Abstract
Aims
Cardiac allograft vasculopathy (CAV) is a major limitation to long-term survival following cardiac transplantation. Conventional imaging modalities such as angiography and intravascular ultrasound fail to characterize CAV plaque morphology. Our aim was to characterize CAV in vivo using the high spatial resolution of intracoronary optical coherence tomography (OCT).
Methods and results
We prospectively enrolled 53 cardiac transplant patients to undergo OCT of the left anterior descending coronary artery (LAD) in addition to annual CAV screening by coronary angiography and intravascular ultrasound (IVUS). The proximal 30 mm of the LAD was divided into three segments of 10 mm each (n = 156). Segments with CAV plaque on IVUS were analysed by OCT for specific CAV morphological characteristics within the framework of three groups according to follow-up time after heart transplantation: (i) 0–3 months (n = 18), (ii) 12–36 months (n = 55), and (iii) ≥48 months (n = 83). The prevalence of atherosclerotic characteristics such as eccentric plaques, calcification, and lipid pools increased from 6, 0, and 6% in group 1 to 78, 42, and 61% in group 3, respectively (all P < 0.001). The prevalence of vulnerable plaque features such as thin-cap fibroatheroma, macrophages, and microchannels increased from 0% in group 1 to 12, 29, and 33% in group 3, respectively (P = 0.19, P = 0.006, and P = 0.003). Complicated coronary lesions such as intimal laceration, intraluminal thrombus, and layered complex plaque increased from 0% in group 1 to 18, 19, and 57% in group 3 (P = 0.009, P < 0.001, and P < 0.001). Plaque rupture was identified in 4% of group 3 segments.
Conclusion
The current study gives new insight into CAV that extends far beyond the current concept of concentric and fibrosing vasculopathy, that is, the development of atherosclerosis with vulnerable plaque and complicated coronary lesions.
Keywords: Cardiac allograft vasculopathy, Transplant, Optical coherence tomography, Atherosclerosis, Vulnerable plaque, Thrombus
Introduction
Cardiac allograft vasculopathy (CAV) is a unique yet quite universal disease process after heart transplantation. In distinction from coronary atherosclerosis, which is marked by focal, eccentric fibro-fatty atheroma, CAV is thought to involve the entire coronary vasculature diffusely with marked intimal proliferation and concentric vascular thickening and fibrosis.1,2 Typically, cardiac transplant recipients do not experience angina but may present with left ventricular dysfunction as a consequence of myocardial ischaemia. Thus, CAV is an important modulator of cardiac allograft function and is the leading cause of morbidity and mortality in heart transplant recipients.3 This underscores the need for early clinical detection and characterization of this prognostically important disease.
Routine surveillance for CAV has been advocated with coronary angiography as the strategy of choice.4 However, angiography provides only a reflection of the luminal dimensions, which can appear relatively preserved even with an extensive burden of vascular disease. Intravascular ultrasound (IVUS) was introduced to assess the coronary vasculature in a cross-sectional manner allowing earlier detection of CAV at the stage of intimal thickening.5 Optical coherence tomography (OCT) is a novel intracoronary imaging technique using an optical analogue of ultrasound which has a resolution of 10–20 μm which is 10 times greater than IVUS. Optical coherence tomography has been shown, using histological controls, to have a sensitivity and specificity of 92 and 91%, respectively, for the detection of lipid-rich plaques in native coronaries6 and can provide more accurate information on plaque characteristics.7,8 Most recently, Garrido et al.9 have compared OCT to IVUS for the measurement of maximal intimal thickness (MIT) and luminal area in 21 patients with CAV showing good correlation but better inter-observer variability with OCT. Hou et al.10 demonstrated higher sensitivity of OCT than IVUS for early detection of CAV in seven patients. The current study was designed to characterize CAV with OCT so as to provide further insight into the disease process with potentially important implications for therapy and clinical outcomes.
Methods
After approval by the Mayo Clinic Institutional Review Board, heart transplant recipients who were scheduled to have routine yearly CAV screening with coronary angiography and IVUS at the Mayo Clinic, Rochester, MN, USA, were approached to obtain informed consent for OCT of the left anterior descending (LAD) coronary artery. All cardiac transplant recipients ≥18 years of age were eligible for study inclusion unless they had chronic kidney disease ≥stage IV (GFR <30 mL/min), active infection, active rejection, or were unable to give informed consent. Between August 31st 2011 and April 26th 2012, a total of 62 subjects were enrolled in this study. One subject did not have OCT due to technical difficulties and eight subjects were excluded from the final analysis because of poor image quality secondary to inadequate blood washout with contrast. The remaining 53 subjects were included in the study and divided into three pre-specified groups according to time after transplant: 0–3 months (group 1, n = 6), 12–36 months (group 2, n = 19), and ≥48 months (group 3, n = 28). Demographic and clinical patients' data were obtained from the medical records. Six-month rejection scores on group 2 and 3 patients were performed to quantify early cellular rejection as previously described.11 The proximal 30 mm of the LAD was analysed and divided longitudinally into three segments of 10 mm each (n = 156).10,12 Segments with CAV plaque on IVUS were analysed by OCT for specific CAV morphological characteristics within the framework of three groups according to follow-up time after heart transplantation: (i) 0–3 months (n = 18), (ii) 12–36 months (n = 55), and (iii) ≥48 months (n = 83).
Image acquisition
After routine coronary angiography and the administration of 100–200 mg of intracoronary nitroglycerine and i.v. heparin for an activated clotting time of 250–300 s, a 0.014 inch guide-wire was advanced into the distal segment of the LAD artery. Intravascular ultrasound images were recorded (s5 IVUS™ system, Volcano Corporation, Rancho Cordova, CA, USA) for offline analyses from the mid-LAD coronary artery (at least 30 mm distal to the ostium of the LAD) to the left main coronary artery with an automated pullback system at a speed of 0.5 mm/s using 2.9-F, 20-MHz, phased array IVUS catheters (Eagle Eye Gold™, Volcano Corporation). Subsequently, the IVUS catheter was replaced with a 2.7F OCT catheter (C7 DragonFly™, St Jude Medical, St Paul, MN, USA). During contrast injection for the clearance of blood (14 mL at 4 mL/s power injection or by manual injection according to operator preference), OCT images (C7-XR™ system, St Jude Medical) were recorded from at least 30 mm distal to the ostium of the LAD to 54 mm proximally at a pull-back speed of 20 mm/s and a frame rate of 100/s and were stored digitally for subsequent offline analysis.
Image interpretation
Cardiac allograft vasculopathy by coronary angiography was defined as any angiographically detected lesion in any part of the coronary tree. It was furthermore classified in accordance with the International Society of Heart & Lung Transplantation (ISHLT) guidelines4 as ISHLT CAV0 (no disease), ISHLT CAV1 (mild disease), ISHLT CAV2 (moderate disease), and ISHLT CAV3 (severe disease).
Offline volumetric analysis of IVUS data was performed by two experienced operators (A.C. and Y.M.) using Volcano Image Analysis Software version 3.1 (Volcano Corporation) as described previously.11,13,14 Automatic detection of both the lumen and media-adventitia interface was corrected manually frame by frame. Maximal intimal thickness was the largest observed distance from the lumen to the external elastic membrane. For each patient, we analysed the first 30 mm of the LAD by starting from the first complete vascular ring of the LAD distal to the bifurcation with the left circumflex artery lumen until 30 mm into the LAD. We then divided this longitudinally into three segments: the first, second, and third 10 mm (0–10, 10–20, and 20–30 mm). Pathological studies on normal subjects have shown that the normal thickness of the coronary artery intima should be <0.3 mm15,16 and thus we used an MIT of ≥0.3 mm which extended over at least 1.5 mm by IVUS as a definition of CAV plaque.17 Quantitative volumetric IVUS greyscale analysis was performed and plaque volume index (vessel volume − lumen volume/length) and per cent plaque burden (plaque volume/vessel volume × 100) were determined for each 10 mm segment and for each patient (30 mm LAD).
Offline analysis of OCT images was performed by two blinded investigators (A.C. and Y.M) using validated software (St Jude Medical/LightLab Imaging, Inc., Westford, MA, USA) and criteria for OCT plaque characterization.7,8,18 Maximal intimal thickness was defined as the distance from the leading edge of the intima to the external elastic membrane.19 For every 10 mm segment of LAD, we diagnosed CAV by OCT if there was an intimal thickness of ≥0.3 mm (or when the external elastic membrane could not be visualized such as in lipid pools, as plaque that disrupted the normal three layers of intima, media, and adventitia) which extended over at least 1.5 mm. For every 10 mm segment of LAD in which CAV was present by IVUS criteria, we further assessed and recorded the presence of the following 10 OCT-based characteristics (Figure 1A–L): (i) eccentric plaque (Figure 1A), (ii) calcification (Figure 1B), (iii) lipid pools (Figure 1C), (iv) thin-cap fibroatheroma (Figure 1D), (v) macrophage image (Figure 1E), (vi) microchannels (Figure 1F), (vii) intimal laceration (Figure 1G), (viii) plaque rupture (Figure 1H), (ix) intraluminal thrombus (Figure 1I and J), and (x) layered complex plaque (Figure 1K and L).
Figure 1.
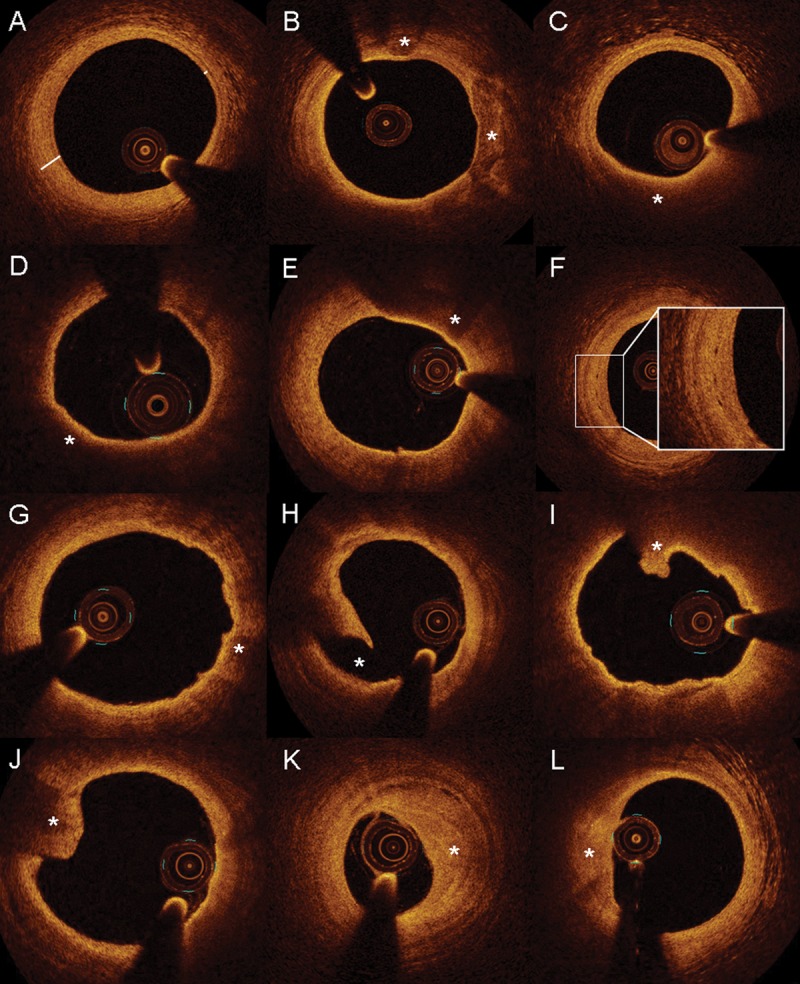
Morphological characteristics of cardiac allograft vasculopathy by optical coherence tomography. Optical coherence tomography images revealing atherosclerotic plaque characteristics (A, eccentric intimal hyperplasia; B, calcification; and C, lipid pool) with vulnerable plaque features (D, thin-cap fibroatheroma; E, macrophage image; and F, Microchannels) as well as complicated lesions (G, intimal laceration; H, plaque rupture; I–J, intraluminal thrombus; and K–L, layered complex plaque).
Eccentric plaque was defined as the minimal intimal thickness/MIT ratio of <0.5 or lipid pool in ≥1 quadrants with normal intimal thickness (<0.3 mm) in the other quadrants (Figure 1A). Heterogenous signal poor and signal-rich regions which were sharply delineated with low attenuation were considered calcium depositions (Figure 1B). Lipid pools were defined as homogenous, diffusely bordered, signal poor regions with overlying signal-rich bands (corresponding to a fibrous cap) (Figure 1C). Thin-cap fibroatheroma was defined as a large lipid pool (two or more quadrants) covered with a thin (<65 micrometre) fibrous cap (Figure 1D). Macrophage image was defined as signal-rich regions on plaque surfaces accompanied by attenuation (Figure 1E). Microchannels were defined as sharply delineated signal-poor tubuloluminal structures that could be followed on three consecutive frames (Figure 1F). Intimal laceration was characterized by severe irregularity of the superficial intimal lining without frank rupture (Figure 1G). Plaque rupture was defined as disruption of the intimal fibrous cap with an underlying empty cavity (Figure 1H). Intraluminal thrombus was defined as a protruding mass attached to the luminal surface (Figure 1I and J). Layered complex plaque was defined as a heterogenous signal region in the intima in a layered pattern with low attenuation. The luminal surface was concave and could be smooth or irregular and the abluminal aspect frequently had microchannels (Figure 1K and L).
Statistical analysis
Continuous variables are presented as means ± standard deviation or median (inter-quartile range) as appropriate and discrete variables are summarized as frequency (group percentage). Comparisons between groups were made using the analysis of variance or Kruskal–Wallis test for continuous variables and Fisher's exact test for discrete variables. McNemar's test was used to test whether CAV was diagnosed more often with one modality over another. A Bland–Altman plot was used to test correlation between OCT and IVUS for MIT. Linear or logistic regression was used to test associations after adjusting for years after transplant. The Kappa statistic was used to define the level of intra- and inter-observer agreement. All tests are two-tailed with a 0.05 significance level. Analyses were completed with JMP9 software (SAS Institute, Inc., Cary, NC, USA).
Results
The patient characteristics are shown in Table 1. The population was predominantly Caucasian with an average age of 53 years. More than two-thirds of the subjects were male. The most common reason for heart transplantation was dilated cardiomyopathy followed by ischaemic cardiomyopathy. Patient groups by time from heart transplantation did not differ in demographics, donor age, or cold ischaemic time. Differences in calcineurin inhibitor and sirolimus usage are explained by our practice to change heart transplant patients from a calcineurin inhibitor to sirolimus after 6 months. Aspirin use was low (26%) and statin use increased with time from transplant from 67% in group 1 to 96.4% in group 3.
Table 1.
Patient characteristics
| Variable | All (n = 53) | Group 1 (n = 6) | Group 2 (n = 19) | Group 3 (n = 28) | P-value |
|---|---|---|---|---|---|
| Age, years | 52.8 ± 13.8 | 48.3 ± 16.1 | 50.4 ± 12.6 | 55.4 ± 14.1 | 0.33 |
| Male, n (%) | 38 (71.7) | 3 (50.0) | 14 (73.7) | 21 (75.0) | 0.46 |
| BMI, kg/m2 | 28.4 ± 4.4 | 29.2 ± 3.7 | 29.5 ± 4.4 | 27.4 ± 4.4 | 0.25 |
| Caucasian, n (%) | 51 (96.3) | 5 (83.3) | 18 (94.7) | 28 (100) | 0.09 |
| Reason for transplant, n (%) | 0.85 | ||||
| Ischaemic CM | 11 (20.8) | 2 (33.3) | 2 (10.5) | 7 (25) | |
| Dilated CM | 27 (50.9) | 3 (50.0) | 11 (57.9) | 13 (46.4) | |
| Restrictive CM | 6 (11.3) | 0 (0.0) | 3 (15.8) | 3 (10.7) | |
| Other (hypertrophic CM, valvular, congenital) | 9 (17.0) | 1 (16.7) | 3 (15.8) | 5 (17.9) | |
| Years from Tx, median (Q1,Q3) | 4.0 (1.0, 9.0) | 0.0 (0.0, 0.0) | 2.0 (1.0, 2.0) | 9 (6.0, 13.8) | |
| Donor age, years | 31.2 ± 13.1 | 27.5 ± 5.7 | 29.8 ± 11.6 | 33.1 ± 15.2 | 0.55 |
| Cold ischaemic time, min | 186.7 ± 41.5 | 201.3 ± 24.8 | 181.7 ± 41.6 | 187.0 ± 44.1 | 0.60 |
| Renal Insufficiency, n (%) | 24 (45.3) | 2 (33.3) | 8 (42.1) | 14 (50.0) | 0.79 |
| Hypertension, n (%) | 44 (83.0) | 3 (50.0) | 16 (84.2) | 25 (89.3) | 0.08 |
| Hyperlipidaemia, n (%) | 50 (94.3) | 5 (83.3) | 17 (89.5) | 28 (100) | 0.12 |
| Diabetes Mellitus, n (%) | 18 (34.0) | 1 (16.7) | 7 (36.8) | 10 (35.7) | 0.84 |
| Smoking, n (%) | 0.09 | ||||
| Never | 23 (43.4) | 4 (66.7) | 6 (31.6) | 13 (46.4) | |
| Past | 28 (52.8) | 1 (16.7) | 12 (63.2) | 15 (53.6) | |
| Current | 2 (3.8) | 1 (16.7) | 1 (5.3) | 0 (0.0) | |
| Cytomegalovirus viraemia, n (%) | 11 (20.8) | 1 (16.7) | 4 (21.1) | 6 (21.4) | 1.00 |
| ACE inhibitors, n (%) | 21 (39.6) | 0 (0.0) | 8 (42.1) | 13 (46.4) | 0.12 |
| Statins, n (%) | 46 (86.8) | 4 (66.7) | 15 (79.0) | 27 (96.4) | 0.06 |
| Aspirin, n (%) | 14 (26.4) | 1 (16.7) | 3 (15.8) | 10 (35.7) | 0.31 |
| Azathioprine, n (%) | 10 (18.9) | 0 (0.0) | 1 (5.3) | 9 (32.1) | 0.037 |
| Mycophenolate mofetil, n (%) | 38 (71.7) | 5 (83.3) | 18 (94.7) | 15 (53.6) | 0.005 |
| Calcineurin inhibitor, n (%) | 22 (41.5) | 6 (100) | 6 (31.6) | 10 (35.7) | 0.008 |
| Sirolimus, n (%) | 29 (54.7) | 0 (0.0) | 11 (57.9) | 18 (64.3) | 0.014 |
| LV ejection fraction, % | 62.6 ± 5.7 | 63.5 ± 4.5 | 63.4 ± 7.3 | 61.9 ± 4.5 | 0.60 |
ACE, angiotensin converting enzyme; BMI, body mass index; CM, cardiomyopathy; LV, left ventricular; Tx, transplant.
The characteristics of CAV by angiography, IVUS, and OCT per patient analysis are shown in Table 2 and Figure 2. More patients were diagnosed with CAV by OCT than by angiography (72 vs. 47%, P < 0.001) but OCT was not superior to IVUS in this regard (both 72%, with 100% agreement for diagnosing CAV using definitions above). The ISHLT score of 1 by coronary angiography included patients with a wide range of CAV volume indexes on IVUS [1.7–12.2 mm3/mm, median 7.7 (5.2, 9.6) mm3/mm].
Table 2.
Cardiac allograft vasculopathy by angiography and intravascular ultrasound per patient analysis
| Variable | All (n = 53) | Group 1 (n = 6) | Group 2 (n = 19) | Group 3 (n = 28) | P-value |
|---|---|---|---|---|---|
| Coronary angiography | |||||
| CAV by angiography, n (%) | 25 (47.2) | 0 (0.0) | 8 (42.1) | 17 (60.7) | 0.023 |
| CAV ISHLT score | 0.19 | ||||
| ISHLT score 0, n (%) | 28 (52.8) | 6 (100) | 11 (57.9) | 11 (39.3) | |
| ISHLT score 1, n (%) | 19 (35.9) | 0 (0.0) | 7 (36.8) | 12 (42.9) | |
| ISHLT score 2, n (%) | 4 (7.6) | 0 (0.0) | 1 (5.3) | 3 (10.7) | |
| ISHLT score 3, n (%) | 2 (3.8) | 0 (0.0) | 0 (0.0) | 2 (7.1) | |
| IVUS | |||||
| CAV by IVUS, n (%) | 38 (71.7) | 1 (16.7) | 11 (57.9) | 26 (92.9) | <0.001 |
| MIT by IVUS, mm | 0.9 (0.0, 1.3) | 0.0 (0.0, 0.1) | 0.5 (0.0, 1.1) | 1.1 (0.7, 1.4) | <0.001 |
| Vessel volume index (mm3/mm) | 15.9 (14.2, 19.5) | 15.8 (14.9, 15.8) | 17.9 (14.4, 19.4) | 16.4 (13.8, 19.8) | 0.73 |
| Lumen volume index (mm3/mm) | 11.5 (8.6, 13.6) | 13.6 (12.6, 13.8) | 12.4 (10.1, 14.4) | 9.7 (7.0, 14.8) | 0.015 |
| Plaque volume index (mm3/mm) | 4.4 (2.1, 8.1) | 2.1 (1.8, 2.3) | 2.4 (1.9, 6.5) | 6.6 (3.8, 9.4) | 0.005 |
| Plaque burden, % | 26.6 (14.5, 45.0) | 13.0 (12.5, 14.6) | 20.9 (11.9, 32.5) | 38.3 (25.8, 49.1) | <0.001 |
CAV, cardiac allograft vasculopathy; ISHLT, International Society of Heart and Lung Transplantation; IVUS, intravascular ultrasound; MIT, maximal intimal thickness.
Figure 2.
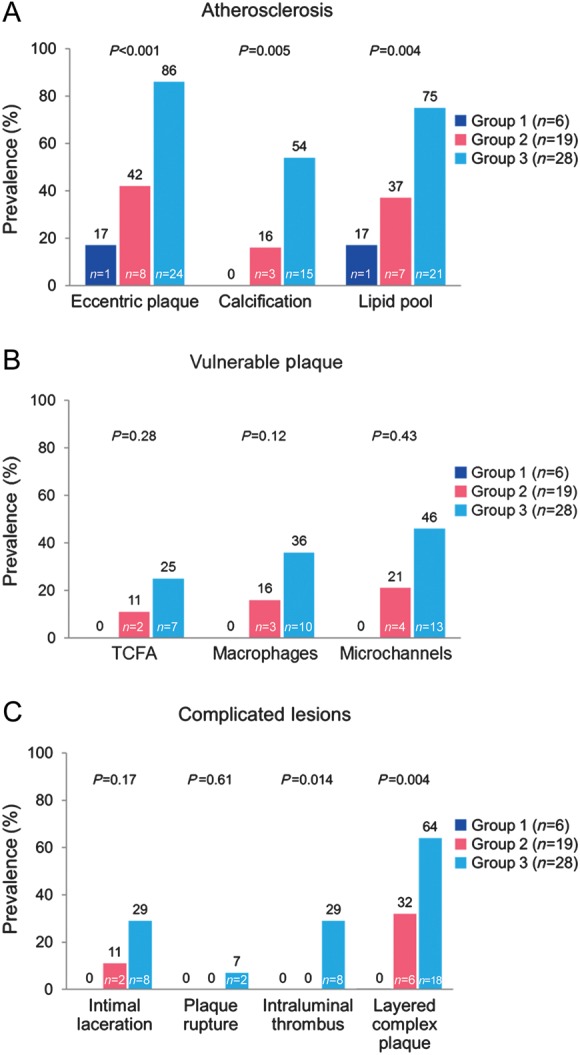
Prevalence of cardiac allograft vasculopathy morphological characteristics by optical coherence tomography with time from transplantation per patient analysis. (A) Atherosclerotic characteristics, (B) vulnerable plaque and (C) complicated lesions. TCFA, thin-cap fibroatheroma.
The characteristics of CAV by IVUS and OCT per segment analysis are shown in Table 3 and Figure 3. There was very strong correlation (r = 0.85) between OCT and IVUS measurements of MIT (mean difference −0.05 ± 0.03 mm, P = 0.10) in segments where measurements could be taken in both modalities (n = 36). Cardiac allograft vasculopathy provided important additional information on the pre-specified 10 CAV characteristics. All 10 CAV characteristics by OCT were significantly (all P < 0.001) associated with increasing CAV volume index by IVUS. There were no differences by OCT in the prevalence of CAV or any of the 10 characteristics between the first, second, and third 10 mm segments. The prevalence of atherosclerotic characteristics such as eccentric plaques, calcification, and lipid pools increased from 6, 0, and 6% in group 1 to 78, 42, and 61% in group 3, respectively (all P < 0.001). The prevalence of vulnerable plaque features such as thin-cap fibroatheroma, macrophages, and microchannels increased from 0% in group 1 to 12, 29 and 33% in group 3, respectively (P = 0.19, P = 0.006, and P = 0.003). Complicated coronary lesions such as intimal laceration, intraluminal thrombus, and layered complex plaque increased from 0% in group 1 to 18, 19 and 57% in group 3 (P = 0.009, P < 0.001, and P < 0.001). Plaque rupture was identified in 4% of group 3 segments. The median number of cross-sectional slices with macrophages and microchannels per 10 mm segment (50 slices) was 9.0 (6.0, 17.8) and 12.0 (6.0, 20.0), respectively. Kappa statistic for intra-observer and inter-observer variability for macrophage images was 0.92 and 0.87, respectively.
Table 3.
Cardiac allograft vasculopathy by intravascular ultrasound per segment analysis
| Variable | All (n = 156) | Group 1 (n = 18) | Group 2 (n = 55) | Group 3 (n = 83) | P-value |
|---|---|---|---|---|---|
| CAV by IVUS, n (%) | 106 (68.0) | 2 (11.1) | 30 (54.6) | 74 (89.2) | <0.001 |
| 1st segment (0–10 mm)a | 37 (72.6) | 1 (16.7) | 10 (55.6) | 26 (93.3) | <0.001 |
| 2nd segment (10–20 mm)a | 36 (67.9) | 1 (16.7) | 10 (52.6) | 25 (89.3) | <0.001 |
| 3rd segment (20–30 mm)a | 33 (63.5) | 0 (0.0) | 10 (55.6) | 23 (82.1) | <0.001 |
| MIT by IVUS, mm | 0.6 (0.0, 1.1) | 0.0 (0.0, 0.0) | 0.3 (0.0, 0.9) | 0.9 (0.4, 1.2) | <0.001 |
| Vessel volume index (mm3/mm) | 16.6 (13.5, 19.7) | 14.6 (13.7, 17.1) | 17.0 (14.2, 19.4) | 16.7 (13.4, 20.4) | 0.27 |
| Lumen volume index (mm3/mm) | 11.2 (8.7, 13.5) | 12.9 (11.8, 14.8) | 12.0 (10.1, 14.6) | 9.7 (6.7, 12.7) | <0.001 |
| Plaque volume index (mm3/mm) | 3.9 (2.0, 8.7) | 2.1 (1.7, 2.4) | 2.8 (1.9, 6.4) | 6.4 (3.5, 9.7) | <0.001 |
| Plaque burden, % | 25.8 (14.1, 44.4) | 12.4 (12.2, 14.3) | 18.3 (12.0, 34.0) | 37.5 (24.4, 49.1) | <0.001 |
CAV, cardiac allograft vasculopathy; IVUS, intravascular ultrasound; MIT, maximal intimal thickness.
a1st segment (0–10 mm), n = 51, 6, 18, 27; 2nd segment (10–20 mm), n = 53, 6, 19, 28; 3rd segment (20–30 mm), n = 52, 6, 18, 28 for all, group 1, group 2, and group 3, respectively.
Figure 3.
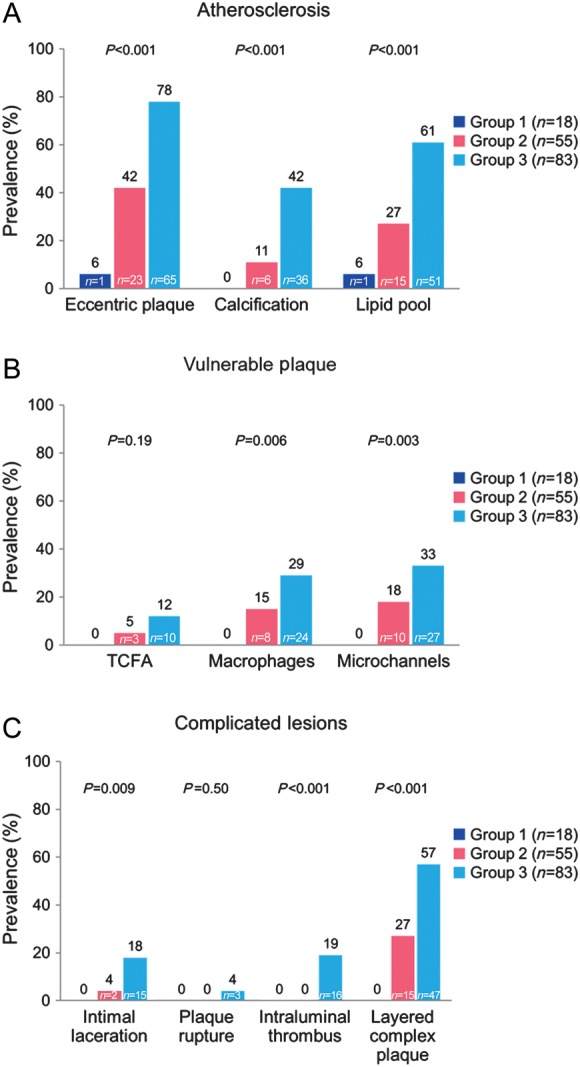
Prevalence of CAV morphological characteristics by optical coherence tomography with time from transplantation per segment analysis. (A) Atherosclerotic characteristics, (B) vulnerable plaque, and (C) complicated lesions. TCFA, thin-cap fibroatheroma.
Complicated lesions were associated with vulnerable plaque features as follows. Lesions with intimal laceration were more likely to have a thin-cap fibroatheroma (P < 0.001) or a macrophage image (P < 0.001) than those without. Microchannels and macrophages were identified more frequently in lesions with layered complex plaque compared with those without (both P < 0.001).
In lesions with intraluminal thrombus (n = 16) (Figure 1I and J), two lesions (12.5%) had OCT evidence of plaque rupture (Figure 1H) and the other 14 patients (87.5%) had no evidence of plaque rupture and are presumed to have had plaque erosion.7,8 Of these 14 lesions with presumed plaque erosion, 9 lesions (56.3%) had OCT images in keeping with intimal laceration (Figure 1G). All the patients with intraluminal thrombus (n = 8) also had evidence of layered complex plaque.
There was no statistically significant difference in the CAV volume index by IVUS [7.7 (2.0, 11) mm3 vs. 4.3 (2.1, 7.5) mm3, P = 0.30] between patients who were transplanted for ischaemic cardiomyopathy and patients who had other indications for heart transplantation. However, patients transplanted for ischaemic cardiomyopathy had a higher prevalence of intraluminal thrombus by OCT (36.4 vs. 9.5%, P = 0.048) even after adjusting for years after transplantation (P = 0.019).
Patients diagnosed with CAV by OCT had a higher mean transplant donor age than patients without CAV (34.8 ± 13.7 vs. 22.8 ± 5.3 years, P = 0.002) which remained significant after adjustment for years after transplantation (P = 0.002). Left ventricular ejection fraction by echocardiography was significantly lower in patients with than without CAV (61.6 ± 6.1 vs. 65.1 ± 3.1%, P = 0.043). There was no association between 6-month rejection score and CAV or macrophage image (P = 0.20 and P = 0.33, respectively) even after adjustment for years after transplantation (P = 0.28 and P = 0.59, respectively). There was no association between immunosuppressive agent (calcineurin inhibitor vs. sirolimus) and CAV or macrophage image (14 vs. 24%, P = 0.13 and 23 vs. 26%, P = 0.80, respectively) even after adjustment for years after transplantation (P = 0.63 and P = 0.77, respectively).
Discussion
The current study is the largest study using high resolution OCT imaging to characterize the presence of CAV characteristics in heart transplant patients. Optical coherence tomography reveals CAV disease aspects that extend far beyond the current concept of diffuse concentric and fibrosing vasculopathy. Features typical of atherosclerosis such as eccentric coronary artery lesions, calcification, and lipid pools were observed. Moreover, vulnerable lesion features such as thin-cap fibroatheroma, macrophages and microchannels were identified and complicated coronary lesions such as layered complex plaque and intraluminal thrombus became increasingly prevalent with time from heart transplantation. Intraluminal thrombi were more commonly associated with intimal laceration (possibly due to plaque erosion) rather than plaque rupture. These novel findings challenge the current pathophysiological concept of CAV and might have important implications for the management of heart transplant patients.
The longitudinal diffuse nature of CAV was confirmed by OCT in this study as there were no differences in the prevalence of CAV or any of its characteristics between the first, second, or third 10 mm segments in the LAD. However, while diffuse concentric fibrotic changes were noted in some segments of the examined coronaries, in patients ≥48 months after transplantation, classic characteristics of atherosclerosis such as eccentric plaques, calcification and lipid pools were highly prevalent. Furthermore, OCT images possibly indicative of plaque vulnerability were also present. These features include thin-cap fibroatheroma, which started to emerge ≥12 months after heart transplantation. Another characteristic of such ‘‘vulnerable’’ lesions is the macrophage image. Optical coherence tomography can identify a high burden of macrophage infiltration and in this study we found this to be present in nearly 30% of segments ≥48 months out from heart transplantation. Macrophages can influence vascular stability in many ways (cytokine production for matrix deposition or proteolytic enzyme production for intimal erosion) and are of key interest at the interface of innate and adaptive immune response. Optical coherence tomography studies on native coronary artery disease have shown that the macrophage image is associated with plaque instability20,21 and plaque progression.18 Our study showed an association of macrophages with both intimal laceration as well as complex layered plaque. Furthermore, plaque progression and vulnerability in native atherosclerosis has been associated with prominent plaque neovascularization18,22 which may supply inflammatory cells, cytokines or result in microvascular leakage into the plaque. Optical coherence tomography is unique in its capability to visualize microchannels7,8 which were present in one-third of segments ≥48 months after transplantation. This study also found an association between microchannels and layered complex plaque confirming the importance of neovascularization in advanced atherosclerotic disease.
A disintegration-thrombosis theory for CAV similar to that in native atherosclerosis can be entertained further based on the observation of intraluminal thrombus on OCT.23 Histologically, intraluminal thrombosis is most likely due to plaque erosion or plaque rupture.24 Most lesions with intraluminal thrombosis also had OCT images in keeping with intimal laceration which was defined as severe irregularity of the superficial intimal lining without frank rupture. It is possible that not all plaque erosions can be imaged by OCT due to the spatial resolution limitation which is less than one endothelial cell dimension. Plaque rupture remains another possibility, and indeed, was observed in the current study as well but less commonly. These observations would suggest that the prevailing mechanism of coronary artery thrombosis in CAV may be plaque erosion. This is in contrast to native coronary atherosclerosis, in which OCT has shown plaque rupture to be more common than plaque erosion in patients with acute coronary syndrome.23
Of particular interest is the visualization of multi-layer patterns within areas of intimal thickening by OCT, which we describe as layered complex plaque. These lesions became highly prevalent in patients ≥48 months from transplantation and are in keeping with alternating layers of elastin and dense collagen enriched by smooth muscle cells and proteoglycans, a pathological hallmark of repeated healed intimal erosions after thrombosis as described by Virmani et al.25 Indeed, the same group proposed repeated thrombosis (in the setting of plaque rupture) as a cause of coronary atherosclerosis progression based on pathological specimens of men with sudden cardiac death.26 Autopsy studies in coronaries of transplanted hearts by Arbustini et al.27,28 confirmed a high prevalence (63–83%) of coronary thrombi, of which 77% were non-occlusive mural thrombus. These mural thrombi were layered on discontinuous or absent endothelium without atheromatous lesions, suggesting repeated plaque erosion as the likely underlying mechanism in CAV.29 We believe that the current study lends support for those pathological observations for the very first time in humans in vivo, made possible by the high spatial resolution of OCT.
The current observations may have important clinical implications. If, indeed, CAV has similarities to atherosclerosis with thrombosis as a key element, the role of antiplatelet therapy needs to be evaluated for the management of heart transplant patients with CAV for prevention of cardiac events. Also, there is increasing prevalence of lipid pools with time from transplant indicating the importance of intensive lipid-lowering therapy in this patient population.30
Study limitations
This study has several limitations including the small study population and cross-sectional design. Longitudinal follow-up of patients would provide more information for the progressive nature of the disease. Immunosuppressive agent use differed among groups with time after transplantation. We acknowledge the lack of direct correlation of OCT with histology in this study allowing for possible misinterpretation or overinterpretation of plaque characteristics such as discerning calcifications from lipid pools.31 Assessment of macrophage image lacks validation studies without use of dedicated algorithms on raw OCT data.7 The sensitivity of OCT falls below the dimension of individual endothelial cells and therefore the diagnosis of plaque erosion cannot be based on the direct visualization of loss of the endothelial layer but rather as lack of fibrous cap rupture identification beneath thrombus by OCT.7,8,29,32 Furthermore, thrombi may reduce the ability to assess underlying structures. Optical coherence tomography is an ideal imaging tool for tissue characterization but has limited penetration depth making total vasculopathy volume as well as deep plaque features (such as beyond lipid pools) difficult to assess. We used IVUS as a complementary imaging tool in this study to overcome this limitation. Limited penetration depth can result in thick fibrous plaques having similar appearance to lipid pools but all lipid pools in our study had cap thickness <300 μm (mean 130 ± 65 μm) making overdiagnosis of lipid pools unlikely.
Conclusions
The current study gives new insight into CAV with OCT that extends far beyond the current concept of concentric and fibrosing vasculopathy, that is, the development of atherosclerosis with vulnerable plaque and complicated coronary lesions such as intraluminal thrombus. This might have important implications for the management of heart transplant patients.
Funding
The work was supported by the National Institute of Health (HL92954, AG31750, HL77131 and HL085307) and the National Center for Advancing Translational Sciences (UL1 TR000135). St Jude Medical, St Paul, Minnesota provided the OCT catheters for the study.
Conflict of interest: none declared.
Acknowledgements
We would like to thank Ms Jasmine A. Sexton and Ms Rebecca E. Nelson for study co-ordination as well as Ms Jonella M. Tilford and Ms Cynthia D. Miller for imaging technical support.
References
- 1.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–815. doi: 10.1161/01.RES.0000246086.93555.f3. doi:10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 2.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. doi:10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 3.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The registry of the international society for heart and lung transplantation: 29th official adult heart transplant report-2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. doi:10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. doi:10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Torres HJ, Merello L, Ramos SA, Aninat MA, Becerra LE, Mora AD, Valenzuela PT, Godoy MJ, Prieto AA. Prevalence of cardiac allograft vasculopathy assessed with coronary angiography versus coronary vascular ultrasound and virtual histology. Transpl Proc. 2011;43:2318–2321. doi: 10.1016/j.transproceed.2011.06.002. doi:10.1016/j.transproceed.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. doi:10.1161/01.CIR.0000029927.92825.F6. [DOI] [PubMed] [Google Scholar]
- 7.Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. doi:10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 8.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudeck D, Falk E, Feldman MD, Fitzgerald P, Garcia H, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonada S, Suter M, Takarada S, Tanaka A, Terashima M, Troels T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. doi:10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 9.Garrido IP, Garcia-Lara J, Pinar E, Pastor-Perez F, Sanchez-Mas J, Valdes-Chavarri M, Pascual-Figal DA. Optical coherence tomography and highly sensitivity troponin T for evaluating cardiac allograft vasculopathy. Am J Cardiol. 2012;110:655–661. doi: 10.1016/j.amjcard.2012.04.047. doi:10.1016/j.amjcard.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Hou J, Lv H, Jia H, Zhang S, Xing L, Liu H, Kong J, Yu B, Jang IK. OCT assessment of allograft vasculopathy in heart transplant recipients. JACC Cardiovasc Imaging. 2012;5:662–663. doi: 10.1016/j.jcmg.2012.01.018. doi:10.1016/j.jcmg.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Raichlin E, Bae JH, Kushwaha SS, Lennon RJ, Prasad A, Rihal CS, Lerman A. Inflammatory burden of cardiac allograft coronary atherosclerotic plaque is associated with early recurrent cellular rejection and predicts a higher risk of vasculopathy progression. J Am Coll Cardiol. 2009;53:1279–1286. doi: 10.1016/j.jacc.2008.12.041. doi:10.1016/j.jacc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Wykrzykowska JJ, Mintz GS, Garcia-Garcia HM, Maehara A, Fahy M, Xu K, Inguez A, Fajadet J, Lansky A, Templin B, Zhang Z, de Bruyne B, Weisz G, Serruys PW, Stone GW. Longitudinal distribution of plaque burden and necrotic core-rich plaques in nonculprit lesions of patients presenting with acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S10–S18. doi: 10.1016/j.jcmg.2012.01.006. doi:10.1016/j.jcmg.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Raichlin E, Bae JH, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, Rihal C, Lerman A, Kushwaha SS. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–2733. doi: 10.1161/CIRCULATIONAHA.107.692996. doi:10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 14.Topilsky Y, Hasin T, Raichlin E, Boilson BA, Schirger JA, Pereira NL, Edwards BS, Clavell AL, Rodeheffer RJ, Frantz RP, Maltais S, Park SJ, Daly RC, Lerman A, Kushwaha SS. Sirolimus as primary immunosuppression attenuates allograft vasculopathy with improved late survival and decreased cardiac events after cardiac transplantation. Circulation. 2012;125:708–720. doi: 10.1161/CIRCULATIONAHA.111.040360. doi:10.1161/CIRCULATIONAHA.111.040360. [DOI] [PubMed] [Google Scholar]
- 15.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, Young JB, Nissen SE. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. doi:10.1161/01.CIR.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 16.Velican D, Velican C. Comparative study on age-related changes and atherosclerotic involvement of the coronary arteries of male and female subjects up to 40 years of age. Atherosclerosis. 1981;38:39–50. doi: 10.1016/0021-9150(81)90102-7. doi:10.1016/0021-9150(81)90102-7. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. doi:10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 18.Uemura S, Ishigami K, Soeda T, Okayama S, Sung JH, Nakagawa H, Somekawa S, Takeda Y, Kawata H, Horii M, Saito Y. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33:78–85. doi: 10.1093/eurheartj/ehr284. doi:10.1093/eurheartj/ehr284. [DOI] [PubMed] [Google Scholar]
- 19.Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary intima–media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J. 2005;69:903–907. doi: 10.1253/circj.69.903. doi:10.1253/circj.69.903. [DOI] [PubMed] [Google Scholar]
- 20.MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, Yabushita H, Shishkov M, Kauffman CR, Houser SL, Aretz HT, DeJoseph D, Halpern EF, Tearney GJ. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004;44:972–979. doi: 10.1016/j.jacc.2004.05.066. doi:10.1016/j.jacc.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 21.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. doi:10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 22.Kitabata H, Tanaka A, Kubo T, Takarada S, Kashiwagi M, Tsujioka H, Ikejima H, Kuroi A, Kataiwa H, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol. 2010;105:1673–1678. doi: 10.1016/j.amjcard.2010.01.346. doi:10.1016/j.amjcard.2010.01.346. [DOI] [PubMed] [Google Scholar]
- 23.Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. doi:10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 24.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. doi:10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 25.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. doi:10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 26.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. doi:10.1161/01.CIR.103.7.934. [DOI] [PubMed] [Google Scholar]
- 27.Arbustini E, Dal Bello B, Morbini P, Gavazzi A, Specchia G, Vigano M. Multiple coronary thrombosis and allograft vascular disease. Transpl Proc. 1998;30:1922–1924. doi: 10.1016/s0041-1345(98)00526-0. doi:10.1016/S0041-1345(98)00526-0. [DOI] [PubMed] [Google Scholar]
- 28.Arbustini E, Dal Bello B, Morbini P, Grasso M, Diegoli M, Fasani R, Pilotto A, Bellini O, Pellegrini C, Martinelli L, Campagna C, Gavazzi A, Specchia G, Vigano M, Roberts WC. Frequency and characteristics of coronary thrombosis in the epicardial coronary arteries after cardiac transplantation. Am J Cardiol. 1996;78:795–800. doi: 10.1016/s0002-9149(96)00424-9. doi:10.1016/S0002-9149(96)00424-9. [DOI] [PubMed] [Google Scholar]
- 29.Arbustini E, Dal Bello B, Morbini P, Gavazzi A, Specchia G, Vigano M. Immunohistochemical characterization of coronary thrombi in allograft vascular disease. Transplantation. 2000;69:1095–1101. doi: 10.1097/00007890-200003270-00013. doi:10.1097/00007890-200003270-00013. [DOI] [PubMed] [Google Scholar]
- 30.Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Krobot K, Steinbeck G, Seidel D, Reichart B. Simvastatin initiated early after heart transplantation: 8-year prospective experience. Circulation. 2003;107:93–97. doi: 10.1161/01.cir.0000043241.32523.ee. doi:10.1161/01.CIR.0000043241.32523.EE. [DOI] [PubMed] [Google Scholar]
- 31.Sawada T, Shite J, Garcia-Garcia HM, Shinke T, Watanabe S, Otake H, Matsumoto D, Tanino Y, Ogasawara D, Kawamori H, Kato H, Miyoshi N, Yokoyama M, Serruys PW, Hirata K. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J. 2008;29:1136–1146. doi: 10.1093/eurheartj/ehn132. doi:10.1093/eurheartj/ehn132. [DOI] [PubMed] [Google Scholar]
- 32.Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122:2505–2513. doi: 10.1161/CIRCULATIONAHA.110.955302. doi:10.1161/CIRCULATIONAHA.110.955302. [DOI] [PubMed] [Google Scholar]


