The ability to learn which stimuli in the environment pose a threat is critical for adaptive functioning. Visual stimuli that are associated with threat when they are consciously perceived can evoke physiological [1] and neural [2] responses consistent with fear arousal even when they are later suppressed from awareness. It remains unclear, however, whether a specific new fear association can be acquired for stimuli that are never consciously seen [3], and whether such acquisition develops differently from conscious learning. It has recently been suggested [4] that, rather than simply affording a degraded version of conscious experience, processing of emotional stimuli without awareness may differ qualitatively from conscious perception, evoking different patterns of neural activity across the brain or differences in the time-course of behavioral and physiological responses. Here, we investigated nonconscious fear acquisition and how it may differ from conscious learning using classical fear conditioning, and found that conscious and unconscious fear acquisition both occur, but evolve differently over time.
We presented observers with monocular conditioned stimuli (CSs, a male and female fearful face) that could be suppressed from awareness for long durations (4 seconds) by salient dynamic stimulation of the other eye (continuous flash suppression, CFS; Figure 1A). One image (CS+) co-terminated with a mild shock to the wrist on 50% of its presentations; the other (CS−) was never paired with shock. (We define fear in this context as an anticipatory physiological response to a stimulus that predicts an aversive outcome. This is measured by phasic increases in skin conductance responses (SCRs), which arise from autonomic nervous system arousal.)
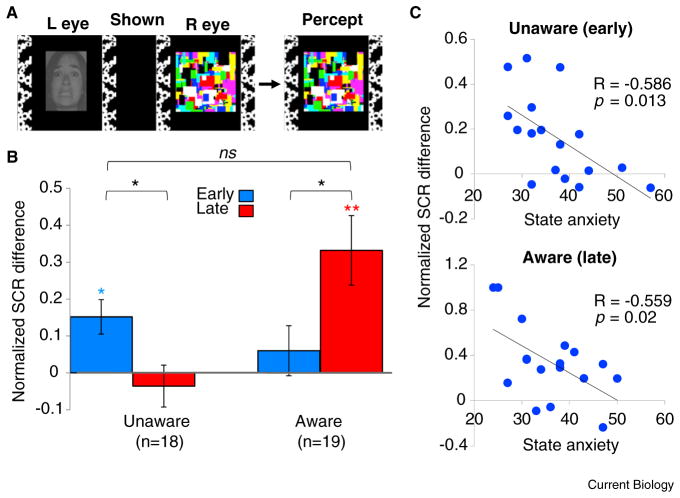
Figure 1. Results of fear learning experiments.
(A) The continuous flash suppression (CFS) display. The aware group saw the same display, without the colorful, dynamic suppressor. (B) Normalized SCR differences for the aware and unaware groups. *p < 0.01; **p < 0.001; ns, not significant; blue/red asterisks, comparison with zero; black asterisks, comparison between indicated bars. (C) Correlations between state anxiety and fear learning during the stage at which learning occurred for each group.
We assessed fear learning using the normalized difference between average SCRs evoked by the CS+ and CS− (see Supplemental Information available on-line with this issue for detailed experimental procedures). Critically, we measured SCRs during early and late acquisition (first versus second half of all non-reinforced trials) to track the development of learning over time [5]. Two groups of participants were conditioned with identical CSs: for one, CFS was used on all trials, suppressing the CSs from awareness (unaware group), whereas for the other CFS was never used (aware group).
To verify successful manipulation of awareness, after each trial participants were asked to indicate which face had been presented, and to rate their confidence from 1 (guess) to 3 (sure). We reasoned that objective (chance-level, 50% identification) and subjective (reported guessing) unawareness, coupled with physiological conditioning (greater SCR to the CS+ than CS−) would indicate nonconscious fear learning. Indeed, the unaware group’s performance (46%) was at chance; participants reported guessing on nearly all trials, and their confidence ratings did not differ between correct (M = 1.09) and incorrect (M = 1.06) responses (p = 0.73). For the aware group, performance was nearly perfect (97%) and confidence was high (M = 2.90; see also Supplemental Information for detailed results and statistical analyses).
We found significantly greater SCRs to the CS+ compared with the CS− in both groups. However, we observed a striking difference in the temporal pattern of these physiological responses. Consistent with previous research [5], learning in the aware group increased over time, only becoming significant during late acquisition. In contrast, participants conditioned without awareness showed significant learning only during early acquisition (Figure 1B, and Supplemental Figure S1) — fear was thus acquired rapidly, but was also quick to decline. Furthermore, at the stage in which learning occurred, the difference between groups in magnitude of learning did not reach significance (p = 0.09).
The groups did, however, differ in the pattern of responses to each CS across the experiment: In the unaware group, there was a specific reduction in the initially large average response to the CS+, whereas average SCRs to the CS− remained consistent across the session’s two halves. Conversely, for the aware group there was a reduction in average responses to the CS−, but not the CS+ (Figure S1). Both groups thus showed conditioning, but differed in the pattern of response to each CS. This additional qualitative difference suggests that the pattern underlying fear-conditioning, rather than just the difference between CS+ and CS−, may be revealing in itself: awareness may allow inhibition of arousal responses to stimuli that predict safety, whereas without awareness, early responses to stimuli that predict danger are amplified.
Participants completed the Spielberger State-Trait Anxiety Inventory before the experiment. Previous research has shown that higher anxiety impairs discrimination between safe and threatening stimuli [6] and anxiety modulates amygdala activity [7]. Indeed, the magnitude of differential conditioning was negatively correlated with state anxiety for both groups, but only during the stage in which learning occurred (early for unaware, late for aware participants; Figure 1C).
What underlies the rapid decline of nonconscious conditioning? Learning may have dissipated due to habituation, whereby differential responses attenuated despite a stable association forming. Alternatively, suppressing the CSs from awareness may have allowed initial differentiation between the CS+ and CS− but prevented the formation of a stable association, leading to rapid forgetting. To distinguish these possibilities, in a second experiment a new unaware group underwent only the early acquisition portion (first half) of the original experiment, to maximize learning. Participants were tested again 24 hours later with the same stimuli, but without reinforcement, so any differential response could only be attributed to learning on the first day. If conditioning in the first experiment had declined because of habituation, it should reemerge a day later; if the decline were due to forgetting, we would not expect differential fear responses to be observed on the second day. Results from day 1 replicated the original experiment: SCRs to the CS+ were significantly greater than for the CS− (p < 0.05; Figure S2), and correlated negatively with state anxiety. On day 2, however, SCRs to the CS+ and CS− no longer differed. Unlike conscious fear learning, which is known to persist over time [5], fear acquired nonconsciously is thus subject to rapid forgetting.
Previous attempts to investigate nonconscious conditioning (for example, [8]) used backward masking to suppress briefly-presented stimuli from awareness. However, the methodological limitations of masking (see Supplemental Information), as well as insufficiently rigorous measures of awareness used in past studies [3], have left the question of whether a new fear association can be learned nonconsciously unresolved. Here we used CFS to suppress long-duration CSs from awareness reliably (as assessed by both objective and subjective measures), and found that although the overall magnitude of nonconscious fear learning is comparable to conscious learning, it is characterized by a distinct temporal pattern. Conscious fear developed progressively over time, whereas nonconscious fear was acquired rapidly and declined swiftly.
The mechanisms underlying conscious and nonconscious fear conditioning may thus fulfill complementary roles: The initial orienting response that allows a stimulus to be associated with threat may not require awareness, but the long-term retention and expression of such learning does. Both conscious and nonconscious conditioning likely involve the amygdala, a brain region critical for the acquisition and expression of fear [9]. The amygdala plays a role in the automatic detection and processing of subliminally-presented affective stimuli [4], but has a tendency to rapidly habituate, especially to emotionally-laden stimuli [10]. Such habituation may, in turn, prevent the formation of a stable fear association, which might lead to rapid forgetting in the absence of other processes that involve awareness. The neural mechanisms that distinguish learning with and without awareness are thus fertile ground for further investigation.
Supplementary Material
Acknowledgments
This research was supported by an International Brain Research Foundation Postdoctoral Fellowship to DC, and NIH Grants RO1 MH062104 to E.A.P. and RO1 EY016200 to M.C. The authors thank Daniela Schiller for helpful discussions.
Footnotes
Supplemental Information includes two figures and supplemental experimental procedures and can be found with this article online at doi:10.1016/j.cub.2012.04.023.
References
- 1.Ohman RJ, Soares JJF. ‘Unconscious anxiety’: Phobic responses to masked stimuli. J Abnorm Psychol. 1994;103:231–240. doi: 10.1037//0021-843x.103.2.231. [DOI] [PubMed] [Google Scholar]
- 2.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 3.Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. J Exp Psychol Anim Behav Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- 4.Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci. 2010;11:697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- 5.Schiller D, Monfils M, Raio CM, Johnson D, LeDoux JE, Phelps EA. Blocking the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteves F, Parra C, Dimberg U, Ohman A. Nonconscious associative learning: Pavlovian conditioning of skin conductance responses to masked fear-relevant facial stimuli. Psychophysiology. 1994;31:375–385. doi: 10.1111/j.1469-8986.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 9.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.