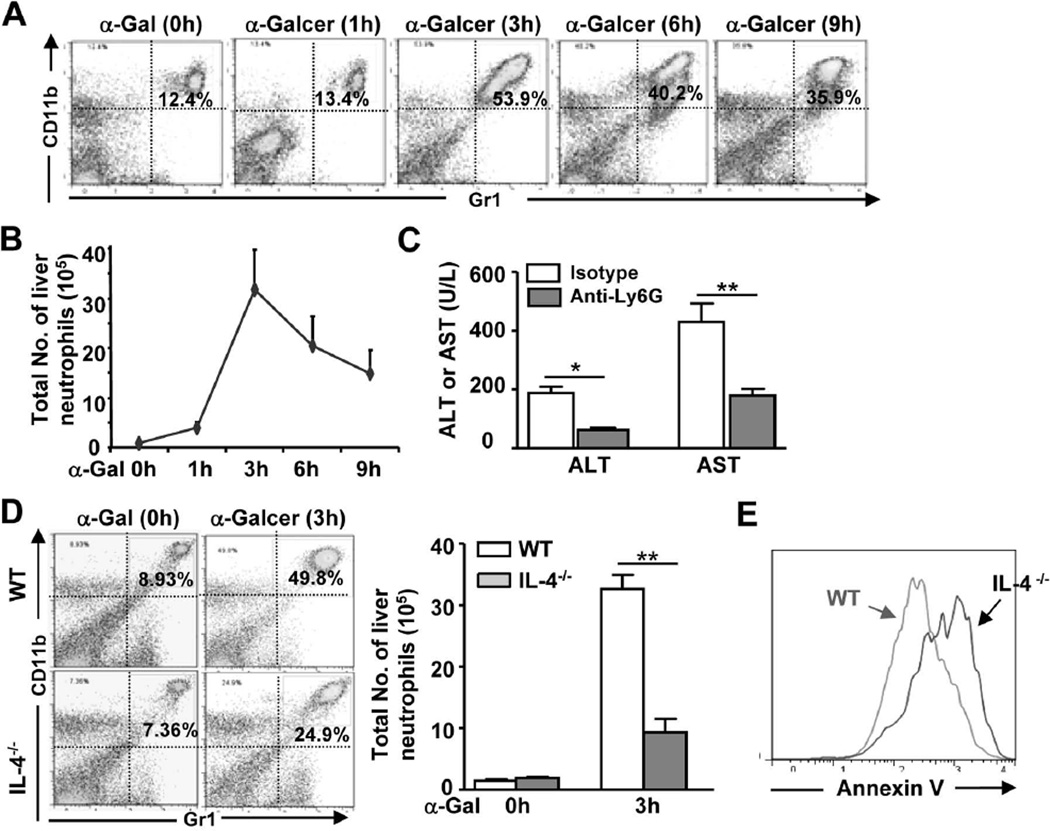
The above findings indicated that the number of inflammatory foci (iNKT expansion) in the liver was lower in IL-4−/− or IL-4R−/− mice than in WT mice 72h post α-Galcer injection, which may have been due to IL-4-mediated promotion of iNKT proliferation, as demonstrated previously.17 Fluorescence-activated cell sorting (FACS) analyses of liver MNCs revealed that WT and IL-4−/− mice had a similar number of iNKT cells at the early time points post α-Galcer injection (data not shown), which does not explain the reduced liver injury in IL-4−/− mice. To further explore the mechanisms underlying α-Galcer-induced liver injury, we examined NK cells and neutrophils in the liver. In this case, FACS analyses revealed that the number of NK cells was not increased post α-Galcer injection and that depletion of NK cells using an anti-ASGM1 antibody did not affect α-Galcer-induced liver injury in mice (data not shown), suggesting that NK cells are not involved in this process. In contrast, there was a striking increase in the percentage and total number of neutrophils in the liver after α-Galcer injection. As illustrated in Figs. 3A–B, the percentage of neutrophils was elevated 4 fold, whereas the total number of neutrophils was elevated 30 fold at 3h post-α-Galcer administration. Moreover, depletion of neutrophils markedly reduced serum ALT and AST levels (Fig. 3C), suggesting that the accumulation of neutrophils contributes to α-Galcer-induced hepatocellular damage.
Fig. 3. α-Galcer treatment induces neutrophil accumulation in the liver, which contributes to liver injury and requires IL-4.
(A, B) Liver PMNs were isolated from α-Galcer-treated WT mice and subjected to FACS analyses. Representative FACS graphs are shown in A. The total number of CD11b+ Gr1hi neutrophils was counted and is shown in B. (C) WT mice were treated with anti-Ly6G to deplete neutrophils or control isotype antibodies, and then injected with α-Galcer. Serum ALT and AST levels were determined 16h post-α-Galcer injection. (D) Liver PMNs were isolated from α-Galcer-treated WT and IL-4−/− mice and subjected to FACS analyses, and the total number of neutrophils was counted. (E) Liver PMNs from panel D (α-Galcer 3h) were subjected to FACS analyses for neutrophil apoptosis. A representative graph is shown. **P<0.01.
Fig. 3D shows that the percentage and total number of hepatic neutrophils were lower in IL-4−/− mice than in WT mice at 3h post-α-Galcer administration. Furthermore, Fig. 3E shows that neutrophils from α-Galcer-treated IL-4−/− mice demonstrated higher levels of apoptosis (Annexin V staining) than those of WT mice, which suggests that the reduced neutrophil accumulation in α-Galcer-treated IL-4−/− mice was due to increased neutrophil apoptosis.
STAT6, the major downstream signaling molecule of IL-4, is required for α-Galcer-induced neutrophil accumulation and liver injury
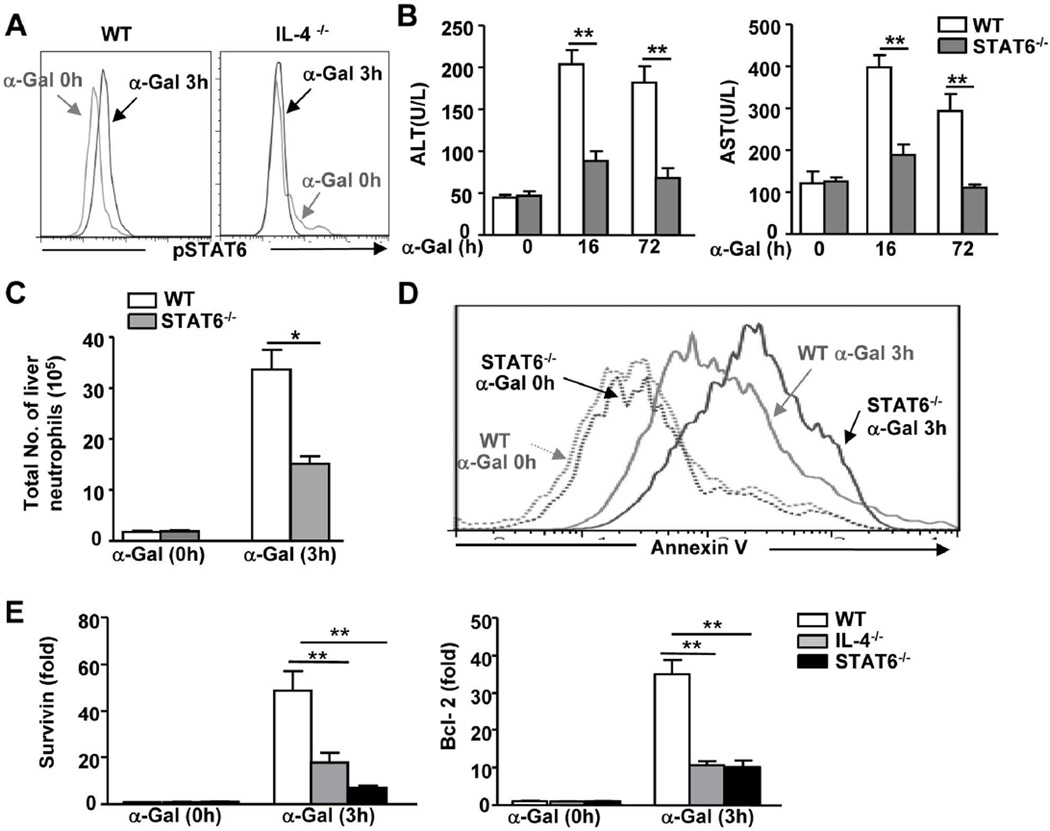
To further understand the mechanisms through which IL-4 contributes to α-Galcer-induced liver injury, we examined the role of STAT6 in this model. As illustrated in Fig. 4A, STAT6 was activated in neutrophils from the livers of α-Galcer-treated WT mice, whereas this activation was diminished in neutrophils from α-Galcer-treated IL-4−/− mice. This result suggests that IL-4 is responsible for the observed STAT6 activation in neutrophils.
Fig. 4. STAT6, a key downstream signaling molecule of IL-4, is required for α-Galcer-induced liver injury and neutrophil accumulation in vivo.
(A) Liver PMNs were isolated from α-Galcer-treated mice and subjected to FACS intracellular staining for pSTAT6 in neutrophils. (B-E) WT and STAT6−/− mice were treated with α-Galcer and euthanized at various time points. (B) Serum ALT and AST levels were measured. (C) Hepatic PMNs were isolated and subjected to FACS analyses. The total number of neutrophils was calculated. (D) Hepatic neutrophil apoptosis was measured by FACS analysis, and a representative graph is shown. (E) Hepatic neutrophils were purified from α-Galcer-treated mice and subjected to real-time PCR analyses. *P<0.05; **P<0.01.
In agreement with the data from IL-4−/− mice, STAT6−/− mice had lower serum levels of ALT and AST (Fig. 4B), fewer inflammatory foci (Supporting Fig. 2), and a reduced number of neutrophils in the liver (Fig. 4C) compared to WT mice post-α-Galcer administration. In addition, neutrophils from α-Galcer-treated STAT6−/− mice demonstrated higher levels of apoptosis than those from WT mice (Fig. 4D). Collectively, our findings suggest that IL-4/STAT6 inhibit neutrophil apoptosis.
To understand the mechanisms underlying the IL-4/STAT6-mediated inhibition of neutrophil apoptosis, we investigated the expression of anti-apoptotic genes in these cells and identified that the expression of survivin and Bcl-2 was significantly upregulated in hepatic neutrophils from α-Galcer-treated WT mice, whereas this upregulation was reduced in hepatic neutrophils from α-Galcer-treated IL-4−/− or STAT6−/− mice (Fig. 4E).
α-Galcer-induced liver injury is exacerbated in IFN-γ−/− or IFNGR−/− mice
The finding that deletion of IL-4 abolished α-Galcer-induced hepatitis cannot explain the exacerbated α-Galcer-induced liver injury observed in IL-4−/− IFN-γ−/− dKO mice. To further understand the mechanisms by which IL-4−/− IFN-γ−/− dKO mice are more susceptible to α-Galcer-induced hepatitis, we examined this model in IFN-γ−/− or IFNGR−/− mice. As illustrated in Fig. 5A, IFN-γ−/− or IFNGR−/− mice were more sensitive to α-Galcer-induced liver injury, as reflected by the higher levels of serum ALT and AST than WT mice. In agreement with the biochemical data, histological examination, as shown in Fig. 5B, confirmed more severe liver injury and inflammation (larger area of necrosis and a larger number of inflammatory foci) in IFN-γ−/− and IFNGR−/− mice at both 16h and 72h after α-Galcer administration than in WT mice. In addition, the number of MPO+ neutrophils was higher in the livers of IFN-γ−/− or IFNGR−/− mice post-α-Galcer injection (Fig. 5B).
Fig. 5. IFN-γ−/− and IFNGR−/− mice are more susceptible to α-Galcer-induced liver inflammation and injury than WT mice.
Mice were treated with a single dose of α-Galcer and euthanized at different time points post injection. (A) Serum ALT and AST levels. (B) Representative photos of H&E- or anti-MPO antibody-stained liver sections from α-Galcer-treated mice (magnification, × 200). The red arrows indicate inflammatory foci. The yellow dotted line indicates the area of necrosis. The graphs represent the mean score or the number of MPO+ cells per field *P<0.05, **P<0.01.
Enhanced α-Galcer-induced liver injury in IFN-γ−/− mice is dependent on neutrophils but not NK cells
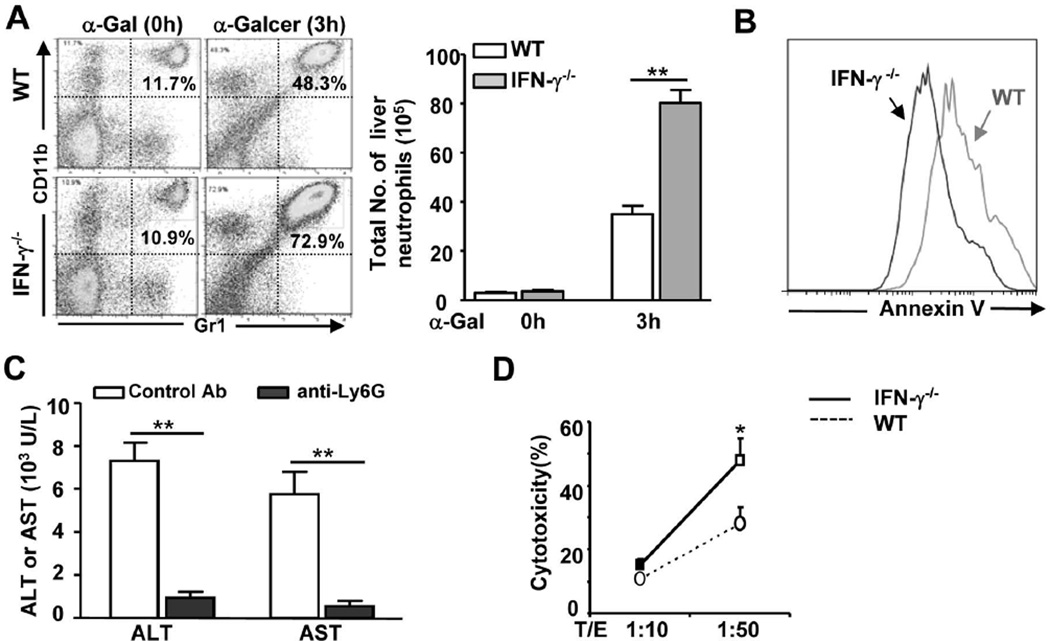
Because it has been shown that NKT and NK cells can kill hepatocytes and contribute to liver injury,18, 19 we hypothesized that the differences in α-Galcer-induced liver injury in WT and IFN-γ−/− mice were due to varying degrees of NKT and NK activation. The data in Supporting Fig. 3 show that mononuclear cells (MNCs) from α-Galcer-treated WT and IFN-γ−/− mice had similar levels of cytotoxicity towards hepatocytes, which could not account for the difference in liver injury between WT and KO mice and suggests that enhanced liver injury in IFN-γ−/− mice is not due to increased MNC (NKT) cytotoxicity. In addition, depletion of NK cells did not affect serum ALT and AST levels in IFN-γ−/− mice (Supporting Fig. 4), indicating that NK cells are not the cause of the severe liver injury in these animals.
FACS analyses showed that the percentage of iNKT cells was markedly decreased, whereas the percentage of macrophages was slightly increased 3h after α-Galcer injection. Such changes were similar in WT and IFN-γ−/− mice (data not shown). Interestingly, the percentage and total number of neutrophils were much higher in IFN-γ−/− mice than in WT mice 3h after α-Galcer injection (Fig. 6A), which was likely due to reduced apoptosis as demonstrated by Annexin V staining (Fig. 6B). Moreover, depletion of neutrophils with an anti-Ly6G antibody reduced α-Galcer-induced elevation of serum ALT and AST levels by 80% in IFN-γ−/− mice (Fig. 6C), and liver histology revealed that depletion of neutrophils completely prevented α-Galcer-induced necrosis in IFN-γ−/− mice (Supporting Fig. 5).
Fig. 6. Increased hepatic accumulation of neutrophils in IFN-γ−/− mice after α-Galcer injection contributes to liver injury.
(A) Liver PMNs were isolated from α-Galcer-treated WT and IFN-γ−/− mice and subjected to FACS analyses. The total number of neutrophils (CD11b+ Gr1hi) neutrophils was counted. (B) Liver PMNs were isolated from α-Galcer-treated WT and IFN-γ−/− mice and stained with annexin V for apoptosis. (C) IFN-γ−/− mice were treated with control or anti-Ly6G antibodies, followed by injection with α-Galcer. Mice were euthanized 16h post α-Galcer injection. Serum ALT and AST levels were measured. (D) Liver PMNs were isolated from α-Galcer (3h)-treated WT and IFN-γ−/− mice and incubated with primary mouse hepatocytes for 4h. Cytotoxicity was measured. **P<0.01.
Next we investigated the mechanisms through which neutrophils contribute to liver injury by examining liver leukocyte cytotoxicity against hepatocytes. As shown in Fig. 6D, liver polymorphonuclear cells (PMNs) isolated from α-Galcer-treated IFN-γ−/− mice demonstrated higher levels of cytotoxic activity against mouse hepatocytes than those from α-Galcer-treated WT mice.
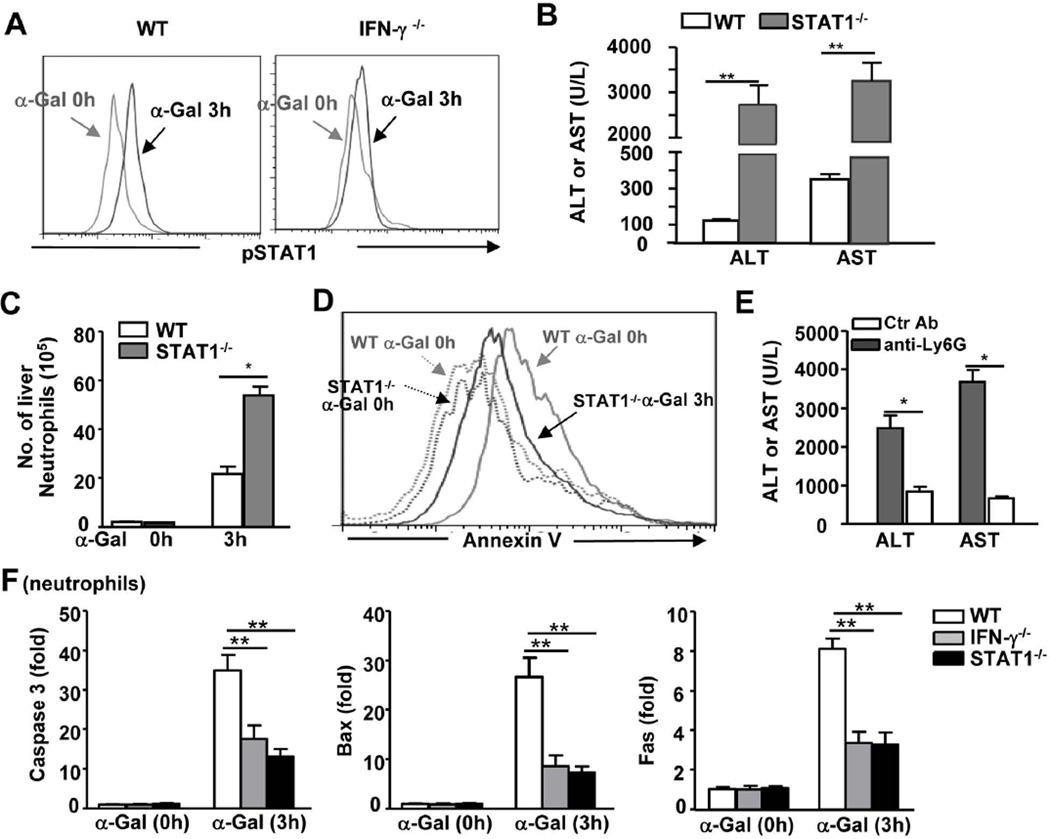
STAT1−/− mice are more susceptible to α-Galcer-induced liver injury and neutrophil infiltration
Figure 7A shows that STAT1 was activated in neutrophils from α-Galcer-treated WT mice but not in neutrophils from IFN-γ−/− mice, suggesting that STAT1 is the key downstream signaling molecule of IFN-γ in this process. To examine the role of STAT1 in α-Galcer-induced hepatitis, we compared the α-Galcer-induced liver injury in STAT1−/− and WT mice. As illustrated in Fig. 7B, α-Galcer administration induced higher levels of serum ALT and AST in STAT1−/− mice than in WT mice at 16h post-injection. Moreover, the liver histology revealed that STAT1−/− mice had larger areas of necrosis in the liver than WT mice at 24h post-α-Galcer injection (data not shown). In agreement with the data from IFN-γ−/− mice, the STAT1−/− mice also had a larger number of liver neutrophils than WT mice 3h after α-Galcer injection (Fig. 7C). Additionally, liver neutrophils from α-Galcer-treated STAT1−/− mice demonstrated reduced levels of apoptosis compared with those of WT mice (Fig. 7D). Finally, the depletion of neutrophils with an anti-Ly6G antibody markedly abolished α-Galcer-induced liver injury in STAT1−/− mice (Fig. 7E).
Fig. 7. STAT1−/− mice are more susceptible to α-Galcer-induced liver injury and neutrophil accumulation than WT mice.
(A) WT and IFN-γ−/− mice were injected with α-Galcer for 3h, after which time liver PMNs were isolated and subjected to FACS intracellular staining for pSTAT1 in neutrophils. (B-D) STAT1−/− and WT mice were treated with a single dose of α-Galcer and euthanized at different time points post-injection. (B) Serum ALT and AST levels 16 h post injection; (C) The total number of liver neutrophils was counted; and hepatic neutrophil apoptosis was examined by FACS analyses with a representative graph shown in (D). (E) STAT1−/− mice were treated with control or anti-Ly6G antibodies, followed by the injection of a single dose of α-Galcer. Serum ALT and AST levels were determined 16h post α-Galcer injection. (F) Liver neutrophils were isolated from WT, IFN-γ−/−, and STAT1−/− mice treated with or without α-Galcer and then subjected to real-time PCR analyses. *P<0.05, **P<0.01.
To understand the mechanisms underlying decreased neutrophil apoptosis in IFN-γ−/− mice post-α-Galcer injection, we investigated the expression of pro-apoptotic genes and identified that the expression of caspase 3, Bax, and Fas was upregulated in neutrophils from α-Galcer-treated WT mice, and this result was observed to a lesser extent in neutrophils from IFN-γ−/− or STAT1−/− mice (Fig. 7F).
Antagonism between IL-4 and IFN-γ in controlling α-Galcer-induced liver injury and neutrophil accumulation
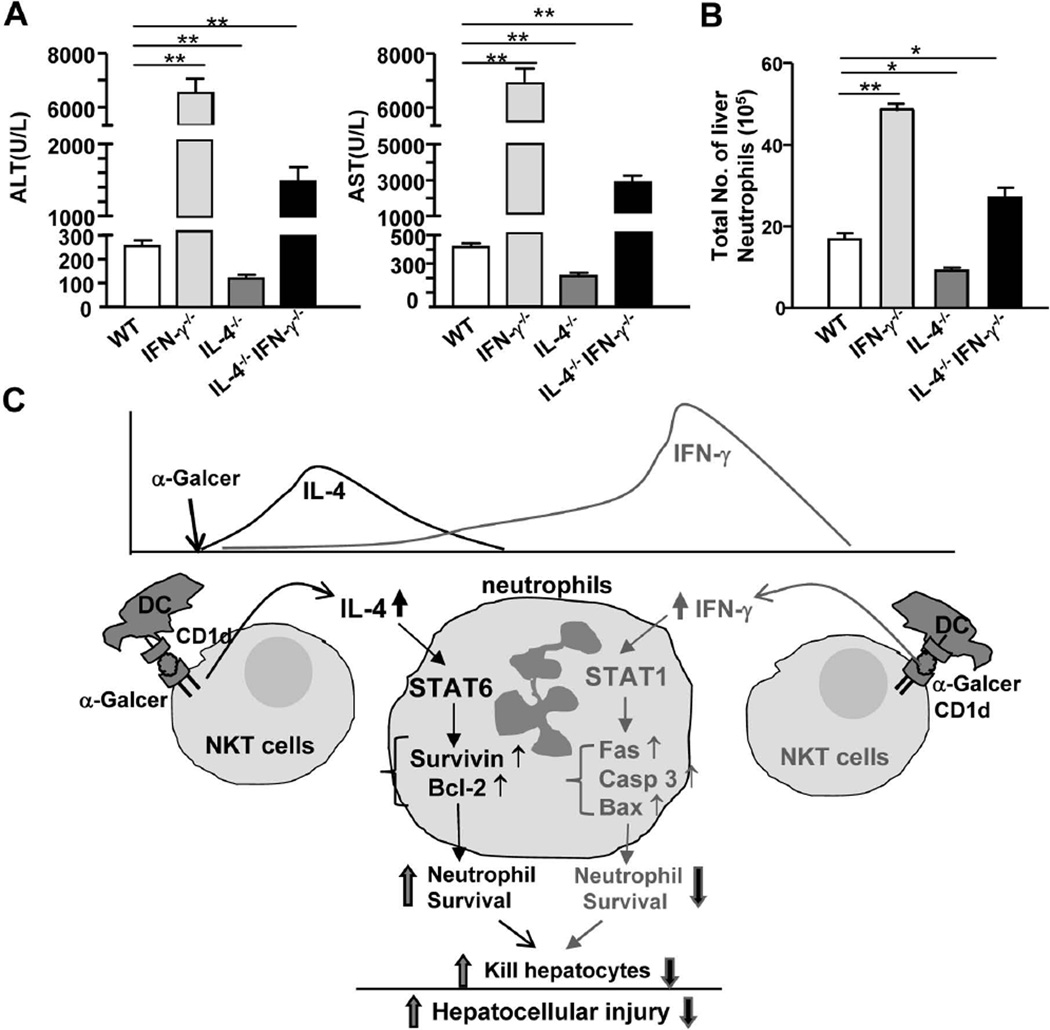
The above data suggest that IL-4 and IFN-γ play opposing roles in controlling α-Galcer-induced liver injury. Next we examined whether IL-4 and IFN-γ antagonize each other to control iNKT-mediated liver injury in vivo by comparing α-Galcer-induced hepatic neutrophil accumulation and injury among IL-4−/− IFN-γ−/−, IL-4−/−, IFN-γ−/−, and WT mice. As shown in Figs. 8A–B, IFN-γ−/− mice had the highest levels of serum ALT and AST and the greatest number of liver neutrophils, whereas IL-4−/− mice had the lowest levels of serum ALT and AST and the lowest number of liver neutrophils. The values from IL-4−/− IFN-γ−/− mice were in between those from IFN-γ−/− and IL-4−/− mice. These findings suggest that IL-4 and IFN-γ antagonize each other to control α-Galcer-induced liver neutrophil infiltration and injury in vivo.
Fig. 8. IL-4 and IFN-γ antagonize each other to control α-Galcer-induced liver neutrophil infiltration and injury in vivo.
(A, B) WT, IL-4−/−, IFN-γ−/−, and IL-4−/− IFN−/− dKO mice were treated with α-Galcer. (A) Serum ALT and AST levels were measured 16h post α-Galcer injection, and (B) liver neutrophils were counted 3h post α-Galcer injection. *P<0.05; **P<0.01. (C) A model depicting the antagonism of IL-4 and IFN-γ in controlling α-Galcer-induced liver neutrophil accumulation and injury. α-Galcer is presented by CD1d on dendritic cells (DC) to iNKT cells, which results in the activation of iNKT cells. Activated iNKT cells rapidly produce IL-4, which induces neutrophil recruitment and survival via a STAT6-dependent mechanism, thereby promoting liver injury. Activated iNKT cells subsequently produce IFN-γ, which activates STAT1 and upregulates pro-apoptotic genes to induce neutrophil apoptosis, thereby ameliorating liver injury.
Discussion
It has long been known that injection of α-Galcer activates iNKT cells, inducing a rapid elevation in the levels of IL-4 and a delayed elevation in the levels of IFN-γ.20 In the present study, we demonstrate 1) that the rapid production of IL-4 by iNKT cells induces liver neutrophil accumulation, which contributes to liver injury, and 2) that the delayed production of IFN-γ attenuates hepatic neutrophil accumulation by inducing neutrophil apoptosis, thereby preventing iNKT-mediated liver injury. We have integrated these findings into a model depicting the opposing roles of IFN-γ and IL-4 in controlling iNKT-mediated neutrophil accumulation and liver injury (Fig. 8C).
iNKT activation induces hepatic neutrophil accumulation, which contributes to liver injury
Although it is well documented that injection of the iNKT ligand α-Galcer induces mild hepatitis, the underlying mechanisms have not been fully understood.15 Previous studies have suggested that Kupffer cells do not contribute to α-Galcer-induced hepatitis.15 In the current paper, we observed a striking increase (30 fold) in neutrophils in the liver 3h after α-Galcer injection and found that depletion of neutrophils prevented α-Galcer-induced liver injury, which suggests that the accumulation of neutrophils contributes to liver injury. However, the mechanism through which neutrophils induce liver injury in this model was not investigated. It has also previously been shown that neutrophils induce hepatocellular damage in several models of liver injury via the oxidative killing of hepatocytes or the induction of liver lymphocyte recruitment.21–23 These mechanisms also likely mediate the neutrophil-mediated liver injury induced by α-Galcer because liver neutrophil-enriched PMNs from α-Galcer-treated mice were able to kill primary hepatocytes in vitro (Fig. 6D).
IL-4 exacerbates α-Galcer-induced liver injury by promoting hepatic neutrophil survival and infiltration
Activation of iNKT cells has been shown to induce neutrophil accumulation in the lung,24 ischemic kidneys via an IL-17-dependent mechanism,25 and in Listeria-infected livers via an IL-17-independent mechanism26 but inhibit neutrophil infiltration in cholestatic liver damage.27 Although α-Galcer injection rapidly stimulates iNKT cells to produce IL-17, the blockade of IL-17 increased, rather than reduced, α-Galcer-induced hepatic neutrophil recruitment, suggesting that IL-17 is not involved in iNKT-induced hepatic neutrophil accumulation and injury.16 Our current findings demonstrate that IL-4/STAT6 signaling plays a critical role in inducing liver neutrophil accumulation by inhibiting neutrophil apoptosis because genetic deletion of IL-4, the IL-4R, or its downstream signaling molecule STAT6 increased neutrophil apoptosis and suppressed neutrophil accumulation in α-Galcer-treated mice (Fig. 3). Although IL-4 has been shown to suppress neutrophil apoptosis in human neutrophils, the underlying mechanisms are not fully understood.28 Here, we demonstrated that the expression of survivin and Bcl-2 in neutrophils was upregulated in α-Galcer-treated WT mice but not in IL-4−/− or STAT6−/− mice (Fig. 4). Because survivin and Bcl-2 play an important role in promoting neutrophil survival and proliferation,28, 29 the induction of survivin and Bcl-2 by IL-4 and STAT6 likely promotes neutrophil survival and accumulation in the liver during α-Galcer-induced iNKT hepatitis.
Additionally, IL-4 has been shown to promote hepatic leukocyte recruitment by augmenting the expression of chemokines in Con A-induced hepatitis via a STAT6-dependent mechanism.30 This mechanism may also apply to IL-4/STAT6 promotion of neutrophil accumulation in α-Galcer-induced iNKT hepatitis because hepatic expression of several chemokines was lower in IL-4−/− or STAT6−/− mice than in WT mice after α-Galcer administration (Supporting Fig. 6). Additionally, hepatic expression of IFN-γ was also lower in IL-4−/− mice than that in WT mice after α-Galcer (Supporting Fig. 7), suggesting IL-4 enhances IFN-γ production. However, this unlikely contributes to IL-4 promotion of hepatic neutrophil accumulation because IFN-γ attenuates hepatic neutrophil infiltration (see below).
IFN-γ prevents neutrophil infiltration and protects against α-Galcer-induced liver injury by accelerating hepatic neutrophil apoptosis
The detrimental effects of IFN-γ/STAT1 signaling have been documented in several models of liver injury, including Con A-induced hepatitis31–33 and LPS/D-galactosamine-induced liver injury.34 However, a previous study found that inhibition of IFN-γ exacerbated α-Galcer-induced liver injury,15 but the underlying mechanisms of this protective effect remain enigmatic. In the present study, we found that genetic ablation of the IFN-γ, IFNGR, or STAT1 genes also exacerbated α-Galcer-induced hepatocellular damage. Our additional findings suggest that the beneficial effect of IFN-γ in α-Galcer-induced liver injury is mediated by the prevention of hepatic neutrophil accumulation. First, as shown in Fig. 6A, the total number of neutrophils in the liver was much higher in α-Galcer-treated IFN-γ−/− and STAT1−/− mice than in WT mice. Second, liver PMNs from α-Galcer-treated IFN-γ−/− mice had higher levels of cytotoxicity against primary mouse hepatocytes than those from WT mice (Fig. 6D). Finally, depletion of neutrophils ameliorated α-Galcer-induced severe liver injury by 90% in both IFN-γ−/− and STAT1−/− mice (Figs. 6 and 7), suggesting that the accumulation of neutrophils contributes to the exacerbated liver injury observed in α-Galcer-treated IFN-γ−/− and STAT1−/− mice.
IFN-γ−/− mice had lower levels of hepatic expression of IL-4 compared to WT mice after α-Galcer injection (Supporting Fig. 7), suggesting that IFN-γ is required for the production of IL-4. However, this unlikely attributes to IFN-γ prevention of hepatic neutrophil infiltration because IL-4 promotes hepatic neutrophil accumulation (see above). Our further findings indicate that IFN-γ attenuates hepatic neutrophil accumulation by inducing neutrophil apoptosis after α-Galcer injection, as neutrophil apoptosis was suppressed in IFN-γ−/− mice (Fig. 6). Mechanistic studies suggest that the pro-apoptotic effect of IFN-γ is mediated by the induction of several pro-apoptotic genes via a STAT1-dependent mechanism (Fig. 7F). Collectively, these findings suggest that IFN-γ stimulates the expression of pro-apoptotic genes in neutrophils via a STAT1-dependent mechanism, thereby playing an important role in preventing hepatic neutrophil accumulation in α-Galcer-induced liver injury.
In addition to their opposing roles in the control of hepatic neutrophil accumulation, IL-4 and IFN-γ have been shown to inversely control NKT cell proliferation in vitro.17 During the course of our studies, we observed the percentage and total number of liver iNKT cells in WT, IL-4−/−, IFN-γ−/−, and IL-4−/− IFN-γ−/− dKO mice were comparable before α-Galcer injection. After α-Galcer injection, liver iNKT cells were rapidly disappeared within 24h. This disappearance was similar among these four strains of mice (data not shown). These findings suggest that the differences in hepatic neutrophil accumulation 3h post α-Galcer injection among WT, IL-4−/−, IFN-γ−/−, and IL-4−/− IFN-γ−/− dKO mice were not caused by the changes in iNKT cells at the early time points after α-Galcer injection. Additionally, expression of activation markers (CD11b and CD62L) and production of reactive oxygen species (ROS) were comparable in neutrophils from α-Galcer-treated WT, IL-4−/−, and IFN-γ−/− mice (Supporting Fig. 8), suggesting IL-4 and IFN-γ regulate hepatic neutrophil accumulation but not activation.
Although IL-4 and IFN-γ mediate many crucial functions of iNKT cells in the liver,6–8 IL-4−/− IFN-γ−/− dKO mice still had significant liver injury after α-Galcer injection, suggesting that mechanisms other than IL-4 and IFN-γ are involved. It was previously reported that α-Galcer treatment induces TNF-α production by iNKT cells and that inhibition of TNF-α ameliorated α-Galcer-induced liver injury and diminished the aggravating effects of IFN-γ neutralization in this liver injury.15 These findings suggest that TNF-α likely contributes to the α-Galcer-induced liver injury in IL-4−/− IFN-γ−/− dKO mice.
Clinical implications
Because activation of iNKT cells by α-Galcer has been shown to inhibit hepatitis viral replication35 and liver cancer growth in animals,36–38 α-Galcer has been tested in clinical trials for the treatment of viral hepatitis and liver cancer.5, 12–14 In general, treatment with α-Galcer in patients was well tolerated but showed few beneficial effects.12–14 Our findings that α-Galcer-induced production of IL-4 and IFN-γ antagonize each other to control liver injury suggest that manipulation of these cytokines may improve the therapeutic potential of α-Galcer in the treatment of liver disease. For example, α-Galcer injection stimulates iNKT cell production of IFN-γ, which is not only absolutely required for the anti-tumor and anti-viral activities of α-Galcer in vivo35, 38 but also protects against α-Galcer-induced liver injury, as demonstrated in this and another study.15 In contrast, IL-4 produced by iNKT cells not only impairs iNKT antitumor activities39 but also exacerbates iNKT-mediated liver injury. Thus, the development of ligands that activate iNKT cells to preferentially produce IFN-γ may have higher anti-viral and anti-tumor activities but lower hepatotoxicity than α-Galcer. Indeed, there is an ongoing intensive effort to identify α-Galcer analogs that stimulate iNKT cells to preferentially secrete IFN-γ or IL-4,5 which may lead to the identification of better iNKT activators for the treatment of liver disease.
Supplementary Material
Acknowledgments
This work was supported by the intramural program of NIAAA, NIH (B Gao).
Abbreviations
- α-Galcer
alpha-Galactosylceramide
- ALT
aspartate aminotransferase
- AST
alanine aminotransferase
- IFN-γ
interferon-gamma
- IL
interleukin
- KO
knockout
- MNC
mononuclear cell
- MPO
myeloperoxidase
- NKT
natural killer T cells
- STAT
signal transducer and activator of transcription
- TCR
T cell receptor
Footnotes
No conflicts of interest exist for any authors.
References
- 1.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 2.Natori TKY, Higa T. Agelasphins, novel alpha-galctosylceramides from the marine sponge Agelas mauritanus. Tetrahedron Lett. 1993;34:5591–5592. [Google Scholar]
- 3.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Van Kaer L, Parekh VV, Wu L. Invariant NK T cells: potential for immunotherapeutic targeting with glycolipid antigens. Immunotherapy. 2011;3:59–75. doi: 10.2217/imt.10.85. [DOI] [PubMed] [Google Scholar]
- 5.Duwaerts CC, Gregory SH. Targeting the diverse immunological functions expressed by hepatic NKT cells. Expert Opin Ther Targets. 2011;15:973–988. doi: 10.1517/14728222.2011.584874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santodomingo-Garzon T, Swain MG. Role of NKT cells in autoimmune liver disease. Autoimmun Rev. 2011;10:793–800. doi: 10.1016/j.autrev.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Dennert G, Aswad F. The role of NKT cells in animal models of autoimmune hepatitis. Crit Rev Immunol. 2006;26:453–473. doi: 10.1615/critrevimmunol.v26.i5.50. [DOI] [PubMed] [Google Scholar]
- 9.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao BWH, Lafdil F, Feng D. STAT proteins - Key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol. 2012;57:430–441. doi: 10.1016/j.jhep.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneiders FL, Scheper RJ, von Blomberg BM, Woltman AM, Janssen HL, van den Eertwegh AJ, et al. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140:130–141. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Woltman AM, Ter Borg MJ, Binda RS, Sprengers D, von Blomberg BM, Scheper RJ, et al. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther. 2009;14:809–818. doi: 10.3851/IMP1295. [DOI] [PubMed] [Google Scholar]
- 14.Veldt BJ, van der Vliet HJ, von Blomberg BM, van Vlierberghe H, Gerken G, Nishi N, et al. Randomized placebo controlled phase I/II trial of alpha-galactosylceramide for the treatment of chronic hepatitis C. J Hepatol. 2007;47:356–365. doi: 10.1016/j.jhep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Biburger M, Tiegs G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 16.Wondimu Z, Santodomingo-Garzon T, Le T, Swain MG. Protective role of interleukin-17 in murine NKT cell-driven acute experimental hepatitis. Am J Pathol. 2010;177:2334–2346. doi: 10.2353/ajpath.2010.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iizuka A, Ikarashi Y, Yoshida M, Heike Y, Takeda K, Quinn G, et al. Interleukin (IL)-4 promotes T helper type 2-biased natural killer T (NKT) cell expansion, which is regulated by NKT cell-derived interferon-gamma and IL-4. Immunology. 2008;123:100–107. doi: 10.1111/j.1365-2567.2007.02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochi M, Ohdan H, Mitsuta H, Onoe T, Tokita D, Hara H, et al. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology. 2004;39:1321–1331. doi: 10.1002/hep.20204. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 21.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 22.Bonder CS, Ajuebor MN, Zbytnuik LD, Kubes P, Swain MG. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 24.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, et al. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emoto M, Emoto Y, Yoshizawa I, Kita E, Shimizu T, Hurwitz R, et al. Alpha-GalCer ameliorates listeriosis by accelerating infiltration of Gr-1+ cells into the liver. Eur J Immunol. 2010;40:1328–1341. doi: 10.1002/eji.200939594. [DOI] [PubMed] [Google Scholar]
- 27.Wintermeyer P, Cheng CW, Gehring S, Hoffman BL, Holub M, Brossay L, et al. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–1059. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girard D, Paquin R, Beaulieu AD. Responsiveness of human neutrophils to interleukin-4: induction of cytoskeletal rearrangements, de novo protein synthesis and delay of apoptosis. Biochem J. 1997;325(Pt 1):147–153. doi: 10.1042/bj3250147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croker BA, O'Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, et al. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci U S A. 2011;108:13135–13140. doi: 10.1073/pnas.1110358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial Role of IL-4/STAT6 in T Cell-Mediated Hepatitis: Up-Regulating Eotaxins and IL-5 and Recruiting Leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 31.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato J, Okamoto T, Motoyama H, Uchiyama R, Kirchhofer D, Van Rooijen N, et al. Interferon-gamma-mediated tissue factor expression contributes to T-cell-mediated hepatitis through induction of hypercoagulation in mice. Hepatology. 2013;57:362–372. doi: 10.1002/hep.26027. [DOI] [PubMed] [Google Scholar]
- 33.Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 34.Kim WH, Hong F, Radaeva S, Jaruga B, Fan S, Gao B. STAT1 plays an essential role in LPS/D-galactosamine-induced liver apoptosis and injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G761–G768. doi: 10.1152/ajpgi.00224.2003. [DOI] [PubMed] [Google Scholar]
- 35.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Sakamori R, Ohkawa K, et al. Intrahepatic delivery of alpha-galactosylceramide-pulsed dendritic cells suppresses liver tumor. Hepatology. 2007;45:22–30. doi: 10.1002/hep.21447. [DOI] [PubMed] [Google Scholar]
- 37.Tatsumi T, Takehara T, Miyagi T, Sugiyama T, Aketa H, Sasakawa A, et al. alpha-Galactosylceramide activates antitumor immunity against liver tumor. Hepatol Res. 2011;41:160–169. doi: 10.1111/j.1872-034X.2010.00743.x. [DOI] [PubMed] [Google Scholar]
- 38.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, et al. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 39.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.