Abstract
The effects of gamma radiation are investigated by studying plant germination, growth and development, and biochemical characteristics of maize. Maize dry seeds are exposed to a gamma source at doses ranging from 0.1 to 1 kGy. Our results show that the germination potential, expressed through the final germination percentage and the germination index, as well as the physiological parameters of maize seedlings (root and shoot lengths) decreased by increasing the irradiation dose. Moreover, plants derived from seeds exposed at higher doses (≤0.5 kGy) did not survive more than 10 days. Biochemical differences based on photosynthetic pigment (chlorophyll a, chlorophyll b, carotenoids) content revealed an inversely proportional relationship to doses of exposure. Furthermore, the concentration of chlorophyll a was higher than chlorophyll b in both irradiated and non-irradiated seedlings. Electron spin resonance spectroscopy used to evaluate the amount of free radicals induced by gamma ray treatment demonstrates that the relative concentration of radiation-induced free radicals depends linearly on the absorbed doses.
Keywords: Gamma ray, Maize hybrid, Germination potential, Growth parameters, Photosynthetic pigments , Electron spin resonance spectroscopy
Introduction
Research on the basic interaction of ionizing radiation with biological systems has contributed to human society through applications in medicine, agriculture, pharmaceutical uses, and other technological processes. Gamma rays have proved to be more economical and effective compared to other ionizing radiations because of their easy availability and power of penetration [1].
The exposure of a biological system to ionizing radiation activates a number of physical and chemical steps between the initial absorption of energy and the final biological injury. One of the most important targets is the water molecule, which is omnipresent in organisms. The ionized water molecule (H2O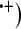 and the radicals H
and the radicals H and
and  OH are produced due to the primary reactions of excitation and ionization. In biological tissues, these ionizations are induced all along the radiation path, leading to chain reactions that produce secondary free radicals as a result of H
OH are produced due to the primary reactions of excitation and ionization. In biological tissues, these ionizations are induced all along the radiation path, leading to chain reactions that produce secondary free radicals as a result of H and e
and e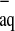 becoming trapped [2]. These radicals can damage or modify important components of plant cells and affect certain physiological and biochemical processes that might be vital for organism survival. Previous studies [3, 4] have revealed that seed exposure to high doses of gamma rays disturbs the protein synthesis, hormone balance, leaf gas exchange, and enzyme activity. The morphological, structural, and functional changes depend on the strength and duration of gamma doses of exposure.
becoming trapped [2]. These radicals can damage or modify important components of plant cells and affect certain physiological and biochemical processes that might be vital for organism survival. Previous studies [3, 4] have revealed that seed exposure to high doses of gamma rays disturbs the protein synthesis, hormone balance, leaf gas exchange, and enzyme activity. The morphological, structural, and functional changes depend on the strength and duration of gamma doses of exposure.
The measurements of free radicals in food and biological systems by electron spin resonance spectroscopy (ESR) are widely used in the fields of food irradiation, lipid oxidation, antioxidants, and food processing [5, 6]. Free radicals are chemical compounds that possess an unpaired electron in the outer shell of a molecule and due to this unpaired electron, these species are paramagnetic [7].
Zea mays is one of the world’s most important crop plants, boasting a multibillion dollar annual revenue. In addition to its agronomic importance, maize has been a keystone model organism for basic research for nearly a century. Within the cereals, which include other plant model species such as rice (Oryza sativa), sorghum (Sorghum bicolor), wheat (Triticum spp.) and barley (Hordeum vulgare), maize is the most thoroughly researched system. As a model organism, maize is the subject of far-ranging biological investigations such as plant domestication, genome evolution, developmental physiology, epigenetics, pest resistance, heterosis, quantitative inheritance, and comparative genomics [8].
Considering the effects of radiation on plants, the present study was conducted to determine the effects of radiation on maize growth and development and on the content of photosynthetic pigments. Furthermore, we used ESR spectroscopy to study the behavior of a radiation-induced free radical in gamma-irradiated maize seeds and to correlate it with the germination pattern.
Materials and methods
Plant material
Seeds of maize hybrid Turda Star were obtained from the Agricultural Research and Developmental Station, Turda, Romania, and were submitted to gamma treatment.
Gamma irradiation
Irradiation of maize seeds was performed using a 60Co (Cobalt 60) gamma source (Gamma Chamber 900) in ambient conditions at the Physics Faculty, “Babeş-Bolyai” University. The doses of exposure were 0.1, 0.2, 0.3, 0.5, 0.7, and 1.0 kGy. The control maize seeds were not irradiated.
Radiation sensitivity test
Three replicates of 20 seeds each/treatment were sowed on moistened filter paper and then germinated at 20 °C with an 8 h photophase, in laboratory conditions. Seeds were considered germinated when they exhibited a radical extension of >2 mm. Counts of germinated seeds were made daily for 7 days to determine both the final germination percentage and germination index. The final germination percentage (FGP) was calculated according to [9] as follows:
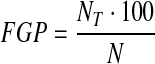 |
1 |
where NT = the proportion of germinated seeds in each treatment for the final measurement; N = number of seeds used in the bioassay.
The germination index is a quantitative expression of germination that relates the daily germination rate to the maximum germination value. The germination index (GI) was calculated as described by the Association of Official Seed Analysts [10] by the following formula:
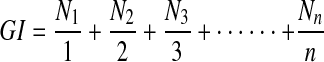 |
2 |
where N1, N2, N3 and Nn represent the number of seeds that germinated in day 1, 2, 3...,n
The root and shoot lengths of control and irradiated samples were measured on the seventh day after the start of the experiment. Shooting and rooting were defined as the protrusion of the shoot and root to the extent of at least 0.5 mm. The root/shoot length data represent the mean of the three replicated treatments.
Pigment extraction
Pigment extraction was carried out essentially as described by [11]. Twenty-eight-day fresh maize leaves were cut into small pieces (2–3 mm), which were suspended in DMF (N,N- dimethylformamide), approximately 100 mg vegetal material/2 ml DMF. The samples were incubated for 48 h in the dark at 4 °C. Absorbance measurements were read in a spectrophotometer (ultraviolet- visible [UV/VIS] spectrophotometer SP8001 Metertech Spectrophotometer provided by the Department of Experimental Biology and Biochemistry, Institute of Biological Research, Cluj-Napoca, Romania) at 664-, 647-, and 480-nm wavelengths. Using the absorbance mean values chlorophyll a, chlorophyll b, and carotenoid pigments were calculated based on equations proposed by [12] and expressed in μg/g fresh weight [FW]. The data represent the averages of the three replicated treatments.
ESR measurement
Gamma-irradiated maize seed samples were placed in ESR quartz tubes in order to register paramagnetic species. ESR spectra were recorded at room temperature with the Bruker Biospin EMX spectrometer operating at X-band (9–10 GHz). The ESR parameters were set at 100-KHz modulation frequency, microwave power of 2 mW, modulation amplitude of 2 G, time constant of 2.56 ms; scan time of 61 s, number of scans, five, and receiver gain 104. The variation of the steady state of the relative concentration of the paramagnetic species generated at different absorbed doses was obtained through double integration of the experimental spectra divided by the sample weight expressed in milligrams.
Statistical analysis
Experimental data were subjected to one-way analysis of variance (one-way ANOVA) with Dunnett’s post test, at a 5% level of probability, to determine the differences in average of all tested parameters between irradiated and non-irradiated plantlets. Statistical analysis was performed using GraphPad Prism (version 5.00 for Windows, GraphPad Software, San Diego, CA, USA).
Results and discussion
Radiation sensitivity test
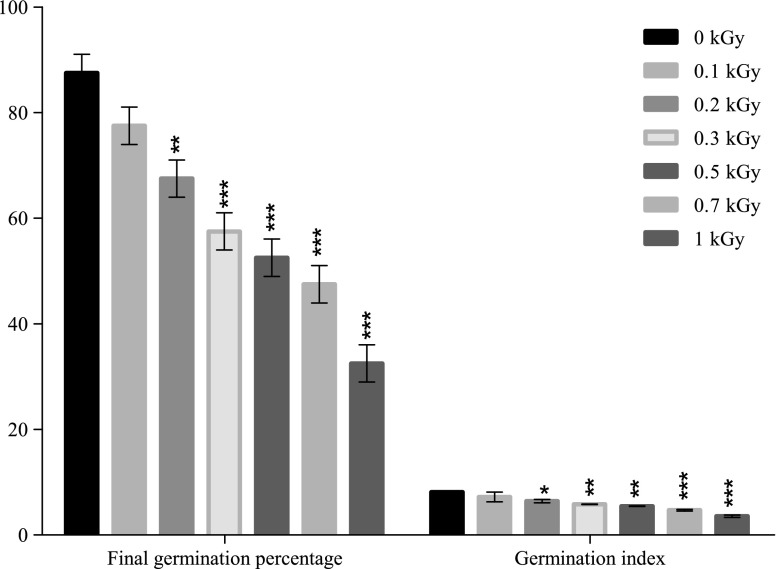
The seed germination test after gamma irradiation (0.1–1.0 kGy) revealed that the maximum germination percentage was observed in control seedlings. As illustrated in Fig. 1, the final germination percentage decreased with increasing gamma ray doses by 11% at 0.1 kGy, 22% at 0.2 kGy, 34% at 0.3 kGy, 40% at 0.5 kGy and 45% at 0.7 kGy. The maximum decrease, by 62%, of the germination percentage was observed at 1 kGy. Statistical analysis revealed that exposure at doses higher than 0.1 kGy significantly decreased the seeds’ germination capacity at 7 days from gamma radiation exposure.
Fig. 1.
Effect of gamma irradiation on final germination percentage and germination index of maize (Each bar represents Mean ±SD [Standard deviation] for average of n = 3 independent experiments; ∗ p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001)
Seed germination potential, expressed through the germination index (Fig. 1), revealed the same decreasing pattern by increasing the radiation doses, like FGP. Exposure to a dose of 0.1 kGy decreased insignificantly the germination index by 11% but a statistically significant inhibition of the germination potential was noticed at higher doses, where the germination index decreased by 21% for 0.2 kGy, 28% for 0.3 kGy, 32% for 0.5 kGy and 41% for 0.7 kGy, comparing with the control. The highest inhibition of the germination process, by 51%, was recorded at 1 kGy.
The biometric measurements of roots emerged from irradiated seeds show a significant decrease of length after 7 days from the start of the experiment (Table 1). The maximum radical length was recorded in the control samples, while the radical length of samples exposed to 0.1, 0.2, 0.3, 0.5 and 0.7 kGy decreased by 9, 31, 41, 47, and 56%, respectively. The maximum reduction of radical length, by 71%, was observed at 1 kGy. Results show that gamma treatment with doses higher than 0.1 kGy significantly inhibited the length of the radicular system of plants derived from irradiated seeds.
Table 1.
Effects of gamma radiation on root and shoot mean length (cm) of maize (Zea mays)
| Gamma irradiation dose (Gy) | Root length (cm) | Shoot length (cm) |
|---|---|---|
| 0 | 1.85 ± 0.23 | 0.81 ± 0.01 |
| 100 Gy | 1.67 ± 0.18 | 0.73 ± 0.01 |
| 200 Gy | 1.26 ± 0.07∗ | 0.49 ± 0.08∗∗ |
| 300 Gy | 1.08 ± 0.18∗∗ | 0.42 ± 0.14∗∗ |
| 500 Gy | 0.97 ± 0.14∗∗ | 0.38 ± 0.01∗∗ |
| 700 Gy | 0.81 ± 0.04∗∗∗ | 0.32 ± 0.001∗∗∗ |
| 1000 Gy | 0.52 ± 0.03∗∗∗ | 0.30 ± 0.03∗∗∗ |
Data are the means of three replicates ± SD [Standard Deviation]; ∗ Significant difference (p < 0.05); ∗∗ Highly significant difference (p < 0.01); ∗∗∗ Extremely significant difference (p < 0.001)
The gamma rays (≥0.2 kGy) imposed a significant impact on the shoot length. The highest shoot length was observed in the unirradiated plants. Following exposure to gamma rays, shoot lengths decreased by 11, 40, 48, 53, and 60% at 0.1, 0.2, 0.3, 0.5, and 0.7 kGy, respectively. The maximum decrease of shoot length, by 63%, was observed at 1 kGy (Table 1).
Plants derived from seeds exposed to higher doses (≥0.5 kGy) did not survive, so it was not possible for further investigations.
These results are in accordance with the findings of previous researchers who reported that the seed germination potential of different crops decreased by increasing the irradiation dose. Mashev et al. [13] showed that irradiation with doses above 0.1 kGy caused a significant decline in the grain yield of wheat. Gamma treatment (0.1, 0.2, 0.3, 0.4 kGy) of three wheat cultivars caused a delay of the germination process and decrease of the survival percentage and plant height [14].
Growth inhibition induced through high-dose irradiation has been attributed to cell cycle arrest in the G2/M phase during somatic cell division and/or to a variety of damages in the entire genome [15]. Processes like auxin destruction, changes of the ascorbic acid content, and physiological and biochemical disturbances could induce the inhibition of plant germination and development [16]. Chaudhuri [17] reported that when radiation is sufficient to reduce the rooting percentage, the root lengths do not exceed a few millimeters in length. Due to metabolic disorders in the seeds after gamma irradiation, the seeds are unable to germinate or to survive more than a few days. Plant survival to maturity depends on the nature and extent of chromosomal damage. The frequency of chromosomal damage with increasing dosage may be responsible for less germinability and reduction in plant survival and development [18].
Photosynthetic pigment content
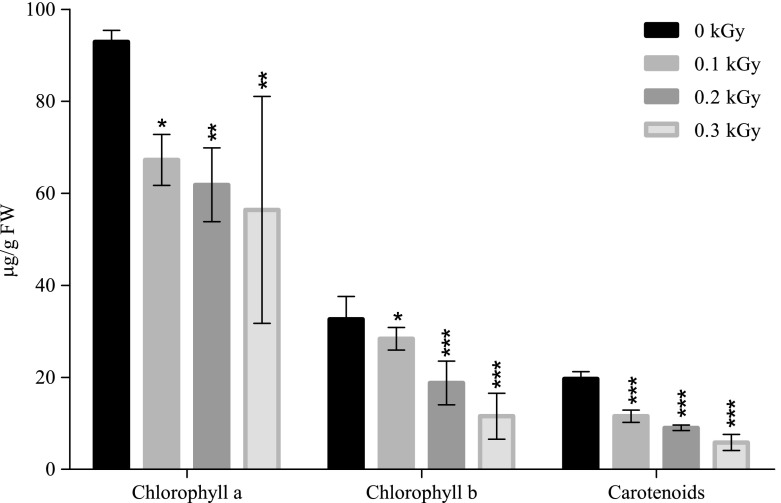
The contents of chlorophyll a, chlorophyll b, and carotenoids were determined only for samples exposed to 0.1, 0.2, and 0.3 kGy, because the plants derived from seeds exposed at higher doses (≥0.5 kGy) did not survive long enough to allow leaf growth and development.
The contents of assimilatory pigments, both for the control and irradiated samples, are presented in Fig. 2. Statistical analysis showed that the chlorophyll a content has significantly decreased in plant leaves derived from irradiated seeds. Comparing with the control, 92.92 μg/g FW, the chlorophyll a content decreased by 27% at 0.1 kGy (67.27 μg/g FW), 33% at 0.2 kGy (61.85 μg/g FW) and the maximum decrease, by 39%, was recorded at 0.3 kGy (56.38 μg/g FW).
Fig. 2.
Effect of gamma irradiation doses on chlorophyll a, chlorophyll b, carotenoids, total chlorophyll, and total assimilatory pigments content (μg/g FM) of maize (Each bar represents Mean ±SD for average n = 3 independent experiments; ∗ p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001)
Regarding chlorophyll b, a similar trend of decreasing content with increasing gamma dosage as in chlorophyll a (Fig. 2) is noticed. Chlorophyll b significantly decreased by 13% and 42% at 0.1 kGy (28.38 μg/g FW) and 0.2 kGy (18.79 μg/g FW), respectively. A remarkable decline of chlorophyll b was recorded with 0.3 kGy plantlets, which exhibited a content of 11.56 μg/g FW, which was 64% lower compared with non-irradiated samples, 32.63 μg/g FW.
Carotenoids were also negatively influenced by gamma treatment. Compared with the control (19.68 μg/g FW), gamma treatment with 0.1 kGy (11.56 μg/g FW) and 0.2 kGy (9.03 μg/g FW) induced a significant decrease of the carotenoids content by 41 and 54%, respectively. As seen in Fig. 2, the minimum content of carotenoids is noticed in samples exposed to 0.3 Gy, 5.83 μg/g FW, which represents a decrease by 70% comparing with the control.
Our data are in agreement with the results of previous works [19, 20] where chlorophyll concentrations were found to be lower in irradiated plants than in control ones.
Photosynthetic pigments can be destroyed by gamma rays with a concomitant loss of photosynthetic capacity. Saha et al. [21] reported that the chlorophyll content of plants decreased gradually after irradiation, which can result from the release of chlorophyll from its protein complex with subsequent dephytolization and possibly pheophytinization. In addition, it can be observed that the concentration of chlorophyll a was relatively higher than the concentration of chlorophyll b in both irradiated and unirradiated seedlings. A number of researchers [18, 22] have already reported that gamma irradiation results in greater destruction of chlorophyll b than chlorophyll a due to disturbance of its biosynthesis or degradation of its precursors.
ESR measurements
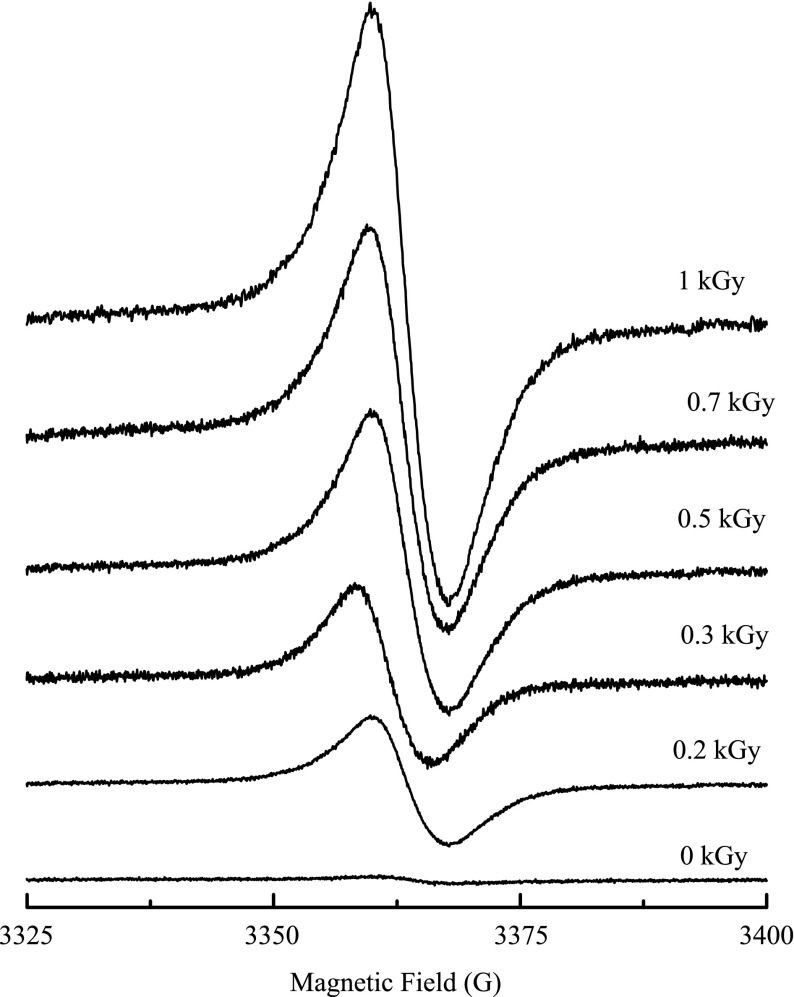
The ESR spectra of typical gamma-irradiated glass powder samples measured at room temperature are shown in Fig. 3 and are characterized by a structureless signal, centered on a g-factor of 2.0073 and a line width of 8.1 G.
Fig. 3.
ESR spectra of gamma-irradiated maize at some absorbed doses
The nature of the paramagnetic species in the non-irradiated samples has been attributed to oxidation of fatty acids visible in some vegetables, phenols, especially flavonoids or as free radicals of semi-quinones or lignin [23].
Gamma irradiation was reported to induce oxidative stress with overproduction of reactive oxygen species such as superoxide radicals, hydroxyl radicals, and hydrogen peroxides, which react rapidly with almost all structural and functional organic molecules including proteins, lipids, and nucleic acids causing disturbance of cellular metabolism [24].
Reactive oxygen species can react with nearly all cell constituents. Such interaction triggers free radical chain reactions, eventually causing membrane lipid peroxidation. As a result, the membrane loses its stability and its permeability is enhanced leading to damages of the cell structure and disturbances of normal physiological functions [25]. Massive doses of ionizing radiation have been shown to induce physiological changes in plants, such as enhancement of respiration, increase in ethylene production and induction of enzyme activities (particularly for phenolic metabolisms and accumulation specific protein species). These effects are considered a consequence of both the direct interactions between the ionizing radiation and the macromolecular structures and the indirect action of ROS generated by water radiolysis [26].
Polovka et al. [27] and Suhaj et al. [28] confirmed, using EPR spectroscopy, that the gamma radiation treatment of cellulose-containing spice samples such as ground black pepper (Piper nigrum L.), allspice berries (Pimenta officinalis L.), ginger root (Zingiber officinale Rosc.), dried clove buds (Caryophyllus aromaticus L.) and dried oregano leaves (Origanum vulgare L.) resulted in the dose-dependent generation of paramagnetic species of different structures and properties.
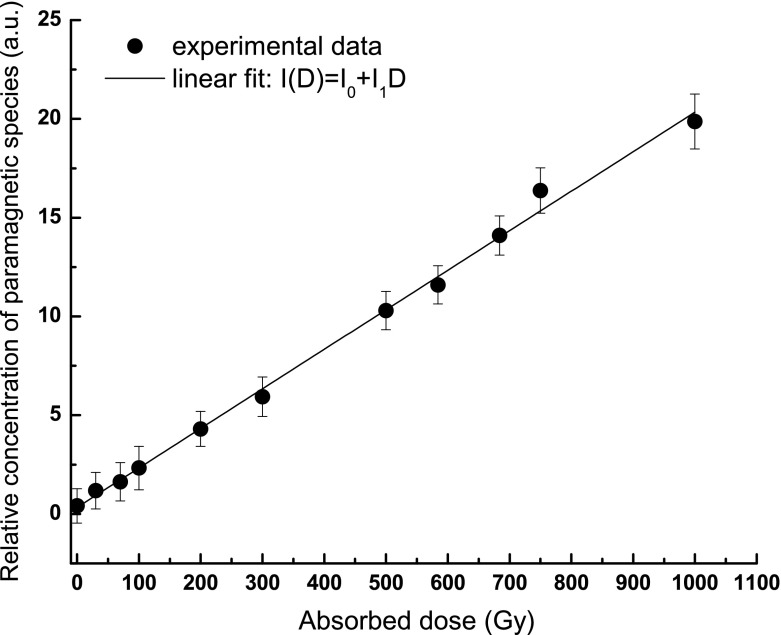
The double integrated ESR signal per 1 mg of samples was used to follow the variation of paramagnetic species as a function of absorbed dose (i.e., dose–response curve). A linear increase of the relative concentration of the paramagnetic species is observed (Fig. 4).
Fig. 4.
Variation of relative concentration of paramagnetic species as a function of the absorbed dose
Conclusions
In the present study, treatment of maize seeds with high doses of gamma rays (60Co) showed the same decreasing pattern of all studied parameters (final germination percentage, germination index, root and shoot lengths, photosynthetic pigment content) with an increase of irradiation dose. Gamma radiation exposure at doses ranging from 0.1 to 1 kGy lead to a decrease by 11–62% of the final germination percentage, by 11–51% of the germination index, by 9–71% of the root length, and by 9–62% of the shoot length. Plants derived from seeds exposed to higher doses (0.5, 0.7, and 1 kGy) did not survive to allow further investigations. The photosynthetic pigment content was also decreased by 26–49% at 0.1, 0.2, and 0.3 kGy compared with the control.
Unirradiated and irradiated ground maize was studied using ESR spectroscopy. Gamma radiation induced detectable amounts of free radicals in the studied samples. The relative concentration of the radiation-induced free radicals was found to depend linearly on the absorbed doses. Results of the present study substantiate the suitability of employing the ESR technique for detection of free radicals present naturally or produced after radiation and/or conventional processing.
The present results show that seed treatment with 60Co gamma radiation (0.1–1 kGy) decreased plant germination, development, and the photosynthetic pigment content with a concomitant increase of the relative concentration of the paramagnetic species. These findings confirm the results obtained by earlier studies that showed the inhibitory effects of plant growth and development exposed to high doses of gamma radiation, simultaneous with the increase of reactive oxygen species generated through water radiolysis.
Acknowledgement
The authors wish to express their gratitude to the Department of Experimental Biology and Biochemistry, Institute of Biological Research, Cluj-Napoca, Romania, for providing some infrastructure facilities to carry out the experiment and providing access to laboratory equipment.
References
- 1.Moussa JP. Role of gamma irradiation in regulation of NO3 level in rocket (Eruca vescaria subsp. sativa) plants. Russ. J. Plant Physiol. 2006;53:193–197. doi: 10.1134/S1021443706020075. [DOI] [Google Scholar]
- 2.Esnault AM, Legue F, Chenal C. Ionizing radiation: advances in plant response. Environ. Exp. Bot. 2010;68:231–237. doi: 10.1016/j.envexpbot.2010.01.007. [DOI] [Google Scholar]
- 3.Al-Salhi M, Ghannam MM, Al-Ayed MS, El-Kameesy SU, Roshdy S. Effect of gamma irradiation on the biophysical and morphological properties of corn. Nahrung. 2004;48:95–98. doi: 10.1002/food.200300331. [DOI] [PubMed] [Google Scholar]
- 4.Hameed A, Mahmud TS, Atta BM, Haq MA, Sayed H. Gamma irradiation effects on seed germination and growth, protein content, peroxidase and protease activity, lipid peroxidation in desi and kabuli chickpea. Pak. J. Bot. 2008;40:1033–1041. [Google Scholar]
- 5.Dadayli D, Sunnetcioglu MM, Koksel H, Celik S. Detection of irradiated wheat using the electron paramagnetic resonance. Cereal. Chem. 1997;74:375–378. doi: 10.1094/CCHEM.1997.74.4.375. [DOI] [Google Scholar]
- 6.Sünnetçioğlu MM, Dadayli D, Çelik S, Köksel H. Application of the electron paramagnetic resonance spin probe technique for detection of irradiated wheat. Cereal Chem. 1998;75:875–878. doi: 10.1094/CCHEM.1998.75.6.875. [DOI] [Google Scholar]
- 7.Damian G, Miclăuş V. Study of free radicals in gamma-irradiated metoclopramide using spin trapping ESR spectroscopy. Rom. J. Biophys. 2005;15:121–126. [Google Scholar]
- 8.Strable, J., Scanlon, J.M.: Maize (Zea mays): a model organism for basic and applied research in plant biology. In: Emerging Model Organisms: A Laboratory Manual, vol. 2, pp. 33–41. Cold Spring Harbor Laboratory Press (1999) [DOI] [PubMed]
- 9.Anjum T, Bajwa R. Importance of germination indices in interpretation of allelochemical effects. Int. J. Agric. Biol. 2005;7:417–419. [Google Scholar]
- 10.Dezfuli P, Sharif-Zadeh F, Janmohammadi M. Influence of priming techniques on seed germination behaviour of maize inbred lines (Zea mays L.) J. Agric. Biol. Sci. 2008;3:22–25. [Google Scholar]
- 11.Moran R, Porath D. Chlorophyll determination in intact tissues using N,N-Dimethylformamide. Plant Physiol. 1980;65:478–479. doi: 10.1104/pp.65.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wellburn A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- 13.Mashev N, Vassilev G, Ivanov K. A study of N-allyl N-2 pyridyl thiourea and gamma radiation treatment on growth and quality of peas and wheat. Bulg. J. Plant. Physiol. 1995;21:56–63. [Google Scholar]
- 14.Irfaq M, Nawab K. Effect of gamma irradiation on some morphological characteristics on three wheat (Triticum aestivum L.) cultivars. J. Biol. Sci. 2001;1:935–937. doi: 10.3923/jbs.2001.935.937. [DOI] [Google Scholar]
- 15.Preuss SB, Britt AB. A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics. 2003;164:323–334. doi: 10.1093/genetics/164.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah TM, Mirza JI, Haq MA, Atta BM. Radio sensitivity of various chickpea genotypes in M1 generation I-Laboratory studies. Pak. J. Bot. 2008;40:649–665. [Google Scholar]
- 17.Chaudhuri SK. A simple and reliable method to detect gamma-irradiated lentil (Lens culinaris Medik.) seeds by germination efficiency and seedling growth test. Radiat. Phys. Chem. 2002;64:131–136. doi: 10.1016/S0969-806X(01)00467-4. [DOI] [Google Scholar]
- 18.Kiong ALP, Lai AG, Hussein S, Harun AR. Physiological responses of Orthosiphon stamineus plantlets to gamma irradiation. Am-Eurasian. J. Sustain. Agric. 2008;2:135–149. [Google Scholar]
- 19.Dale MF, Griffiths DW, Bain H, Goodman BA. The effect of gamma irradiation on glycoalkaloid and chlorophyll synthesis in seven potato cultivars. J. Sci. Food Agr. 1997;75:141–147. doi: 10.1002/(SICI)1097-0010(199710)75:2<141::AID-JSFA848>3.0.CO;2-L. [DOI] [Google Scholar]
- 20.Ling APK, Chia JY, Hussein S, Harun AR. Physiological responses of Citrus sinesis to gamma irradiation. World Appl. Sci. J. 2008;5:12–19. [Google Scholar]
- 21.Saha P, Raychaudhuri SS, Chakraborty A, Sudarshan M, PIXE analysis of trace elements in relation to chlorophyll concentration in Plantago ovata Forsk. Appl. Radiat. Isot. 2010;68:444–449. doi: 10.1016/j.apradiso.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Strid A, Chow WS, Anderson JM. Effects of supplementary gamma irradiation on photosynthesis in Pisum sativum. Biochem. 1990;1020:260–268. [Google Scholar]
- 23.Yarbaşi Z, Karabulut B, Karabulut A. An EPR study of gamma-irradiated medicinal plants: cress seeds and mistletoe. Gazi Univ. J. Sci. 2011;24:203–207. [Google Scholar]
- 24.Al-Rumaih MM, Al-Rumaih MM. Influence of ionizing radiation on antioxidant enzymes in three species of Trigonella. Am. J. Environ. Sci. 2008;4:151–156. doi: 10.3844/ajessp.2008.151.156. [DOI] [Google Scholar]
- 25.Moghaddam SS, Jaafar H, Ibrahim R, Rahmat A, Aziz MA, Philip E. Effects of acute gamma irradiation on physiological traits and flavonoid accumulation of Centella asiatica. Molecules. 2011;16:4994–5007. doi: 10.3390/molecules16064994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata T, Todoriki S, Hayashi T, Shibata Y, Mori M, Kanegae H, Kikuchi S. Gamma-radiation induces leaf trichome formation in Arabidopsis. Plant Physiol. 1999;120:113–119. doi: 10.1104/pp.120.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polovka M, Brezova V, Staško A, Mazur M, Suhaj M, Šimko P. EPR investigations of gamma-irradiated ground black pepper. Radiat. Phys. Chem. 2006;75:309–321. doi: 10.1016/j.radphyschem.2005.07.007. [DOI] [Google Scholar]
- 28.Suhaj M, Rácová J, Polovka M, Brezová V. Effect of gamma-irradiation on antioxidant activity of black pepper (Piper nigrum L.) Food Chem. 2006;97:696–704. doi: 10.1016/j.foodchem.2005.05.048. [DOI] [Google Scholar]