Abstract
A prey–predator discrete-time model with a Holling type I functional response is investigated by incorporating a prey refuge. It is shown that a refuge does not always stabilize prey–predator interactions. A prey refuge in some cases produces even more chaotic, random-like dynamics than without a refuge and prey population outbreaks appear. Stability analysis was performed in order to investigate the local stability of fixed points as well as the several local bifurcations they undergo. Numerical simulations such as parametric basins of attraction, bifurcation diagrams, phase plots and largest Lyapunov exponent diagrams are executed in order to illustrate the complex dynamical behavior of the system.
Keywords: Prey–predator model, Holling type I, Prey refuge, Chaotic dynamics, Population outbreaks
Introduction
The simplest population model in continuous time was first introduced by Verhulst [1] and describes the growth rate of the population of a single species. This model is known as the logistic differential equation. Lotka [2] and Volterra [3] were the first who described predator-prey interaction by introducing the known Lotka–Volterra predator–prey continuous-time model. A drawback of the Lotka–Volterra model, which makes this model unrealistic, is that the predator never becomes saturated. This problem was solved by Holling [4, 5], who suggested three kinds of functional responses for different species of predator, which are called Holling type I, II, and III. The function indicates the number of prey killed by one predator at various prey densities. Thus, Rosenzweig and MacArthur [6] later studied the Lotka–Volterra model with a logistic growth rate of prey, while the saturation of the predator was taken into account by a Holling type II functional response. The Rosenzweig–MacArthur model is one of the basic models since prey–predator coexistence is not limited to a stable equilibrium; a limit cycle appears when the stable equilibrium undergoes the Hopf bifurcation. For many years, the studies of prey–predator interactions focused mainly on continuous-time models, where the dynamics could produce only a stable equilibrium or limit cycles. However, in recent years the research has turned to discrete-time prey–predator models, as it seems their dynamics may include a much richer set of patterns. Maynard Smith [7] first studied the Lotka–Volterra prey–predator model with a logistic growth rate of prey in discrete time. Hadeler and Gerstmann [8] studied the discrete-time version of the Rosenzweig–MacArthur model. Later, Danca et al. [9] studied a simple discrete-time prey–predator model with Holling type I taking place and showed that such a simple discrete model can exhibit chaotic dynamics. Liu and Xiao [10] studied the Rosenzweig–MacArthur prey–predator model with Holling type I, proving once again that the discrete system exhibits far richer dynamics compared to the continuous model. However, since many prey populations incorporate some form of refuge available, a prey refuge provides a more realistic prey–predator model. Maynard Smith [11] showed that a constant proportion of refuge did not alter the dynamics of the neutrally stable Lotka–Volterra model, while a constant number of refuge of any size replaced the neutrally stable behavior with a stable equilibrium. Also, Hassel [12] showed that a large refuge in a model, which in the absence of a refuge exhibits divergent oscillations, replaces the oscillatory behavior with a stable equilibrium. Thus, we observe that many studies have shown that refugia have a stabilizing effect on predator–prey interactions. However, as Taylor [13] mentioned, it would be an oversimplification to assume this is always the case.
In the present paper, we extend Danca et al.’s [9] Lotka–Volterra model with a Holling type I functional response incorporating prey refuge. The Holling type I functional response mainly refers to passive predators like spiders, which wait for their prey to come close in order to capture them [4]. The spiders are the main predators of insects. A single spider eats at least 100 insects in a year and therefore it is estimated that in temperate countries, the annual total weight of insects the spiders eat is larger than the weight of the human population [14]. However, a huge number of many infectious diseases are usually transmitted into human blood by insects (vectors), which feed on blood such as biting flies, mosquitoes, ticks, and other pests, which spiders also eat [15]. For instance, the infectious disease tularemia, which can be transmitted by deer flies and ticks [16], sleeping sickness, which can be transmitted by the tsetse fly [17], the viral disease yellow fever, which is a mosquito-borne disease [18], or the parasitic disease malaria, which can be transmitted by mosquitoes of the genus Anopheles, which has also been associated with causing brain tumors [19]. So from this perspective, spiders in the role of predators, which prey on vectors, can be really useful to humans.
However, by incorporating a prey refuge into Danca et al.’s [9] model, we assume that pests are able to protect themselves from spiders and escape from predation in some way, but in the real world pest insects are one of those insects that may show population outbreaks [14]. So, if a prey refuge leads to population outbreaks, then serious infectious diseases, which pests may carry, could spread through human populations across a large region and a pandemic could be possible. Does a prey refuge stabilize the prey–predator interactions as many studies have shown or leads eventually to population outbreaks? This is the main question we try to answer in this study.
The model system
Danca et al. [9] studied the following discrete prey–predator model with a Holling type I1 functional response taking place:
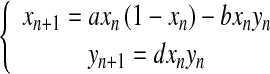 |
1 |
They showed that as the growth rate of the prey increases, chaotic dynamics appear in the system and the prey–predator interactions become irregular. Particularly, they showed that for the parameter values b = 0.2, d = 3.5, as parameter a varies in the interval 0 < a < 4, the dynamical system (1) exhibits a strange attractor (Fig. 1).
Fig. 1.
The strange attractor without a prey refuge (m = 0) for a = 3.99654
Extending Danca et al.’s [9] Lotka–Volterra model by incorporating a prey refuge, we take the following prey–predator system:
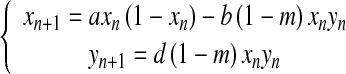 |
2 |
where,
- xn:
the prey population after n generations with 0 < xn < 1; yn: the predator population after n generations. Thus we extend the basic model (1) by adding the new terms (+bmxnyn) and (−dmxnyn) associated with a refuge protecting the prey from the predator.
- a > 0:
the growth rate of the prey in the absence of predators, in the presence of sufficient food and all other requirements.
- b > 0:
the foraging efficiency of the predator; it measures the intensity of the predator’s negative impact on the prey population’s growth.
- d > 0:
the growth rate of the predator.
- mx:
a refuge protecting the prey from the predator; (1 − m)x: the prey available to the predator, where m ∈ [0, 1).
Analytical stability analysis
The dynamical system (2) has the following three fixed points: the origin (E1), a boundary fixed point (E2) and a fixed point for which both populations survive (E3):
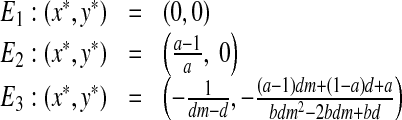 |
The Jacobian matrix at any point (x, y) is:
 |
The determinant of the Jacobian matrix is:
 |
If 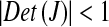 the system is dissipative, while if
the system is dissipative, while if  the system is conservative.
the system is conservative.
In order to study2 the local behavior around each of the three fixed points, we calculate the Jacobian matrix at E1, E2, E3. If λ1, λ2 are the eigenvalues of the Jacobian matrix at each fixed point, then the fixed point is stable, if ∣ λ1 ∣ < 1 and ∣ λ2 ∣ < 1. By using Vieta’s equations 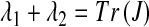 ,
,  and applying Jury’s conditions [20] the fixed point is linearly asymptotically stable if and only if:
and applying Jury’s conditions [20] the fixed point is linearly asymptotically stable if and only if:
 |
3 |
Local stability of the fixed point E1
The Jacobian matrix at E1:(x ∗ , y ∗ ) = (0, 0) is:
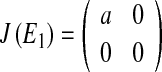 |
with eigenvalues λ1 = a, λ2 = 0, determinant DetJ(E1) = 0 and trace TrJ(E1) = a. For a < 1 the origin is a stable node (λ1, λ2 < 1), while for a > 1 the origin is a saddle (λ1 > 1, λ2 < 1). For a = 1 the fixed point is non-hyperbolic (λ1 = 1, λ2 < 1); this parameter value is associated with the stability condition TrJ = 1 + DetJ (3) and a real eigenvalue crossing the unit circle at +1. So a = 1 is a bifurcation point at which a fold bifurcation occurs.
Local stability of the fixed point E2
The Jacobian matrix at  is:
is:
 |
The eigenvalues of the Jacobian matrix at E2 are given by  and λ2 = 2 − a.
and λ2 = 2 − a.
The determinant and the trace are  .
.
Using stability conditions (3), we obtain the following:
If
 and a < 1 then E2: unstable node (∣λ1 ∣, ∣λ2 ∣ >1)
and a < 1 then E2: unstable node (∣λ1 ∣, ∣λ2 ∣ >1)If
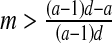 and
and  then E2: saddle
then E2: saddle 
If
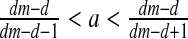 then E2: stable node (∣λ1 ∣, ∣λ2 ∣ <1)
then E2: stable node (∣λ1 ∣, ∣λ2 ∣ <1)If
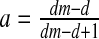 then E2: non-hyperbolic (λ1 = 1); this parameter value is associated with the stability condition TrJ = 1 + DetJ (3) and a real eigenvalue crossing the unit circle at +1. So this is another bifurcation point at which a fold bifurcation occurs in the system.
then E2: non-hyperbolic (λ1 = 1); this parameter value is associated with the stability condition TrJ = 1 + DetJ (3) and a real eigenvalue crossing the unit circle at +1. So this is another bifurcation point at which a fold bifurcation occurs in the system.
Local stability of the fixed point E3
The Jacobian matrix at 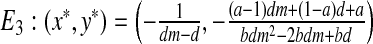 is:
is:
 |
with eigenvalues
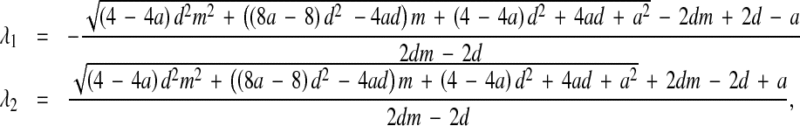 |
determinant 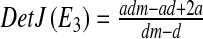 and trace
and trace  .
.
Using stability conditions (3) we obtain the following:
If
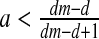 and
and 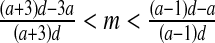 then E3: saddle (∣λ1 ∣ <1, ∣λ2 ∣ >1)
then E3: saddle (∣λ1 ∣ <1, ∣λ2 ∣ >1)If
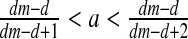 and
and  then E3: stable focus (complex eigenvalues with DetJ(E3) < 1) or stable node (∣λ1 ∣, ∣λ2 ∣ <1).
then E3: stable focus (complex eigenvalues with DetJ(E3) < 1) or stable node (∣λ1 ∣, ∣λ2 ∣ <1).If
 and
and 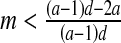 then E3: unstable focus (complex eigenvalues with DetJ(E3) >1).
then E3: unstable focus (complex eigenvalues with DetJ(E3) >1).If
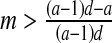 then E3: unstable node (∣λ1 ∣, ∣λ2 ∣ >1).
then E3: unstable node (∣λ1 ∣, ∣λ2 ∣ >1).If
 then E3: non-hyperbolic (λ2 = 1); this parameter value is associated with the stability condition TrJ = 1 + DetJ (3) and a real eigenvalue crossing the unit circle at +1. So this is a bifurcation point at which a fold bifurcation occurs in the system.
then E3: non-hyperbolic (λ2 = 1); this parameter value is associated with the stability condition TrJ = 1 + DetJ (3) and a real eigenvalue crossing the unit circle at +1. So this is a bifurcation point at which a fold bifurcation occurs in the system.If
 and
and 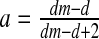 then E3: center (complex eigenvalues with DetJ(E3) = 1 and −2 < TrJ(E3) < +2); this parameter value is associated with the stability condition DetJ = 1 (3) and two complex eigenvalues crossing the unit circle simultaneously. So this is a bifurcation point at which a Neimark–Sacker bifurcation occurs in the system.
then E3: center (complex eigenvalues with DetJ(E3) = 1 and −2 < TrJ(E3) < +2); this parameter value is associated with the stability condition DetJ = 1 (3) and two complex eigenvalues crossing the unit circle simultaneously. So this is a bifurcation point at which a Neimark–Sacker bifurcation occurs in the system.If
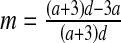 then E3: non-hyperbolic (λ1 = −1); this parameter value is associated with the first stability condition −TrJ = 1 + DetJ (3) and one real eigenvalue crossing the unit circle at −1. Hence, this parameter value corresponds to a bifurcation point at which a flip bifurcation occurs in the system.
then E3: non-hyperbolic (λ1 = −1); this parameter value is associated with the first stability condition −TrJ = 1 + DetJ (3) and one real eigenvalue crossing the unit circle at −1. Hence, this parameter value corresponds to a bifurcation point at which a flip bifurcation occurs in the system.
Numerical simulations
We use various numerical simulation3 tools (parametric basins of attraction, bifurcation diagrams, phase plots and largest Lyapunov exponent diagrams) to study the complex dynamics of the dynamical system (2). In order to see the effect of a refuge on prey–predator interactions, we choose the initial conditions x0 = 0.83 and y0 = 0.55. We also assume b < d since observational studies of spiders have shown that while many species have very low capture rates of prey (parameter b) [22, 23], they produce many offspring (parameter d) (i.e., female spiders lay up to 3,000 eggs in one or more silk egg sacks [24]). This paradox of “low foraging efficiency–high offspring production” is likely related to the fact that spiders have very low metabolic rates compared to other animals. On average, the resting metabolic rate of spiders is 70% of that of comparable ectothermic arthropods [25].4 This is ecologically an important factor that allows spiders to survive extended periods of time (which are sometimes in excess of 200 or 300 days) without food [27].5 Furthermore, even when spiders elevate their metabolic rate during activities (such as searching for food,6 producing webs,7 producing eggs,8 courtship9), the increase in respiration and metabolic rates is relatively low compared to other animals; two to six times resting rates [32]. Hence, the general low metabolic rates make them very efficient in their use of energy, so that despite their low foraging efficiency, they have high reproduction rates. Thus, the parameters b = 0.2 and d = 3.5 [9] are fixed10 and we vary the parameters of the growth rate of prey a and refuge m.
Parametric basins of attractions
This plot is a numerical analysis tool that matches different colors to periodic cycles of different periods in a two-dimensional parameter space [21]. So, we plot the parametric basins of attraction for the parameter values a ∈ [0, 4.35] and m ∈ [0, 1] to see how the dynamics of the system change as the refuge increases (Fig. 2).
Fig. 2.
The parametric basins of attraction (a, m)
The different colors in the parametric plane (a, m) correspond to the following stable states: light blue for an attracting fixed point, dark blue for a stable two-period cycle, yellow for a three-period cycle, pink for a four-period cycle, orange for a five-period cycle, red for a six-period cycle, light green for a seven-period cycle, dark green for an eight-period cycle, grey for a nine-period cycle and purple for a ten-period cycle. The white area corresponds to those values of parameters for which the behavior of solutions may be quasi-periodic (invariant curves) or non-periodic (chaos; strange attractors). The black area is the set of parameters for which every orbit diverges to infinity.
Observing the 2D parameter space we point out the following:
For small values of prey refuge, 0 < m < 0.254, as the growth rate of prey a increases, the stable fixed point (light blue area) is giving rise to non-periodic behavior (white area). So, adding a small refuge does not seem to alter significantly the dynamics of the basic model (1).
For slightly higher values of refuge, 0.254 < m < 0.467, we see that as a increases, the aperiodic dynamics is being replaced by a stable equilibrium. So, by adding more prey refuge, the refuge stabilizes the prey–predator interactions.
However, for even higher values of prey refuge, 0.467 < m < 1, the stabilizing effect of refuge is not the case anymore. Now, as a takes higher values, the stable fixed point, through successive period-doubling bifurcations; two-period cycle (dark blue area), four-period cycle (pink area) and eight-period cycle (dark green area), is giving rise to non-periodic behavior (white area). So, adding a large refuge makes the system unstable once again.
Higher values of the refuge parameter, m > 1, do not correspond to a positive equilibrium and therefore it has no realistic ecological significance.
Bifurcation diagrams
In order to see in detail what happens in both populations’ species, as the refuge parameter increases, we use bifurcation diagrams. The bifurcation diagram is another useful numerical analysis tool that illustrates how the characteristics of a fixed point change as the varying parameter increases in a specific interval [33]. We plot the bifurcation diagram as the system’s parameter increases in the interval m ∈ [0, 1] for various values of parameter a and we distinguish the following cases (Fig. 3):
For a low-growth rate of prey, 0 < a < 1.45, as refuge increases in m ∈ [0, 1], the prey population survives in a small quantity, because of the low reproduction rate, while the predators cannot survive because of refuge. As we see in Fig. 3a, with such a low birth rate of prey, a = 1.1, all preys are able to find refuge and the food is enough to feed them all, while predators cannot find food at all and become extinct immediately. So in this case the system has a stable boundary equilibrium point,
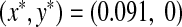 , where both populations are fixed in time.
, where both populations are fixed in time.For an average birth rate of prey, 1.45 < a < 2.3 and a small prey refuge, m < 0.45, both populations survive (x ∗ > 0, y ∗ > 0) temporarily (Fig. 3b). This happens because for such a growth rate, a = 2, the prey density is larger than the amount of refuge. Therefore, the predators are able to find preys to eat and since their population increases faster than the prey population, d = 3.5 > a = 2, the predator population survives in a higher quantity than the prey population (y ∗ > x ∗ ) temporarily. As the prey refuge increases, more and more preys find refuge and therefore the prey population increases, while the predator population decreases. When the amount of refuge is m > 0.45, all preys are able to find refuge and since the food is enough to feed them all, they survive in larger density (x ∗ = 0.514) due to their higher birth rate. On the other hand, predators cannot find food anymore and become extinct (y ∗ = 0). Moreover, this qualitative change in the behavior of solutions, as the refuge parameter increases, is associated with a local bifurcation. For a = 2 (Fig. 3b) in particular and m < 0.4286, the fixed point E3:(x ∗ > 0, y ∗ > 0) is a stable node and the boundary fixed point E2:(x ∗ , y ∗ ) = (0.5, 0) is a saddle (the origin E1:(x ∗ , y ∗ ) = (0, 0) is always a saddle since a = 2 > 1). For m = 0.4286, both fixed points become non-hyperbolic; the eigenvalues of the Jacobian matrix at E3 are (λ1 = −0.0001, λ2 = 1) and the eigenvalues at E2 are (λ1 = 1, λ2 = 0). For m > 0.4286, E3 becomes a saddle and E2 becomes a stable node. Hence, as the varying parameter passes through the critical value m = 0.4286, both fixed points undergo a fold bifurcation.
For a high birth rate of prey, 2.3 < a < 3 and exceptionally small values of refuge, m < 0.11, we observe aperiodic dynamics in both populations’ species (Fig. 3c). This happens because the high reproduction rate of prey, a = 2.8, combined with the exceptionally small amount of refuge results in a large number of preys not being able to find refuge and to protect themselves from predators. The high birth rate of predators results in both populations appearing as irregular oscillations. However, for m > 0.11, refuge stabilizes the system to a positive fixed point (x ∗ , y ∗ > 0), while the predator population is temporarily higher than the prey population (y ∗ > x ∗ ) again. For even higher values of prey refuge, m > 0.56, all preys are able to find refuge and food and because of their higher birth rate, they survive in an even larger density (x ∗ = 0.67). The predators cannot find preys; they do not have food and become extinct (y ∗ = 0) once again.
However, for a higher growth rate of prey, 3 < a < 3.57, the increase of the amount of refuge does not always stabilize the system. As we see in Fig. 3d for m < 0.53, refuge replaces the chaotic regimes with a stable positive equilibrium in both populations (y ∗ > x ∗ > 0). However, once the prey refuge exceeds the threshold m ≈ 0.53, the system loses stability and all orbits in the prey population lie on an attracting four-period cycle x ∗ :(0.387, 0.507, 0.83, 0.88). At the same time, the predator population decreases up to extinction (y ∗ = 0), once again. Particularly, as parameter a increases in the interval (3, 3.57) and for an amount of refuge m > 0.5, successive period-doubling bifurcations appear in the prey population; two-period cycle (Fig. 3e), four-period cycle (Fig. 3d) and eight-period cycle (Fig. 3f). This is because once predators become extinct and the birth rate of preys is significantly high, the available food is not enough to feed the large prey density. Therefore, the prey population becomes slowly unstable, as their birth rate increases continuously.
Finally, for an even higher birth rate of prey, a > 3.57, as the refuge takes higher values, m > 0.523, the successive cycles of higher periodsin the prey’s population lead to chaotic behavior; period-doubling route to chaos (Fig. 3g). Thus, once predators become extinct, the exceptionally high reproduction rate of the prey and the resource limitation compete with each other, leading the prey population sometimes to extinction (x ∗ = 0.01) and sometimes to excessive growth (x ∗ = 1) once again (Fig. 3h). However, now, the chaotic dynamics in the prey population looks random. Particularly, observing both chaotic regimes in Fig. 3h, we see that for a small prey refuge, m < 0.237, the system alternates between chaotic behavior and periodic behavior, while for higher values of the prey refuge, m < 0.372, the system produces dynamics, which seems to have lost almost all its determinism. So, we observe that for such a high reproduction rate, a = 3.983, the refuge not only destabilizes the system but moreover makes the prey’s population behavior almost random.
Furthermore, observing the behavior of the predator’s population for a = 3.7, just before the predators become extinct (Fig. 3g), we see a small chaotic region. This chaotic regime could also be a result of the competition between the reproduction rate of the prey and their resource limitation. Particularly, because of the resource limitation, many preys are not able to find food and therefore die, but the piles of dead preys are food for the predators. While due to the high growth rate of prey, preys die in irregular frequencies, so maybe these two facts lead eventually to the appearance of chaotic behavior in the predator’s population as well.
Fig. 3.
Bifurcation diagrams (m, x ∗ ) and (m, y ∗ ) as m varies in the interval [0, 1] for the parameter values: aa = 1.1, ba = 2, ca = 2.8, da = 3.5, ea = 3.2, fa = 3.56, ga = 3.7 and ha = 3.983
Phase plots
Another numerical tool in investigating prey–predator interactions is the phase diagram (x, y). The strange attractor (Fig. 1) appears in the system for a high-growth rate of prey. In order to see how the refuge replaces the chaotic strange attractor with a stable equilibrium point (i.e., stabilizes the prey–predator interactions), we plot phase diagrams for the parameter value a = 3.986, as the refuge varies in the interval m ∈ [0, 0.24] (Fig. 4).
For m = 0 the origin E1:(x ∗ , y ∗ ) = (0, 0) is a saddle (with eigenvalues (λ1 = 3.986, λ2 = 0); it remains a saddle for every value of the varying parameter m since a = 3.986 > 1), the boundary fixed point E2:(x ∗ , y ∗ ) = (0.7491, 0) is an unstable node (with eigenvalues (λ1 = 2.6219, λ2 = −1.986) it remains an unstable node as m increases), the fixed point E3:(x ∗ , y ∗ ) = (0.2857, 9.2357) is an unstable focus (with eigenvalues λ1,2 = 0.4306 ± 1.2341i, determinant DetJ(E3) = 1.7083 > 1 and trace TrJ(E3) = 0.8611) and all solutions converge to the strange attractor (Fig. 4a).
As the refuge parameter increases in the range 0 < m < 0.0286, the strange attractor deforms and becomes less complicated. The strange attractor splits, locks into a stable four-period cycle and near the value m ≈ 0.02868 reappears more deformed (Fig. 4b).
For the refuge parameter values 0.029 < m < 0.092, the strange attractor becomes less and less complicated. Particularly, near the value m ≈ 0.065, the strange attractor evolves into a strange contiguous band (Fig. 4c).
The contiguous strange band breaks apart into a motion of period 5 near the value m ≈ 0.0926 (Fig. 4d) and then it splits again into a motion of period 10 at m ≈ 0.1067 (Fig. 4e).
For higher values of the parameter, 0.11 < m < 0.1777, a series of period-halving bifurcations takes place in both populations (twenty-period cycle at m ≈ 0.112, ten-period cycle at m ≈ 0.1164, and a five-period cycle at m ≈ 0.133). The system has an ordered behavior (Fig. 4f).
For even higher values of the refuge parameter, 0.1777 < m < 0.235, the locally stable orbit of period 5 gives rise to a kinked curve at m ≈ 0.179 (Fig. 4g). The kinked curve loses and gains stability consecutively, deforms and becomes an invariant circle near the value m ≈ 0.22 (Fig. 4h). At this point, the prey coexists with the predator population and both oscillate among all the states of the invariant circle.
Eventually, the invariant circle diminishes in size and near the value m ≈ 0.2372 the fixed point E3:(x ∗ , y ∗ ) = (0.3746, 9.7863) undergoes a subcritical Neimark–Sacker bifurcation becoming a center (DetJ(E3) = 1 & TrJ(E3) = 0.507 < +2). For 0.2372 < m < 0.443, E3 becomes a stable focus fixed point (with complex eigenvalues and DetJ(E3) < 1), where both populations settle down (Fig. 4i).
In Fig. 5 we plot the phase diagram for the growth rate of prey a = 3.7 as the refuge parameter increases in the interval m ∈ [0.515, 0.55]—the small chaotic region (Fig. 3g). We can observe the fountain phenomenon [8] just before the predators become extinct.
For the parameter value m = 0.515, the origin E1:(x ∗ , y ∗ ) = (0, 0) is a saddle fixed point (a = 3.7 > 1), the boundary fixed point E2:(x ∗ , y ∗ ) = (0.7297, 0) is an unstable node (with eigenvalues (λ1 = 1.2387, λ2 = −1.7); it remains an unstable node as m increases) and all solutions converge to the fixed point E3:(x ∗ , y ∗ ) = (0.5891, 5.3642), which is a stable node (with eigenvalues λ1 = −0.9068, λ2 = 0.7271).
As the refuge parameter increases in the range 0.515 < m < 0.522, the attracting fixed point E3:(y ∗ > x ∗ > 0) near the value m ≈ 0.52 undergoes a flip bifurcation (λ1 = −1), becomes a saddle and a two-period cycle appears surrounding it. The attracting cycle of period 2 loses stability via another period-doubling bifurcation giving rise to a stable orbit of period 4 at m ≈ 0.522 (Fig. 5a).
Close to the value m ≈ 0.5224, we observe a period-doubling route to chaos and the trajectory mimics the Feigenbaum bifurcation diagram (Fig. 5b).
Chaos appears in both populations and as the refuge increases in the interval 0.5224 < m < 0.5364, the trajectory is pushing upward (Fig. 5c).
Near the value m ≈ 0.53647, the chaotic motion is being replaced by a six-period cycle (Fig. 5d).
For higher values of the refuge parameter 0.5365 < m < 0.55, chaos reappears in the system (Fig. 5e) and the predator population decreases continuously until, finally, it becomes extinct (Fig. 5f).
Fig. 4.
Phase plots (x, y) for a growth rate of prey a = 3.986 and refuge parameter values: am = 0, bm = 0.0267, cm = 0.0848, dm = 0.097, em = 0.1067, fm = 0.1791, gm = 0.1794, hm = 0.2351 and im = 0.24
Fig. 5.
Phase plots (x, y) for a growth rate of prey a = 3.7 and refuge parameter values: am = 0.52278, b m = 0.52282, cm = 0.53266, dm = 0.536003, em = 0.54436 and fm = 0.54696
Lyapunov exponent diagrams
A Lyapunov exponent diagram illustrates for which values of a varying parameter two orbits, with neighboring initial conditions, converge or diverge from each other exponentially, depending on the sign11 of the Lyapunov exponents. Moreover, the largest value of the Lyapunov exponents indicates whether the system is chaotic or non-chaotic. The higher the maximum Lyapunov exponent (MLE), the more chaotic the system is and the lower the possibility of predictability [34]. So, we plot the Lyapunov exponent diagram (a, ℓ) as the growth rate of the prey increases in the interval a ∈ [2.2, 4.1] for three cases of the refuge parameter: (a) if there is no prey refuge (m = 0) (Fig. 6), (b) if we have a small refuge (m = 0.1) (Fig. 7) and (c) if we have a large refuge (m = 0.783) (Fig. 8).
Fig. 6.
Lyapunov exponent diagram (a, ℓ) without a refuge m = 0
Fig. 7.
Lyapunov exponent diagram (a, ℓ) along with the bifurcation diagram (a, x ∗ ) with a small refuge m = 0.1
Fig. 8.
Lyapunov exponent diagram (a, ℓ) along with the bifurcation diagram (a, x ∗ ) with a large refuge m = 0.783
If there is no prey refuge (m = 0), for a low birth rate of prey 2.329 < a < 3.195, the populations oscillate between quasi-periodic and periodic behavior and the Lyapunov exponents vary among negative and exceptionally small positive values ℓi ≤ 0.001. For an average to high birth rate of prey, 3.195 < a < 3.578, the system alternates between chaotic and high-periodical behavior and the Lyapunov exponents vary among negative and rather higher positive values, ℓi ≤ 0.086, in comparison to the previous interval of the birth rate of prey. For an exceptionally high birth rate of prey, 3.578 < a < 4, chaotic dynamics appear in both populations and the Lyapunov exponent reaches its maximum value, ℓ max ≈ 0.296364, for a growth rate of prey, a ≈ 4.002179.
For a small prey refuge (m = 0.1), periodic and quasi-periodic dynamics along with exceptionally small Lyapunov exponent values ℓi ≤ 0.002 appear for an average to high birth rate of prey, 2.738 < a < 3.67. For a high birth rate of prey, 3.67 < a < 3.77, the populations oscillate among chaotic and periodic behavior, while the Lyapunov exponents vary among negative and small positive values, ℓi ≤ 0.037. For a higher birth rate of prey, 3.77 < a < 4.047, the period-doubling route to an order of chaotic bands and the largest value of the Lyapunov exponent, which corresponds to a growth rate of prey a ≈ 4.043010, is remarkably low ℓ max ≈ 0.096923.
For a large prey refuge (m = 0.783), for which predators inevitably become extinct, dynamical system (2) becomes the well-known discrete-time logistic map. For an average to high birth rate of prey, 3 < a < 3.57, the system goes through successive period-doubling bifurcations and the Lyapunov exponents take only negative values ℓi < 0. For a high birth rate of prey, 3.57 < a < 3.83, the period-doubling route to chaos and the Lyapunov exponents vary among negative and significantly high positive values, ℓi ≤ 0.4434. For an exceptionally high birth rate of prey, 3.83 < a < 4.002, another series of period-doubling (3 · 2n) routes to chaos and the largest Lyapunov exponent reaches the remarkably high value ℓ max ≈ 0.673651, for a growth rate of prey a ≈ 3.998271.
Comparing the Lyapunov exponent diagrams without a prey refuge to those with small and large refuges, we point out the following:
-
i
The minimal addition of a small refuge gives rise to periodic and quasi-periodic dynamics, even for a high birth rate of prey a ≈ 3.67, while chaotic dynamics appears only for exceptionally higher birth rates. Moreover, the maximum value of the Lyapunov exponent is considerably lower than the corresponding one without a prey refuge ℓ max ≈ 0.0969 < 0.2964 (Figs. 6 and 7). Thus, a small prey refuge stabilizes the system for high birth rates of prey, while for exceptionally even higher birth rates, the prey–predator interactions become much less chaotic. Consequently, the oscillations of the prey’s population are much smaller x ∗ ∈ (0.14, 0.65) and preys do not tend anymore to extinction or to overgrowth (Fig. 7).
-
ii
However, by adding a large refuge the system has a lot of similarities with having only a single species with limited resources, while the situation changes drastically for a high birth rate of prey a > 3.57. For such a high birth rate, the dynamics of the system looks random and therefore the prey population tends to extinction or to overgrowth x ∗ ∈ (0, 1) with almost random changes (Fig. 3h). The maximum Lyapunov exponent is higher than in the corresponding case without refuge ℓ max ≈ 0.6737 > 0.2964 (Figs. 6 and 8), the system is extremely chaotic and because of its dependence on initial conditions, it is almost impossible to predict the behavior of the prey’s population.
Concluding remarks
We have shown that although the addition of a small prey refuge stabilizes prey–predator interactions, the addition of a large refuge makes the prey population to behave even more chaotic than without a refuge. While a small refuge could control the prey population, a large refuge leads to almost unpredictability (i.e., random-like prey population outbreaks). Therefore, taking into consideration that the prey population could be insects, which feed off of human blood like any kind of biting flies and that they are able to protect themselves effectively from passive predators like spiders, we may conclude that a pandemic could be a possible scenario.
Of course this is just a simple mathematical prey–predator model and in the real world there are other predators, which also prey on insects like biting flies. So, even if spiders would become extinct, other predators could control the biting flies’ population (biological control) [14]. Moreover, humans have developed various chemical methods in order to control pest populations. However, history has shown that despite any kind of pest control, significant pandemics have been recorded such as the medieval Black Death pandemic, which was transmitted by fleas living on rats [14]. So, considering the usually devastating cost of treatment and the high growth rate of the human population, even infectious diseases, which can be cured, could be extremely dangerous for humanity.
Footnotes
The shape of the Holling type I functional response is linear. So if the predators are spiders and the preys are biting flies, the number of flies killed by one spider is proportional to the flies’ density [4].
The results of the stability analysis have been exhibited by using the software package Maxima 5.27.0 (http://maxima.sourceforge.net/).
All numerical simulations were executed using the software package E&F Chaos [21].
This low-resting metabolic rate may be due to the fact that they use hydrostatic pressure for extending their appendages [26].
In addition, spiders can further reduce their metabolic rate below their already low levels when they experience periods of food limitation [27].
Many spiders, including both web-building and wandering spiders, are sit-and-wait predators that spend very little time in active locomotion [28]. For example, for the wolf spider Pardosa amentata, the daily energy loss attributed to locomotion was estimated to be only 1% of the daily energy usage of spiders [29].
The energetic costs of web production are relatively small because web building is often a short process and some spiders are able to recycle web proteins, which can substantially reduce the metabolic cost of silk production [29].
For example for the wolf spider Pardosa lugubris, the females invest 26% in reproduction and males invest only 16% [30].
Male spiders invest 81% of their ingested energy into respiration, while females invest 73% [30]. This difference in respiration rate between the sexes is likely related to the high energetic needs of males for agonistic encounters with competing males and for courting females [31].
We use the same parameter values that were also used in Danca et al.’s [9] paper, so that our results are comparable with those of the basic model (1).
Positive Lyapunov exponents correspond to diverging neighboring orbits, negative Lyapunov exponents correspond to converging orbits and zero Lyapunov exponents correspond to bifurcations that occur in the system [34].
References
- 1.Verhulst PF. Notice sur la loi que la population suit dans son accroissement. Corresp. Math. et Phys. 2000;10:113–121. [Google Scholar]
- 2.Lotka AJ. Elements of Physical Biology. Baltimore MD: Williams and Wilkins; 1925. [Google Scholar]
- 3.Volterra V. Fluctuations in the abundance of a species considered mathematically. Nature. 1929;118(2972):558–560. doi: 10.1038/118558a0. [DOI] [Google Scholar]
- 4.Holling CS. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959;91(5):293–320. doi: 10.4039/Ent91293-5. [DOI] [Google Scholar]
- 5.Holling CS. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 1965;45:1–60. doi: 10.4039/entm9745fv. [DOI] [Google Scholar]
- 6.Rosenzweig ML, MacArthur RH. Graphical representation and stability conditions of predator–prey interactions. Am. Nat. 1963;97(895):209–223. doi: 10.1086/282272. [DOI] [Google Scholar]
- 7.Maynard SJ. Mathematical Ideas in Biology. Cambridge: Cambridge University Press; 1968. [Google Scholar]
- 8.Hadeler KP, Gerstmann I. The discrete Rosenzweig model. Math. Biosci. 1990;98(1):49–72. doi: 10.1016/0025-5564(90)90011-M. [DOI] [PubMed] [Google Scholar]
- 9.Danca M, Codreanu S, Bako B. Detailed analysis of a nonlinear prey–predator model. J. Biol. Phys. 1997;23(1):11–20. doi: 10.1023/A:1004918920121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Xiao D. Complex dynamic behaviors of a discrete-time predator–prey system. Chaos, Solitons Fractals. 2007;32(1):80–94. doi: 10.1016/j.chaos.2005.10.081. [DOI] [Google Scholar]
- 11.Maynard SJ. Models in Ecology. London: Cambridge University Press; 1974. [Google Scholar]
- 12.Hassel MP. The Dynamics of Arthropod Predator–Prey Systems. Princeton: Princeton University Press; 1978. [PubMed] [Google Scholar]
- 13.Taylor RJ. Predation. New York: Chapman & Hall; 1984. [Google Scholar]
- 14.Pollock, S.T.: Ecology, vol. 10. Dorling Kindersley, London, Eyewitness Science (1993)
- 15.Last, J.M.: A Dictionary of Epidemiology. Oxford University Press (2000)
- 16.Ryan, K.J., Ray, C.G.: Sherris Medical Microbiology: An Introduction to Infectious Diseases, 4th edn. McGraw-Hill Medical (2003)
- 17.Mandell, G.L., Bennett, J.E., Dolin, R.: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th edn. Churchill Livingstone (2009)
- 18.Oldstone MBA. Viruses, Plagues, & History, 1st edn. USA: Oxford University Press; 1998. [Google Scholar]
- 19.Lehrer S. Anopheles mosquito transmission of brain tumor. Med. Hypotheses. 2010;74(1):167–168. doi: 10.1016/j.mehy.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jury EI. Inners and Stability of Dynamic Systems. New York: Wiley; 1974. [Google Scholar]
- 21.Diks C, Hommes C, Panchenko V, van der Weide R. E&F Chaos: a user-friendly software package for nonlinear economic dynamics. Comput. Econ. 2008;32(1–2):221–244. doi: 10.1007/s10614-008-9130-x. [DOI] [Google Scholar]
- 22.Edgar WD. Prey and predators of wolf spider Lycosa lugubris. J. Zool. Lond. 1969;159:405–411. doi: 10.1111/j.1469-7998.1969.tb03897.x. [DOI] [Google Scholar]
- 23.Nyffeler M, Breene RG. Evidence of low daily food consumption by wolf spiders in meadowland and comparison with other cursorial hunters. J. Appl. Entomol. 1990;110:73–81. doi: 10.1111/j.1439-0418.1990.tb00097.x. [DOI] [Google Scholar]
- 24.Ruppert EE, Fox RS, Barnes RB. Invertebrate Zoology: A Functional Evolutionary Approach. 7. Belmont, CA: Brooks Cole Thomson; 2004. [Google Scholar]
- 25.Anderson JF. Metabolic rates of spiders. Comput. Biochem. Phys. 1970;33:51–72. doi: 10.1016/0010-406X(70)90483-4. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JF, Prestwich KN. The fluid pressure pumps of spiders (Chelicerata, Araneae) Z. Morph. Tiere. 1975;81:257–277. doi: 10.1007/BF00298488. [DOI] [Google Scholar]
- 27.Anderson JF. Responses to starvation in the spiders Lycosa lenta Hentz and Filistate hibernalis (Hentz) Ecology. 1974;55:576–585. doi: 10.2307/1935148. [DOI] [Google Scholar]
- 28.Prestwich KN. The energetics of web-building in spiders. Comput. Biochem. Phys. A. 1977;57:321–326. doi: 10.1016/0300-9629(77)90199-2. [DOI] [Google Scholar]
- 29.Ford MJ. Energy costs of the predation strategy of the web-spinning spider Lepthyphantes zimmermanni Bertkau (Linyphiidae) Oecologia. 1977;28:341–349. doi: 10.1007/BF00345989. [DOI] [PubMed] [Google Scholar]
- 30.Edgar WD. Aspects of the ecological energetic of the wolf spider Pardosa (Lycosa) lugubris (Walckenaer) Oecologia. 1971;7:136–154. doi: 10.1007/BF00346356. [DOI] [PubMed] [Google Scholar]
- 31.Kotiaho JS, Alatalo RV, Mappes J, Nielsen MG, Parri S, Rivero A. Energetic costs of size and sexual signaling in a wolf spider. Proc. R. Soc. B. 1998;265:2203–2209. doi: 10.1098/rspb.1998.0560. [DOI] [Google Scholar]
- 32.Anderson JF, Prestwich KN. Respiratory gas exchange in spiders. Physiol. Zool. 1982;55:72–90. [Google Scholar]
- 33.Blanchard, P., Devaney, L.R., Hall, R.G.: Differential Equations. Thomson Brooks/Cole (2006)
- 34.Shone R. Economic Dynamics: Phase Diagrams and Their Economic Application. 2. New York: Cambridge University Press; 2002. [Google Scholar]













