Abstract
Touch is a fundamental, but complex, element of everyday interaction that impacts one’s sensory and affective experience via interoceptive processing. The insular cortex is an integral component of the neural processes involved in interoception, i.e. the generation of an “emotional moment in time” through the sensing of the internal body state (Craig, 2002). Here, we examine the contribution of different parts of the insular cortex in the representation of both affective and sensory aspects of touch. To that end, subjects were administered a cued application of touch during functional MRI. We find that stimulus-related activation occurs in the mid-to-posterior insula, whereas anticipatory related activation is seen mostly in anterior insula. Moreover, the degree of activation in anterior insula during anticipation is correlated with the degree of activation in the posterior insula and caudate during stimulus processing. Finally, the degree of activation in the anterior insula during anticipation is also correlated with experienced intensity of the touch. Taken together, these results are consistent with the hypothesis that the anterior insula is preparing for the sensory and affective impact of touch. This preparatory function has important implications for the understanding of both anxiety and addictive disorders because dysfunctions in anticipatory processing are a fundamental part of the psychopathology.
Introduction
Touch impacts both sensory and affective processes and, as such, acts as an important component of daily human interactions. In particular, human touch can attenuate stress-related activity in both the brain and body (Coan et al., 2006; Devries et al., 2003) and provides for supportive social behavior and. Moreover, touch by a human hand can produce pleasure and is often associated with significant reward value (Rolls, 2000). Finally, touch intensifies emotional displays from other modalities (Knapp and Hall, 1997) and is even able to convey distinct types of emotion (Hertenstein et al., 2006).
Touch by an external object, including another human being, affects a number of subcutaneous receptors, which signal a change in the internal body state. Therefore, touch is a quintessential example of an exteroceptive stimulus that affects interoceptive processing. Interoception, i.e. the sensing of the internal body state, is mediated via afferent unmyelinated C-fibers that converge within the posterior insular cortex (Craig, 2002). The C-fiber ascending pathway has been linked traditionally to pain processing. However, the function of these fibers has been widely expanded to include a range of sensations such as temperature (Craig and Bushnell, 1994), itch (Schmelz et al., 1997), tickle (Lahuerta et al., 1990), and sensual touch (Olausson et al., 2002; Robinson et al., 2005; Vallbo et al., 1995). One important function of the interoceptive system is to evaluate the emotional intensity of an experience and to modulate subsequent assessment of subjective value of the stimulus, providing a link between the internal body state and cognitive-affective processes.
The insular cortex is a critical neural substrate for interoceptive processing and the production of a general feeling state based on the physiological condition of the body (Craig, 2003). It has also been suggested to play a key role in evaluating the impact that upcoming environmental stimuli may have on the interoceptive body state (Craig, 2002). Thus, the insular cortex is implicated in both anticipatory and stimulus related processing. The anterior insula receives afferent projections from structures involved in emotion processing and motivated behaviors, such as the entorhinal cortex, striatum, and periamygdaloid areas. In comparison, posterior insula receives afferent projections from the primary somatosensory cortex, superior temporal sulcus, and the thalamus (Dupont et al., 2003), providing it with somatosensory and visceral input. Recent literature has suggested a parallel functional organization of insular cortex, implicating the anterior insula in interoceptive awareness and motivation, and the posterior insula in the processing of sensory input (Craig, 2002; Craig, 2005; Critchley et al., 2004; Knapp and Hall, 1997).
Here, we use a cued application of different types of touch (real and latex hand) (Figure 1) during functional magnetic resonance imaging (fMRI) to investigate the role of the insular cortex in anticipation of and stimulation by an emotionally relevant stimulus. By employing a cued application of touch, we are able to focus our analysis on areas active in expectation of touch—a purely affective process—versus areas active during stimulation by touch—a combination of affective and sensory processes.
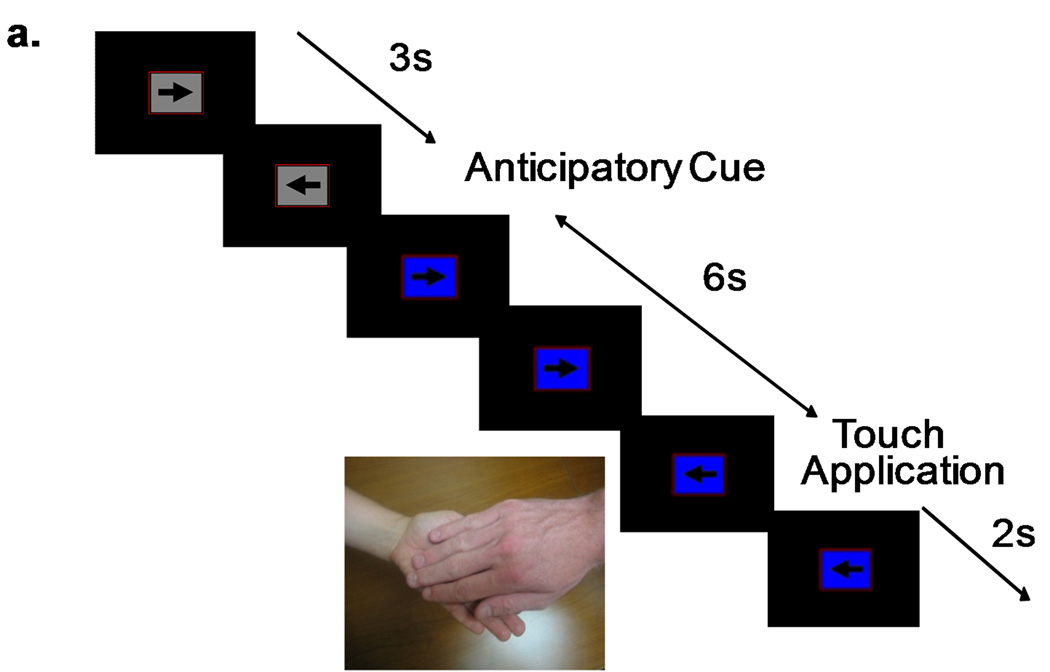
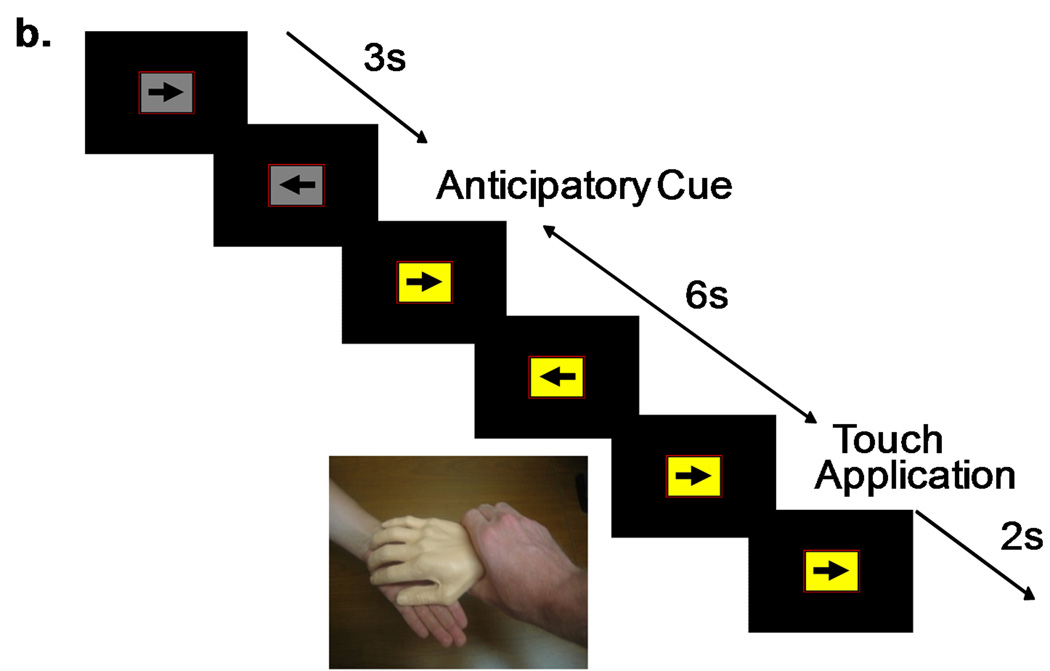
Figure 1.
fMRI paradigm combining a continuous performance task—responding to left or right pointing arrows—and a cued application of touch. The arrows were presented in three second intervals. A background color change to blue indicated an upcoming real hand touch (a); a background color change to yellow indicated an upcoming latex hand touch (b). The anticipatory cue lasted six seconds, and the following touch stimulus lasted two seconds.
We have previously hypothesized that the anterior insula not only receives interoceptive information but is also able to generate a predictive model (Paulus and Stein, 2006), which provides the individual with a signal of how the body will feel, similar to the “as if” loop in the Damasio somatic marker model (Damasio, 1994). In this formulation, Damasio’s theory extends the James Lang theory of emotion because the insula can instantiate body sensation without necessarily receiving peripheral inputs. Therefore, in understanding the anticipatory processing of an emotional stimulus, like touch, we may gain further insight into the abnormal processing in mood and addiction disorders that have expectancy modulation as one of the main psychopathologies.
Materials and Measures
Subjects
Twenty-one non-smoking, right-handed healthy volunteers were interviewed to participate in the study. Nineteen were chosen to complete the fMRI session (11 females; mean age 23.1 years, s.d. ± 2.6 years; average education level 15.9 years, s.d. ± .03 years). All participants denied a history of drug or alcohol dependence or regular use of prescription medications other than oral contraceptives. Additionally, subjects gave written informed consent, as approved by the University of California, San Diego School of Medicine Human Research Protection Program.
Tactile Stimuli
For each subject an experimenter of opposite sex, trained prior to scanning, administered the tactile stimuli. During fMRI, the experimenter received a voice instruction via headphones about the stimulus type and stimulus administration timing. The real (human) hand stimulus consisted of the application of light touch held stationary for two seconds to the subject’s left palm by the experimenter’s own right hand, while the latex hand stimulus consisted of a 170 gm latex right hand—held by the experimenter— applied to the left palm for two seconds. The administrators were trained to apply both real and latex stimuli with equal pressure.
Measures
Thirteen of the nineteen participants completed a questionnaire aimed at quantifying various aspects of the touch sensations using a visual analog scale (VAS)—six subjects were run on the fMRI task before the addition of VAS scales to the protocol. Both prior to and after the fMRI session, each individual received a touch by a real and a latex hand and was instructed to rate his/her experience from “not at all” (rating of 0) to “extremely” (rating of 10) on the following dimensions: pleasant, unpleasant, intensity, tickle, warm, cold, and soft.
Experimental Task Design
During the fMRI session, individuals performed a behavioral task that combined a continuous performance task and a cued stimulus presentation, to examine the effects of anticipation and of the stimulus administration (Figure 1). The continuous performance task was administered to assure that the individual was attending to the visual stimuli, as well as to provide for a baseline in further analysis. Accordingly, a screen was used to present a left or right pointing arrow every three seconds, and subjects were instructed to identify the direction of the arrow by pressing the left or right button on a button box. The cued stimulus presentation had two phases: an anticipatory phase, when the characteristics of the background behind the arrow changed and signaled the impending presentation of a tactile stimulus (detailed below) and a stimulation phase, when subjects received the real or latex touch stimulus. Additionally, the paradigm had two trial types, one in which a touch was administered (stimulus trial) and one in which the subjects expected to receive a touch but none was presented (no-stimulus trial). Throughout the baseline of the task (continuous performance task only), a black arrow on a gray background was presented on a screen. For the anticipation phase, subjects were informed that a blue background on the screen predicts the subsequent touching by a real hand, whereas a yellow background predicts touching by a latex hand. The anticipation phase consisted of a 6 sec period of arrows surrounded by either a blue or yellow background, and was followed by a real or latex 2 second stimulus, respectively, or no stimulus at all. The arrow and background from the anticipation period continued through the stimulus. As the colors used present minimal bias, the background colors were not counterbalanced across subjects. Although the real cue for the human hand was presented thirty times, the human touch occurred during only twenty of these trials, while the remaining ten cues resulted in the no-stimulus trial. Similarly, the latex cues and stimuli were administered with the same frequency. Thus, in total, sixty cues were presented throughout the task (thirty real, thirty latex), followed by a corresponding stimulus type two-thirds of the time (twenty real, twenty latex), or no stimulus one-third of the time (ten real no-stimulus, ten latex no-stimulus).
fMRI Protocol Analysis Pathway
The blood oxygen level dependent (BOLD) fMRI data were collected during the task using a Signa EXCITE 3.0 Tesla-GE scanner (T2*-weighted echo planar imaging (EPI) scans, TR = 2000ms, TE = 32 ms, FOV = 230 × 230 mm3, 64 × 64 matrix, thirty 2.6mm axial slices with a 1.6mm gap, 315 whole-brain axcquisitions). The experimental task was performed over two 10 min and 30 sec. EPI runs. For anatomical reference, a high-resolution T1-weighted image (spoiled gradient recalled (SPGR), TI = 450, TR =8ms, TE = 3ms, FOV = 250 mm, flip angle = 12°, 176 sagitally acquired slices 1 0.97 0.97 mm3 voxels) was obtained during the same session.
All image processing and analysis was done with the Analysis of Functional Neuroimages Software (AFNI) package (Cox, 1996). For preprocessing, EPI images were interpolated to correct for three-dimensional motion, time-corrected for non-simultaneous slice acquisition, and normalized to Talairach coordinates (Talairach and Tournoux, 1988). Time series data for each individual were then analyzed using a multiple regression model. Six regressors of interest were created to measure the neural substrates contributing to each element of the task: (1) real hand anticipation condition (AntR), the 6 second anticipatory phase marked by the background color change to blue prior to real hand stimulus, (2) latex hand anticipation condition (AntL), the 6 second anticipatory phase following background color change to yellow prior to the touch by the latex hand, (3) the real touch stimulus condition (StimR), which consisted of a 2 second stimulation phase, (4) the latex touch stimulus condition (StimL), also a 2 second phase (5) the real no-stimulus condition, the 2 second phase when the individual expected a real touch but did not receive any stimulation (StimNR), and (6) the latex no-stimulus condition when the individual expected a touch by the latex hand but did not receive any stimulation (StimNL). Additionally, three movement-related nuisance regressors were used to account for residual motion (in the roll, pitch, and yaw directions), a white matter mask was used to control for physiological noise (Strigo et al., 2006), and baseline and linear trend nuisance regressors were used to eliminate slow signal drifts.
The regressors of interest were convolved with a modified gamma variate function to account for the delay and dispersion relating presumed neural activation to hemodynamic changes measured by the BOLD response (Boynton et al., 1996). The AFNI program 3dDeconvolve calculated the estimated voxel-wise response amplitude for each regressor of interest, in addition voxel-wise linear contrast were created for specific planned comparison: (1) anticipation phase (AntR+AntL), (2) stimulation phase (StimR+StimL), (3) no-stimulus phase (StimNR+StimNL), (4) difference between the stimulation phase in the real stimulus and the real no-stimulus trials (StimR-StimNR), and (5) difference between the stimulation phase in the latex stimulus and the latex no-stimulus trials (StimL-StimNL). Subsequently, a Gaussian filter with FWHM 6 mm was applied to voxel-wise percent signal change data to account for individual anatomical variations. Finally, statistical significance for the planned comparisons specified above was determined through a series of one-sample t-tests, which included: (1) AntR+AntL, (2) StimR+StimL, (3) StimNR+StimNL, (4) StimR-StimNR, and (5) StimL-StimNL.
Voxel-wise percent signal change data for the whole brain were entered into a t-test to examine effects of real and latex touch anticipation (AntR+AntL) and stimulation (StimR+StimL). Due to the unconstrained nature of whole brain investigation, we aimed to identify only peak areas of activation; thus, we chose to only consider regions of activation significant at the p < 0.001 level in order to remain conservative in our results. A threshold adjustment method based on Monte Carlo simulations was used to guard against identifying areas of false positive activation (Forman et al., 1995). Based on these calculations, a prior voxel-wise probability of p < 0.001 in a 448 µL cluster resulted in cluster-wise false positive probability of p < 0.001. Thus, only activations which satisfied both the volume and voxel connection constraints were used for further analysis.
Additionally, a priori regions of interest (defined by the Talairach demon atlas (Lancaster et al., 2000)) in bilateral amygdala, cingulate, and insular cortex were used as anatomical masks in a region of interest (ROI) based analysis. Within these constrained search regions, a voxel-wise a priori probability of p < 0.01 resulted in a corrected cluster-wise activation probability of p < 0.01 when using a minimum volume of 128 µL for an amygdala cluster, or 256 µL for an insular or cingulate cortex cluster.
A secondary analysis was conducted to examine the overlap of activation during the anticipation phase (AntR+AntL) and the stimulation phase (StimR+StimL) in the insular cortex, amygdala, anterior cingulated cortex (ACC). Clusters of significant activation found in the ROI analysis for the anticipation condition and stimulation condition were submitted to a conjunction analysis, and regions active during “anticipation only,” “stimulation only,” and the intersection of the two (“anticipation ∩ stimulation”) were identified. The signal from within these regions during the anticipation and stimulation phases were extracted and entered into three paired t-tests.
Finally, voxel-wise correlation analyses were carried out to find regions of activation during the stimulation phase that correlated to anterior insular activation during the anticipation phase—found by extracting the anterior regions (y > 0) of the “anticipation only” ROI. Using the statistical package R (Ihaka and Gentleman, 1996), clusters of significant correlation (V > 256 µL, p < 0.01) in the stimulation phase were found using an anatomically defined ROI based analysis, including insula, amygdala, cingulate, and striatal regions. The percent signal change found in these areas was extracted and used for subsequent scatter plots. This same voxel-wise correlation technique was used to correlate VAS scale ratings and fMRI data for the anticipation phase as well as the stimulation phase.
Statistical Analysis
All analyses were carried out using Statistical Package for the Social Sciences (SPSS) 11.0 (Norusis, 2002). The seven dimensions of the VAS from before and after fMRI session were entered into a within-subjects repeated measures analysis of variance (ANOVA). Additionally, behavioral data regarding task accuracy and reaction time during fMRI were collected and entered into within-subjects repeated measures ANOVA to compare data across task condition (Baseline, AntR, AntL, StimR, StimL, StimNR, or StimNL) and touch type. One subject was removed from behavioral analysis due to complication with the button box, resulting in inaccurate recording of the continuous performance task. To account for multiple contrasts, a Bonferroni correction was used for between condition comparisons.
Results
Subjective Assessment of Touch
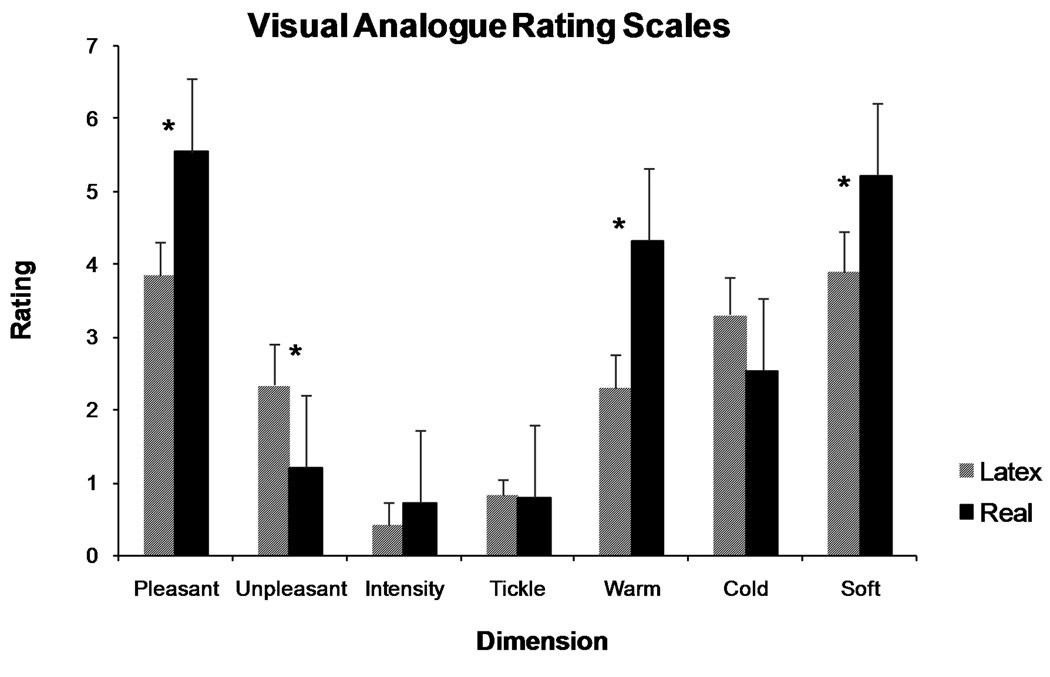
Subjects were asked to rate the real and latex touch on seven dimensions—pleasant, unpleasant, intensity, tickle, warm, cold, and soft—using VAS outside of the fMRI session. Results from the VAS, completed by subjects before and after the task, revealed significant differences in the affective qualities of real and latex touch. There was no significant difference in ratings taken before or after fMRI (F = 0.556, p = .470), thus ratings were averaged for further analysis (Figure 2). Across all subjective dimensions, subjects rated touch by a real hand significantly different from that of the latex hand (F(1, 12) = 15.27, p < 0.01, partial eta-squared = 0.56). Post-hoc analyses revealed that individuals rated the real hand touch more pleasant (t = 3.999, p < 0.01), less unpleasant (t = −2.711, p = 0.019), warmer (t = 4.639, p < 0.01), and softer (t = 2.853, p = 0.015) than the touch by the latex hand. There were no differences between the real hand touch and the latex hand touch on intensity (t = 0.021, p = 0.98), tickle (t = 0.00, p = 1.00), and cold (t = −1.00, p = 0.34) ratings. These results indicate that the different touch types induced significantly different experiences in subjects, and the real hand touch induced significantly more positive ratings than that of the latex hand.
Figure 2.
Subjects’ reported ratings of the characteristics of real and latex hand touch (n = 13). The real touch was rated significantly higher in the pleasant, warm, and soft dimensions, and significantly lower in the unpleasant dimension.
Task Performance
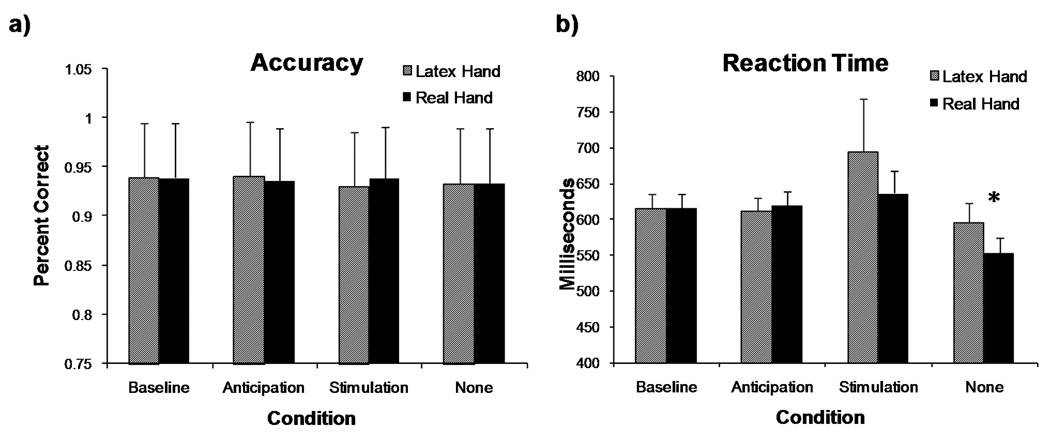
Subjects maintained a high level of accuracy on the continuous performance task across the real and latex anticipation, stimulation, and no-stimulus conditions (Figure 3). Although selecting the correct choice was lowest during the real hand and latex hand touch no-stimulus conditions (mean = 93%, s.d. = 0.06), and highest during the anticipation conditions (mean = 94%, s.d. = 0.06), there were no significant differences across phases (F(2,16) = 0.284, p = 0.754) or across stimulus type (F(1,17) = 0.099, p = 0.756). Alternatively, subjects responded with significantly different latencies during the different task phases (F(2,16) = 4.553, p = 0.018) , although type of stimulus did not significantly alter latency (F(1,17) = 1.286, p = 0.273). Post-hoc analyses revealed that individuals responded significantly faster during the no-stimulus phase relative to the anticipation (t(18) = 4.222, p < 0.01) and stimulation phases (t(18) = 2.632, p = 0.017).
Figure 3.
Percent correct choices made (a) and reaction time (b) in arrow discrimination task during fMRI (n = 18). There were no significant differences in accuracy across stimulus type or phase of the task. The “no-stimulus” conditions for both the real and latex hands produced a significantly faster reaction time (*) compared to the anticipation and stimulation conditions.
fMRI Results
Stimulus Effect
Whole brain analysis revealed robust areas of significant activation and deactivation during the real and latex stimulation conditions (see Supplementary Table 1 online). Relative to the baseline, both the real and latex stimulation produced significant activation (pvoxel < 0.001, V > 448 µL) in primary sensory areas, both cortical (bilateral postcentral gyrus) and subcortical (thalamus), as well as areas associated with reward (caudate and putamen). There were no areas of significantly different activation between the real and latex stimulation conditions in the whole brain analysis.
A combined threshold-volume method (pvoxel < 0.01, V > 256 µL) was used for the ROI based analysis to compare activation across task phase and stimulus type. Bilateral insula and dorsal anterior cingulate cortex (dACC) significantly activated during the stimulation conditions, irrespective of real or latex touch. Touch by the latex hand was associated with significant bilateral amygdala activation. In comparison, touch by the real hand produced significant activation only in the right amygdala. Furthermore, latex, but not real hand touch resulted in significant activation within ventral anterior cingulate cortex (vACC) and subgenual cingulate. There were no areas of significant difference across stimulus type.
Anticipatory Effect
During the real and latex stimulus anticipation conditions, whole brain analysis showed significant activation (pvoxel < 0.001, V > 448 µL) in bilateral insula, cingulate cortex, postcentral gyrus, thalamus, posterior aspects of the rostral medial frontal gyrus, and lateral nucleus. Furthermore, significant activation was seen in the right inferior frontal gyrus and claustrum. In contrast, areas of significant deactivation were found in bilateral superior frontal gyrus, anterior aspects of the rostral medial frontal gyrus, and postcentral gyrus (see Supplementary Table 2 online).
Additionally, the ROI based analysis (pvoxel < 0.01, V > 256 µL) revealed that, relative to the baseline, there was also significant activation in the dACC and bilateral amygdala (pvoxel < 0.01, V > 128 µL) during anticipation of both the real and latex stimulus.
“No-stimulus” Effect
To examine which neural substrates were specific to expected, but not received, stimulation, and also to verify our stimulus condition outcome as resultant of touch and not some other factor, we analyzed the no-stimulus trials relative to the real and/or latex touch trials. Consistent with the stimulation effect findings, both the real and latex stimulation conditions had significantly greater activation in posterior insula, cingulate cortex, right amygdala, and somatosensory cortex than their respective no-stimulus condition. Additionally, the latex touch produced significantly greater activation in subgenual cingulate and vACC than the latex no-stimulus condition; whereas the real touch did not. Thus, areas found to have significantly greater activation when comparing stimulation condition to baseline were also found to have significantly greater activation when comparing the stimulation trials to the no stimulation trials. The consistency of these results supports that the regions active in the stimulus condition can be attributed to tactile stimulation and not interference of the continuous performance task.
Conjunction Analysis
A conjunction analysis was carried out to clarify which portions of the insula were active during the anticipation phase, the stimulation phase, and the intersection of the two. While the middle and posterior of the insula was active during both anticipation and stimulus, it is interesting to note that the anticipation phase displayed significant activation in the anterior portions of the insula while the stimulus phase did not. Post-hoc t-tests were conducted to directly compare anticipation and stimulation processing in each of the three areas (Figure 4). Activation during the anticipatory phase was found to be greater than activation during the stimulation phase in the “anticipation only” region of the insula (Right Insula: t = 2.354, p = 0.03; Left Insula: t = 1.360, p = 0.191), whereas activation during the stimulation phase was found to have significantly greater activation in the “stimulation only” (Right Insula: t = −4.733, p < 0.0001; Left Insula: t = −4.223, p = 0.001 ) and “anticipation ∩ stimulation” (Right Insula: t = −3.765, p = 0.013; Left Insula: t = −2.386, p = 0.028) regions. To further verify this activity, the time course of activity for the “anticipation only”, “stimulation only”, and “both” was extracted and plotted (Supplementary Figure 1). Data from the conjunction analysis for bilateral amygdala and ACC can be found in Supplementary Figure 2.
Figure 4.
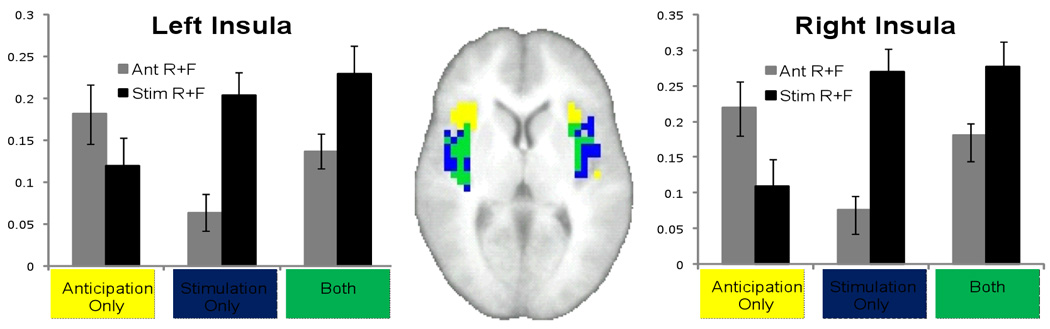
The distinct regions of insular activation during anticipation only and stimulation only (yellow and blue, respectively), and the intersection of these areas of activation (green). The signal within these regions during the anticipation and stimulation phases of the task (AntR+AntL and StimR+StimL) was extracted and entered into a paired t-test for each ROI. Activation during stimulation was significantly (p < 0.017) greater than anticipation in the stimulation only and anticipation ∩ stimulation regions.
Correlation Analyses
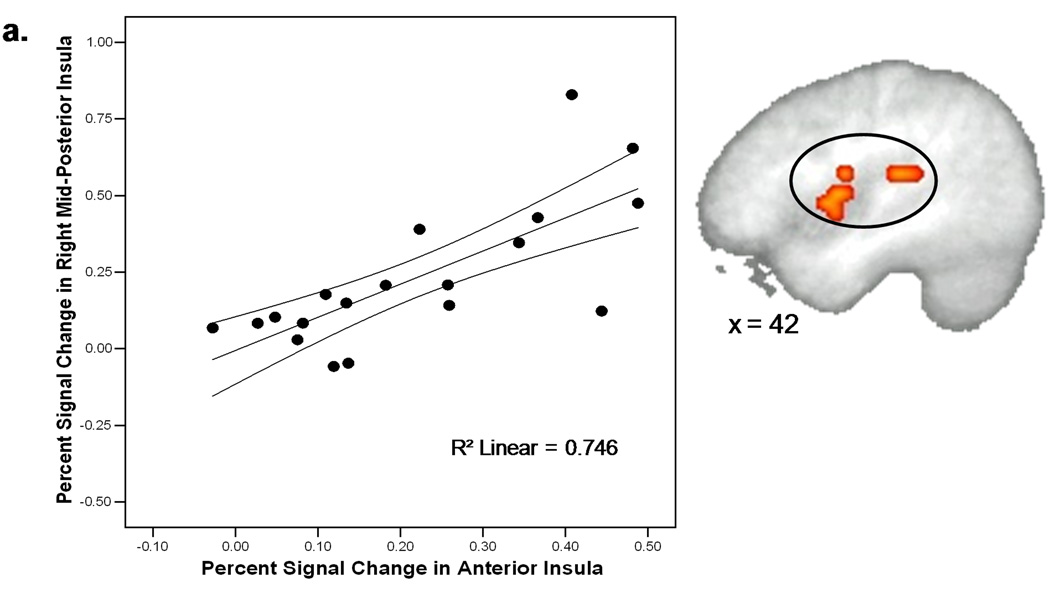
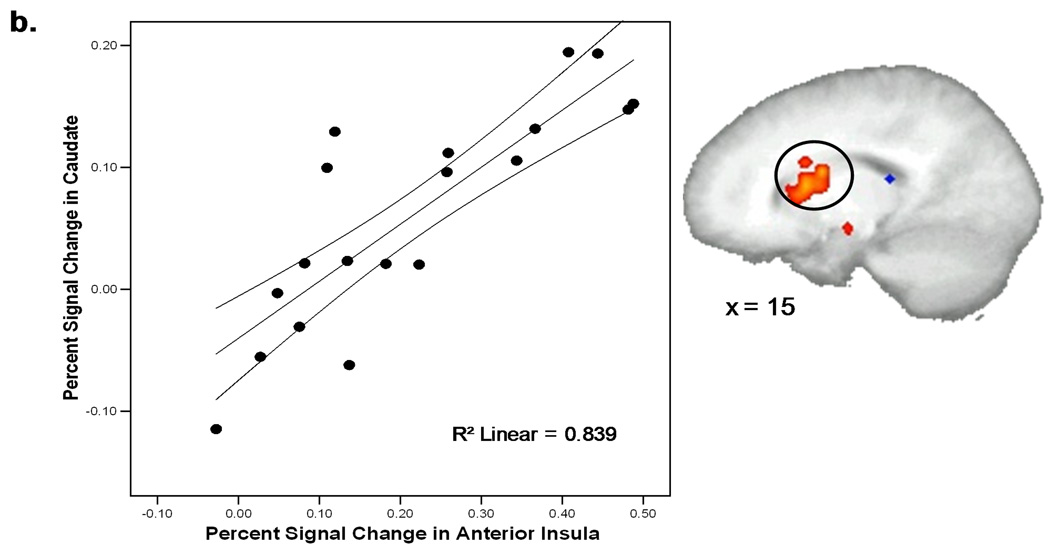
A voxel-wise correlation analysis between activation in the anterior insular portion of the “anticipation only” ROI during the anticipation phase (AntR+AntL) of the task and activation in other regions of interest—insula, striatum, and cingulate cortex—during the stimulation phase (StimR+StimL) of the task was carried out to examine any modulatory functions the anterior insula might be contributing to during anticipation (Figure 5). A significant positive correlation was found in regions of the right mid and posterior insula (r = 0.5, p < 0.01) as well as the caudate (r = 0.84, p < 0.01).
Figure 5.
Activity in the right anterior (34, 17, 2) insula during the anticipation phase was significantly correlated (p < 0.01) with a right mid (42, −3, 6) and posterior (42, −28, 17) region of the insula (a) as well as the caudate (b) during the stimulation phase.
Brain Behavior Relationships
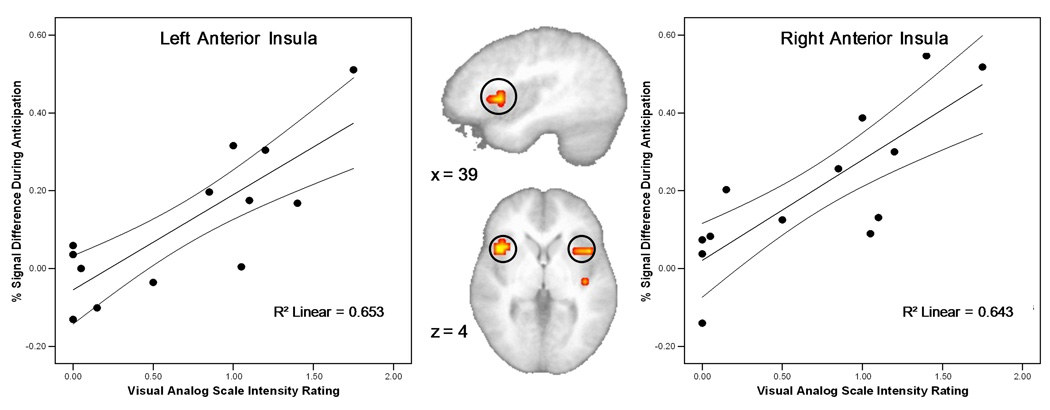
A voxel-wise regression analysis was carried out to determine whether the degree of activation in the insular cortex was related to subjective assessment of the touch experience recorded in the VAS scales. We found that the degree of activation during the anticipation phase (AntR+AntL) in bilateral anterior insula was significantly correlated (right insula: r = 0.78, p < 0.01; left insula: r = 0.81, p < 0.01) with the magnitude of intensity ratings using the VAS (Figure 6). No correlation was found between continuous performance task time or accuracy and activation during anticipation or stimulation conditions.
Figure 6.
A significant correlation (p < 0.01) was found between the reported intensity rating of touch and activation in bilateral anterior insula during the anticipation condition.
Discussion
This investigation yielded three main results. First, stimulation by both the real and latex hand resulted in activation of insula, caudate, cingulate, and amygdala—areas commonly related to emotion and reward—as well as thalamus and somatosensory cortices. Thus, the touch paradigm engaged systems involved in both affective and sensory processing. Second, the anterior portion of the insula was notably active during anticipation of touch, whereas the mid-to-posterior insular cortex was active for both anticipation and stimulation. Third, the degree of activation during anticipation in the bilateral anterior insular cortex was associated with stimulus related processing in the mid and posterior insula and caudate, as well as the ratings of intensity acquired in the VAS scales. Taken together, the data support the hypothesis that the anterior insula generates an anticipatory signal of expected stimulus intensity and thus may have a preparatory function to integrate the affective and sensory experiences associated with interoceptive processing.
During both the real and latex hand stimulation, areas implicated in affective processing, along with areas responsible for sensory processing were significantly activated. In previous studies, the insular cortex has been implicated in interoceptive (Critchley et al., 2004) and emotion-related (Phan et al., 2002) processes. Cingulate cortex and medial frontal gyrus have previously been linked to self-focus and monitoring emotional state (Amodio and Frith, 2006; Beauregard et al., 2001; Gusnard et al., 2001; Mars et al., 2005; Northoff and Bermpohl, 2004; Wicker et al., 2003), as well as in stimulation by pleasant touch (Francis et al., 1999). The amygdala has also been shown to activate during pleasant sensory stimuli(O'Doherty et al., 2001), and during pleasant mood induction (Schneider et al., 1997a). Moreover, caudate and putamen have continually shown relations to reward processing (Knutson and Cooper, 2005b; O'Doherty, 2004). Finally, primary sensory areas, including thalamus and post-central gyrus, were active during the stimulation phase. The activation of all these areas during the stimulation phase support that the touch paradigm utilized here provided for a stimulus that acts on both affective and sensory systems.
Whereas activation was seen in areas related to affective and sensory processing during the stimulation phase, there was a much greater recruitment of areas implicated in affective processing during the anticipation phase. Similar to the stimulus condition, anterior cingulate cortex, medial frontal gyrus, and amygdala were significantly activated during anticipation. Additionally, a region of the insular cortex was found to be significantly active during anticipation; however, this region appeared to extend more into anterior regions than those found active during stimulation.
In order to better segregate these topographical differences, we performed a conjunction analysis to identify regions of the insula active during anticipation only, stimulation only, and the intersection of the two. Our results show that the anterior insula was active only during the anticipation phase of the task. Moreover, post-hoc t-tests revealed that there was greater activation in this “anticipation only” region during anticipation than stimulation, as opposed to the mid-to-posterior insular “stimulation only” region in which stimulation resulted in significantly greater activation than anticipation. Taken into consideration that the anterior insular cortex has been implicated in modulation of the affective response (Craig, 2005; Craig, 2008) and given the current finding of anticipatory signaling in this structure, we suggest that this anticipatory period may be critical for various aspects of affective encoding of the stimulus.
Additionally, the anterior insula activation during anticipation was correlated with the stimulus related activation in a mid and posterior region of the insula, as well as with a region in the caudate. The posterior insula is part of SII and therefore is directly involved in sensory and interoceptive processing (Craig, 2002; Craig, 2003; Critchley et al., 2004). Therefore, this correlation is consistent with the notion that the anterior insula may have a preparatory function to set the sensitivity of the posterior insula when processing the sensory component of the upcoming stimulus. Moreover, the correlation with the caudate, which is important for processing rewarding stimuli (Knutson and Cooper, 2005a), may indicate that the level of anticipatory activity in the anterior insula could function to alter the degree to the rewarding properties of the upcoming stimulus. Finally, the correlation between reported intensity ratings of touch and activation in bilateral anterior insula during anticipation relates the subjective experience to the modulatory role of the anterior insula. In sum, these results are in support of the notion that the anterior insula anticipates the affective value of the stimulus, whereas a more mid-posterior region is primarily involved in processing sensory components of a stimulus.
Contrary to our expectation, we did not find strong differences between the real and latex stimuli. This lack of differential processing may be due to the design of stimulus delivery. Specifically, the latex hand touch was administered by a person holding the hand, which adds an interpersonal element to the stimulus and renders the latex and real hand condition less different than initially conceptualized. Therefore, future studies will need to parse out this element in order to gain further understanding of different dimensions of affective processing during anticipation of and stimulation by a touch stimulus.
This study examines the affective nature of human touch, and the role the insular cortex plays in anticipating and processing this emotionally significant stimuli. The insular cortex is well suited, both anatomically and functionally, to integrate the sensory and psychological self (Craig, 2002; Paulus and Stein, 2006). Specifically, the anterior inferior insula has an agranular columnar organization, whereas posterior insular is characterized by a granular cortical architecture. This type of transition is found elsewhere in the brain where cortical re-representations are based on modulatory or selective feedback circuits (Shipp, 2005). Furthermore, previous studies have outlined the role of anterior insula in interoceptive processing, emotional awareness (Critchley et al., 2004; Schneider et al., 1997b), and meditation of urges (Evans et al., 2002). Together with our results, it appears that the insular cortex is an important neural substrate for the integration of the affective and sensory aspects of touch. The evaluation of stimulus valence occurs in anterior insula prior to stimulation, and this activity is correlated to that of a more mid-to-posterior region that responds during stimulation, suggesting some type of modulatory relationship between the regions.
The functional division of the insula seen in this study not only provides interesting information on the topography of this region of the brain, but also provides a greater understanding of the temporal processing of an exteroceptive/interoceptive stimulus, as well as the association between anticipatory and affective processes. The role of anterior insula in setting a tone for an emotionally significant experience has strong implications for various social phobias, anxiety disorders, and drug abuse. Altered anticipatory processing, frequently related to greater insular activation, has been found in individuals with social phobia and other anxiety disorders (Chua et al., 1999; Lorberbaum et al., 2004; Nitschke et al., 2006b; Simmons et al., 2006). Also, insular activation has been associated with imagery-induced drug craving (Kilts et al., 2001), and has been implicated in maintaining urge related use of drugs (Naqvi et al., 2007). Recently, Nitschke et al showed that expectancy modulates insular response to unpleasant taste (Nitschke et al., 2006a). Thus, understanding the neural substrates of human touch, beyond providing insight into a basic sensory system, may additionally provide a highly useful probe for different stages of emotionally relevant stimulation to examine whether individuals with mood, anxiety, or addiction disorders show altered anticipation or processing of expectancy modulated stimuli.
Supplementary Material
Time series graphs for insular areas found in the conjunction analysis. The portions of the insula found to be significantly active only in the anticipation phase of the trial become active at the onset of anticipatory cue (t=0), and continues increasing as the stimulus is administered (t = 6). In comparison, the portions of the insula found significantly active in the stimulus phase become active only at the onset of stimulus administration. Finally, the area of the insular cortex found to be significantly active during both anticipation and stimulation becomes active slightly prior to stimulus administration, and continues increasing with stimulus administration.
Distinct regions of the amygdala (a) and anterior cingulate cortex (b) were found to be active during anticipation versus stimulation.
Acknowledgments
This research was supported by grants from NIDA (R01DA016663, R01DA018307) and by a VA Merit Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychol. Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Craig AD. Handbook of Emotion. 3rd ed. The Guilford Press; 2008. Interoception and Emotion: a Neuroanatomical Perspective. [Google Scholar]
- Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error and the future of human life. Sci. Am. 1994;271:144. doi: 10.1038/scientificamerican1094-144. [DOI] [PubMed] [Google Scholar]
- Devries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surg. Radiol. Anat. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J. Neurophysiol. 2002;88:1500–1511. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertenstein MJ, Keltner D, App B, Bulleit BA, Jaskolka AR. Touch communicates distinct emotions. Emotion. 2006;6:528–533. doi: 10.1037/1528-3542.6.3.528. [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics. 1996;5:299–314. [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Knapp ML, Hall JA. Fort Worth, TX: Harcourt Brace College; 1997. Nonverbal communication in verbal interaction. [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol. 2005b;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol. 2005a;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Lahuerta J, Bowsher D, Campbell J, Lipton S. Clinical and instrumental evaluation of sensory function before and after percutaneous anterolateral cordotomy at cervical level in man. Pain. 1990;42:23–30. doi: 10.1016/0304-3959(90)91087-Y. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- Mars RB, Coles MG, Grol MJ, Holroyd CB, Nieuwenhuis S, Hulstijn W, Toni I. Neural dynamics of error processing in medial frontal cortex. Neuroimage. 2005;28:1007–1013. doi: 10.1016/j.neuroimage.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, Kosslyn SM, Rose RM, Davidson RJ. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat. Neurosci. 2006a;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006b;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Norusis MJ. Chicago: SPSS. Inc; 2002. Statistical Package for the Social Sciences. SPSS 11. [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J. Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An Insular View of Anxiety. Biol. Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Robinson SK, Viirre ES, Bailey KA, Gerke MA, Harris JP, Stein MB. Randomized placebo-controlled trial of a selective serotonin reuptake inhibitor in the treatment of nondepressed tinnitus subjects. Psychosom. Med. 2005;67:981–988. doi: 10.1097/01.psy.0000188479.04891.74. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J. Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Grodd W, Weiss U, Klose U, Mayer KR, Nagele T, Gur RC. Functional MRI reveals left amygdala activation during emotion. Psychiatry Res. 1997a;76:75–82. doi: 10.1016/s0925-4927(97)00063-2. [DOI] [PubMed] [Google Scholar]
- Schneider F, Grodd W, Weiss U, Klose U, Mayer KR, Nagele T, Gur RC. Functional MRI reveals left amygdala activation during emotion. Psychiatry Res. 1997b;76:75–82. doi: 10.1016/s0925-4927(97)00063-2. [DOI] [PubMed] [Google Scholar]
- Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos. Trans. R. Soc. Lond B Biol. Sci. 2005;360:797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol. Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Strigo I, Simmons AN, Craig AD, Paulus MP. Breathing and BOLD fMRI: watch out. 2006 [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : a 3-dimensional proportional system, an approach to cerebral imaging. In: Thieme G, editor. New York New York: Thieme Medical Publishers, Stuttgart; 1988. [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J. Physiol. 1995;483:783–795. doi: 10.1113/jphysiol.1995.sp020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Res. Brain Res. Rev. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time series graphs for insular areas found in the conjunction analysis. The portions of the insula found to be significantly active only in the anticipation phase of the trial become active at the onset of anticipatory cue (t=0), and continues increasing as the stimulus is administered (t = 6). In comparison, the portions of the insula found significantly active in the stimulus phase become active only at the onset of stimulus administration. Finally, the area of the insular cortex found to be significantly active during both anticipation and stimulation becomes active slightly prior to stimulus administration, and continues increasing with stimulus administration.
Distinct regions of the amygdala (a) and anterior cingulate cortex (b) were found to be active during anticipation versus stimulation.