Abstract
Purpose
Individuals suffering from Fanconi Anemia (FA) exhibit a pronounced hypersensitivity to agents that cause DNA inter-strand crosslinks and frequently also to ionizing radiation. However, fibroblast lines derived from FA patients generally show little or no radiosensitivity in vitro. Here, we sought to elucidate the role of the central FA protein D2 (FANCD2) in determining cellular radioresistance.
Material and methods
Clonogenic radiation survival was assessed in an isogenic pair of human fibroblasts with or without wild-type FANCD2 under varying oxygen concentrations. Additional endpoints included single-cell gel electrophoresis, RAD51 foci formation, and apoptosis.
Results
At 20% oxygen, there was no reduction in the survival of FANCD2-deficient fibroblasts compared to wild-type complemented cells. However, at 0% oxygen FANCD2-deficient cells were more radiosensitive than wild-type cells. Interestingly, at 3% oxygen, which more closely resembles the physiological environment in human tissues, the difference in radiosensitivity was maintained. Our data also suggest that the increased radiosensitivity of FANCD2-deficient cells seen under conditions of reduced oxygen is associated with apoptotic cell death, but not secondary to a defect in the homologous recombination repair pathway that is required for crosslink repair.
Conclusions
Our data may help explain the previously described discrepancy between the clinical and cellular radiosensitivity of FA patients.
Keywords: Fanconi Anemia, FANCD2, radiation sensitivity, hypoxia
Introduction
Fanconi Anemia (FA) is a heterogeneous clinical syndrome characterized by congenital defects, bone marrow failure, and cancer predisposition (reviewed in (Kennedy and D’Andrea 2005, Joenje and Patel 2001, Niedernhofer et al. 2005, W. Wang 2007, Thompson et al. 2005)). The disease is caused by mutations in any of the 13 known genes, FANCA through FANCN. The FA proteins together with BRCA1 cooperate in a common biochemical pathway, the FA/BRCA pathway, which is believed to function mainly in the detection, stabilization, and repair of arrested DNA replication forks (Garcia-Higuera et al. 2001, W. Wang 2007). A multiprotein nuclear core complex of FA components is required for baseline and damage-induced mono-ubiquitination of the downstream effector proteins FANCD2 and FANCI (Smogorzewska et al. 2007). In response to replication-fork blocking DNA lesions, mono-ubiquitinated FANCD2 relocates into chromatin and co-localizes with repair proteins such as BRCA2/FANCD1 and RAD51. These protein accumulations can be visualized as subnuclear foci (Garcia-Higuera et al. 2001, Godthelp et al. 2006, Houghtaling et al. 2003, Tebbs et al. 2005, X. Wang et al. 2004, Digweed et al. 2002). RAD51 foci formation is a surrogate marker for the local activity of the homologous recombination (HR) pathway (Saleh-Gohari et al. 2005, Zhang et al. 2004) that is required for the repair and restart of collapsed replication forks (reviewed by (Li and Heyer 2008)). Cells with a defect in the FA/BRCA pathway or the HR effector pathway exhibit spontaneous and damage-induced chromosomal aberrations, particularly chromatid-type breaks and exchanges (Garcia-Higuera et al. 2001, Howlett et al. 2002, Smogorzewska et al. 2007). A hallmark of these cells is their hypersensitivity to agents that cause replication fork-blocking DNA inter-strand crosslinks (ICL), such as Mitomycin C (MMC).
In addition to ICL hypersensitivity, patients with FA and primary cell cultures derived from these individuals exhibit a hypersensitivity to oxygen, the mechanisms of which remain to be established (reviewed in (Pagano et al. 2005)). This observation has led investigators to passage and transform primary FA cells under conditions of reduced oxygen concentration, i.e., ~1–5%, which generally improves cell growth in vitro (Saito et al. 1993, Kalb et al. 2004, Schindler and Hoehn 1988). Maintaining FA cells under reduced oxygen tension decreases the formation of chromosomal aberrations (Joenje et al. 1981); however, how the response of FA cells to DNA damage introduced at 1–5% oxygen differs from the response at 20% oxygen is not known.
Exposure of cells to ionizing radiation activates the FA/BRCA pathway, which includes post-translational modification of FANCD2 as well as foci formation of the FANCD2 and RAD51 proteins (Garcia-Higuera et al. 2001, Zhang et al. 2004). However, in general, disruption of the function of FANCD2 or other FA proteins does not lead to cellular hypersensitivity to radiation in vitro (Kalb et al. 2004, Arlett and Harcourt 1980, Duckworth-Rysiecki and Taylor 1985, Fornace et al. 1979, Marcou et al. 2001, Weichselbaum et al. 1980, Djuzenova et al. 2004). Exceptions apply to some cell types and systems, such as Chinese hamster ovary cell lines containing mutations of the FANCG/XRCC9 gene (Wilson et al. 2001), or when short-term survival/proliferation assays are used for FA lymphoblasts (Taniguchi et al. 2002). In contrast, there are several reports on increased normal tissue toxicity in patients with FA who received radiation as part of their conditioning regimen prior to bone marrow transplantation (Gluckman 1990, Hows et al. 1989) or as part of therapy for solid cancers (Alter 2002, Marcou et al. 2001, Bremer et al. 2003). It remains to be established why the observed clinical radiosensitivity of FA patients cannot be reliably reproduced in clonogenic survival assays in the laboratory setting. This is a question not only of importance for the management of patients with FA but also for the therapy of sporadic human cancers, which in many instances harbor genetic or epigenetic alterations in the FA pathway (reviewed in (Lyakhovich and Surralles 2006)).
Recently, Begg and colleagues reported that HR proficiency was important for cellular radioresistance under acute hypoxic conditions, i.e., an oxygen concentration of < 0.1% (Sprong et al. 2006). That study also included two primary fibroblast strains from FA group C and G patients, which were more radiosensitive than normal human fibroblasts under hypoxic conditions but not at room air. The authors concluded that the cellular capacity to repair ICL determined hypoxic radioresistance. However, the importance of other FA genes for hypoxic radiosensitivity and the magnitude of the effect in an isogenic cell system are unknown. Furthermore, whether irradiated FA cells display a HR defect under hypoxic conditions remains to be shown.
Therefore, in this study, we sought to test the hypothesis that FANCD2-deficient human fibroblasts are more radiosensitive than cells with wild-type FANCD2 when irradiated under hypoxic conditions. We report that at 0% or 3% oxygen cells without FANCD2 displayed a small but reproducible increase in radiosensitivity compared to isogenic cells complemented with wild-type FANCD2. Furthermore, the radiosensitivity of FANCD2-deficient fibroblasts was not correlated with a defect in HR, but rather associated with increased apoptotic cell death.
Methods and materials
Cell lines
SV40-transformed fibroblasts derived from individuals with FA group D2 (PD20 cells) or A (PD220) and their retrovirally complemented counterparts expressing wild-type protein were obtained from the Fanconi Anemia Cell Repository at the Oregon Health and Science University (Jakobs et al. 1996). Cells were maintained in alpha-minimum essential medium with 2 mM glutamine and 1 μg/ml puromycin (Sigma-Aldrich, St. Louis, MO, USA) using 15% bovine growth serum (HyClone, Logan, UT, USA). All cell lines were tested free of mycoplasma.
X-irradiation under controlled gas conditions
Exponentially growing cells were trypsinized and resuspended in a volume of 10 ml of complete media. Cell suspensions were transferred to custom designed glass flasks through which the appropriate gas mixture was flowed (Held 1985). The flasks were either gassed with room air and 5% CO2 (Figure 1B) or a mixture containing either 0% (v/v) oxygen, 95% nitrogen, and 5% CO2 (Airgas East, Salem, NH, USA; Cat. No. Z02N19542000-36c) (Figure 2A,B), or 3% oxygen, 92% nitrogen, and 5% CO2 (Airgas East; Cat. No. Z03MI9242003486-056) (Figure 2D). Oxygen concentrations were provided by the supplier. A concentration of < 10 ppm was assumed for 0% oxygen (i.e., anoxia) and correlated with the measured oxygen enhancement ratio (OER) (Figure 2E). Gas was humidified by bubbling through a bottle of distilled water, with the system being equilibrated to the appropriate oxygen concentration by bubbling for at least 20 minutes prior to being attached to the cell-containing flasks. Glass flasks were placed on a magnetic stirrer, and cell suspensions were exposed to the respective gas mixture for 1 hour prior to irradiation or mock treatment. Irradiation was performed using a Siemens Stabilipan 2 X-ray generator operated at 250 kVp and 12 mA, and doses ranged from 2 Gy to 24 Gy. A dose rate of 2.08 Gy/min was determined prior to this study and used for all experiments. This dose rate was defined at isocenter for the treatment of adherent cells. Gassing and irradiation was carried out at room temperature. FA cells and the complemented derivatives were irradiated in parallel. For each survival curve experiment, the X-ray generator was paused at individual dose levels to allow removal of a cell aliquot from the glass flask by pushing a needle through the rubber seal. These interruptions in radiation delivery lasted only 1–2 minutes each. After the maximum desired dose was delivered, all cell aliquots were plated for colony formation in 25-cm2 polystyrene tissue culture flasks. In a separate experiment (Figure 2C), incubation under anoxia was maintained for another hour after uninterrupted dose delivery to parallel flasks.
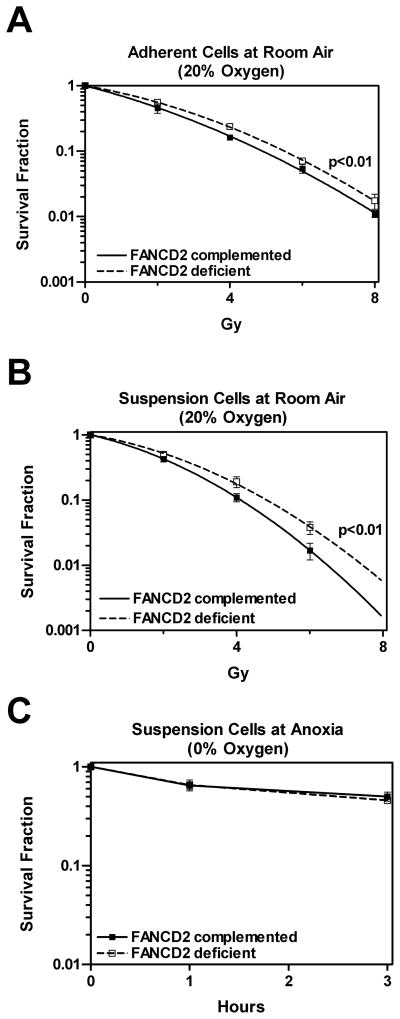
Figure 1.
Clonogenic survival of FANCD2-deficient and wild-type complemented fibroblasts (PD20, filled symbols, and PD20-wtD2, open symbols, respectively). (A) Survival of adherent cells exposed to the indicated doses in air. (B) Survival of cell suspensions following a 1-hour incubation in air and 5% CO2 (see Methods and materials). (C) Survival of cells following exposure to 1–3 hours of anoxia, followed by plating for colony formation at room air (i.e., reoxygenation). Data points represent means +/− standard error based on at least three independent repeats with all gassing and irradiation carried out at room temperature. Radiation survival curves were fitted using the linear-quadratic formula and statistical comparisons between curves were carried out by use of the F test (two-sided) (GraphPad Prism 4.03, GraphPad Software, San Diego, CA, USA). Statistical significance was defined at the level of p = 0.05 or less.
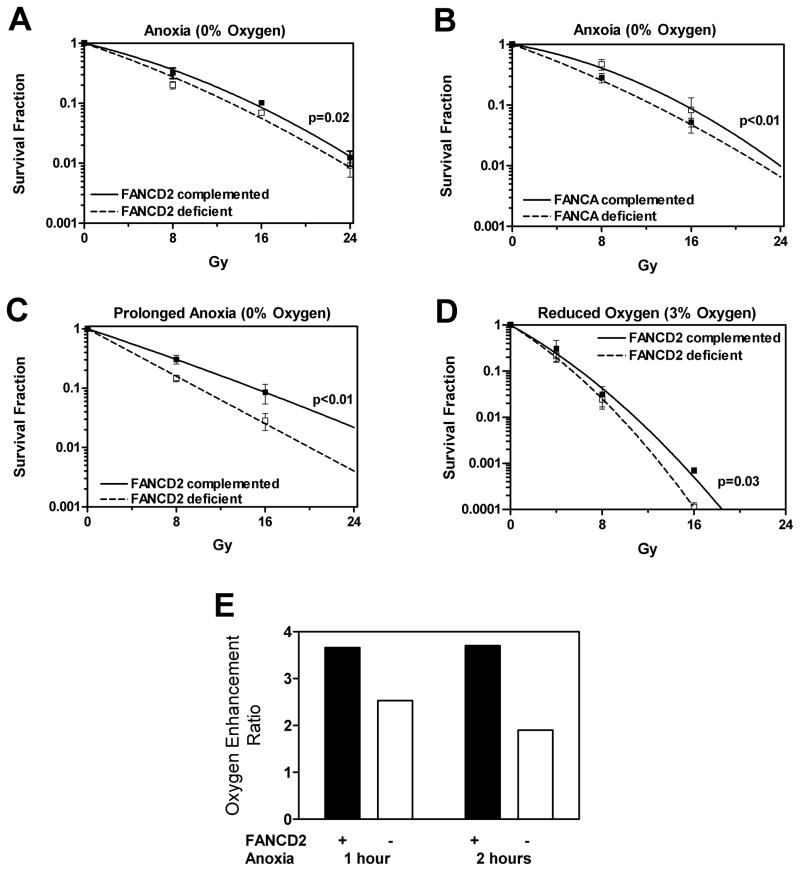
Figure 2.
Sensitivity of fibroblasts to ionizing radiation at reduced oxygen concentrations. (A) Clonogenic survival of FANCD2-deficient (PD20) and wild-type complemented (PD20-wtD2) cells exposed to anoxia (0% oxygen) for 1 hour. (B) Clonogenic survival of FANCA-deficient PD220 fibroblasts and wild-type complemented cells exposed to anoxia (0% oxygen) for 1 hour. (C) Clonogenic survival of PD20 cells as in panel A, except that incubation under anoxia was extended for 1 hour after irradiation. (D) Clonogenic survival as in panel A, except that a gas mixture containing 3% oxygen instead of 0% oxygen was used. (E) Oxygen enhancement ratios (OER) calculated at the 10% survival level using the data shown in Figure 1B and 2A. Data points in panels A–D represent means +/− standard error based on at least three independent repeat experiments with all gassing and irradiation carried out at room temperature. Radiation survival curves and p-values were derived as described for Figure 1.
Colony formation assay
Clonogenic cell survival was assessed by colony formation, as described (Willers et al. 2008, Dahm-Daphi et al. 2005). Colonies were stained with methylene blue after 13 days of incubation in a 37°C incubator supplied with 5% CO2. Colonies containing at least 50 cells were scored under a bright field microscope. Plating efficiencies were calculated as colonies per number of cells plated and surviving fractions as ratios of plating efficiencies for irradiated and unirradiated cells. All experiments consisted of 3–5 independent repeats.
Immunofluorescence microscopy
For the apoptosis experiments, PD20 and PD20-wtD2 cells were plated onto 8-well chamber slides and after 24 hours exposed to 0.5 μg/ml MMC for 1 hour. After 24–48 hours, cells were fixed with 4% paraformaldehyde. Alternatively, following anoxic irradiation with 16 Gy, cell suspensions were plated onto the chamber slides and fixed 24–48 hours later. For scoring of apoptotic nuclei, cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (10 μg/ml). At least 500 nuclei per sample were examined using a fluorescence microscope (Olympus BX51) and assessed for cell morphology and apoptotic bodies (Willers et al. 2008). RAD51 foci formation was assessed 5 hours after MMC treatment or irradiation, as described (Zhang et al. 2004). All experiments consisted of three independent repeats.
Alkaline comet assay
To monitor ICL induction and incision by single-cell gel electrophoresis, we applied a modified comet assay under alkaline conditions as previously reported (Purschke et al. 2004, Rothfuss and Grompe 2004). Cells were mock-treated or subjected to approximately isotoxic doses of MMC as a control (0.5 μg/ml), radiation at room air (5.3 Gy), or anoxic radiation (16 Gy). Treated cells were analyzed at 0–5 hours after the treatment to determine the initial induction of ICL and their uncoupling. Cells were mixed with 120 μl of 0.5% low-melting-point agarose and spread on a chilled microscope slide precoated with agarose. Slides were placed in cold lysis solution for at least 1 hour, washed and treated with proteinase K in dimethyl sulfoxide-free lysis solution in order to remove DNA-protein cross-links that might have been introduced by the treatment. Slides were then incubated for 60 minutes in alkaline unwinding buffer, electrophoresed for 20 minutes at 25 V and 300 mA, and washed in neutralization buffer, followed by drying at room temperature over night. DNA was stained with 50 μl of ethidium bromide solution (20 μg/ml) for 10 minutes. The comets obtained were recorded using a fluorescence microscope and the extent of damage was categorized in five different levels. Slides were coded and at least two slides were measured per data point with 25 cells each.
Results
To investigate the importance of FANCD2 for cellular radioresistance, we employed FANCD2-deficient human fibroblasts derived from a patient with FA group D2 (PD20) and cells complemented with wild-type FANCD2 (PD20-wtD2). Using a standard clonogenic survival assay under room air conditions, we found that the absence of FANCD2 in PD20 cells did not lead to increased radiosensitivity compared to PD20-wtD2 cells (Figure 1A). For reasons unknown, PD20 cells (dotted line) were actually more radioresistant than PD20-wtD2 cells (solid line). In contrast, PD20 cells were hypersensitive to the crosslinking agent MMC (data not shown), as reported previously (Willers et al. 2008, Taniguchi et al. 2002). Given a recent report that FANCC- and FANCG-mutant human fibroblasts were more radiosensitive than normal fibroblasts, but only when irradiated at < 0.1% oxygen (Sprong et al. 2006), we sought to investigate the radiosensitivity of the PD20 cell pair under anoxic conditions. Anoxia was defined as exposure to a balanced gas mixture containing 0% oxygen (see Methods and materials). Exponentially growing PD20 and PD20-wtD2 cells were trypsinized and cell suspensions transferred to gassed glass flasks. Cells were exposed for 1 hour to the desired oxygen concentration, followed by irradiation. Cells were returned immediately to room air and plated for colony formation.
We first confirmed that normoxic irradiation under suspension conditions in the glass flasks itself had no differential effect on the survival of the two PD20 strains, i.e., FANCD2-deficient PD20 cells remained more radioresistant than wild-type complemented cells (Figure 1B, compare to Figure 1A). Plating efficiencies of untreated suspended cells were not reduced compared to adherent cells (data not shown), although radiation survival under suspension conditions was overall decreased compared to adherent cultures. We also determined that anoxic exposure and subsequent reoxygenation at the time of plating for colony formation did not introduce any obvious bias (Figure 1C), though an effect of reoxygenation on colony formation after irradiation cannot be excluded.
Next, PD20 and PD20-wtD2 cells were subjected in parallel to anoxia (0% oxygen) for 1 hour, followed by irradiation and immediate plating to determine clonogenic survival. Figure 2A demonstrates a robust radiosensitization of the PD20 cells under these conditions, especially when compared to the data in Figure 1B. At 20% oxygen, the PD20 survival curve (dotted line) was above the PD20-wtD2 curve (solid line) in Figure 1B, but it shifted below the PD20-wtD2 curve at 0% oxygen (Figure 2A). The corresponding dose enhancement factor (DEF) under anoxic conditions was 1.15, calculated as the ratio of the doses required to produce 10% survival of FANCD2 wild-type cells and FANCD2 deficient cells. The same magnitude of effect was seen in a FANCA wild-type/mutant cell pair, with a DEF of 1.2 (Figure 2B), thus validating our findings.
Since a 1-hour pre-incubation under anoxia only conferred a small radiosensitizing effect, we maintained the PD20 pair after irradiation for another 1 hour under anoxic conditions before plating for colony formation. In parallel, we confirmed that keeping cells in suspension for 2 hours prior to irradiation had no differential effect on survival (data not shown). Strikingly, maintaining cells under anoxia for an additional hour after anoxic irradiation further increased the radiosensitivity of PD20 cells, yielding a DEF of 1.5 (Figure 2C).
Importantly, the physiological oxygen concentration in most human tissues is in the order of 3% (reviewed by (Davies 2000)). Thus, we repeated the experiment under 3% oxygen conditions and found that the difference in radiosensitivity between the two PD20 lines was maintained, i.e., DEF of 1.13 at 10% survival (Figure 2D).
Lastly, Figure 2E demonstrates the oxygen enhancement ratio (OER) for FANCD2 wild-type complemented and deficient cells, calculated as the ratio of doses required to produce 10% cell survival under anoxic and room air conditions. OER values of ~3.5 for wild-type cells are consistent with published data (Palcic and Skarsgard 1984), while the reduced OER of FA cells is in excellent agreement with a previous report by Begg and colleagues (Sprong et al. 2006).
It has been suggested that irradiation under hypoxic conditions produces more ICL than under normoxic conditions, thus offering a possible mechanism to explain the unmasking of the radiosensitivity of FA cells under hypoxia (Sprong et al. 2006). We, therefore, utilized a modification of the alkaline comet assay introduced by Rothfuss and Grompe (2004) to detect the presence of ICL. In this assay, ICL markedly inhibit the denaturation of DNA under alkaline conditions and therefore retard the migration of DNA. However, no relative reduction in DNA migration following anoxic irradiation compared to normoxic irradiation was detected (data not shown). For example, after 16 Gy anoxic irradiation (associated with ~10% clonogenic survival), the fraction of PD20 cells with a comet after 1 hour was 19.8% but after an isotoxic dose at room air it was only 10%. In semi-quantitative analysis of cells with comets, the fraction of cells with the smallest degree of DNA migration was similar at 60% and 70% for anoxia and normoxia, respectively, thus arguing against any significant amount of ICL produced under anoxic irradiation conditions.
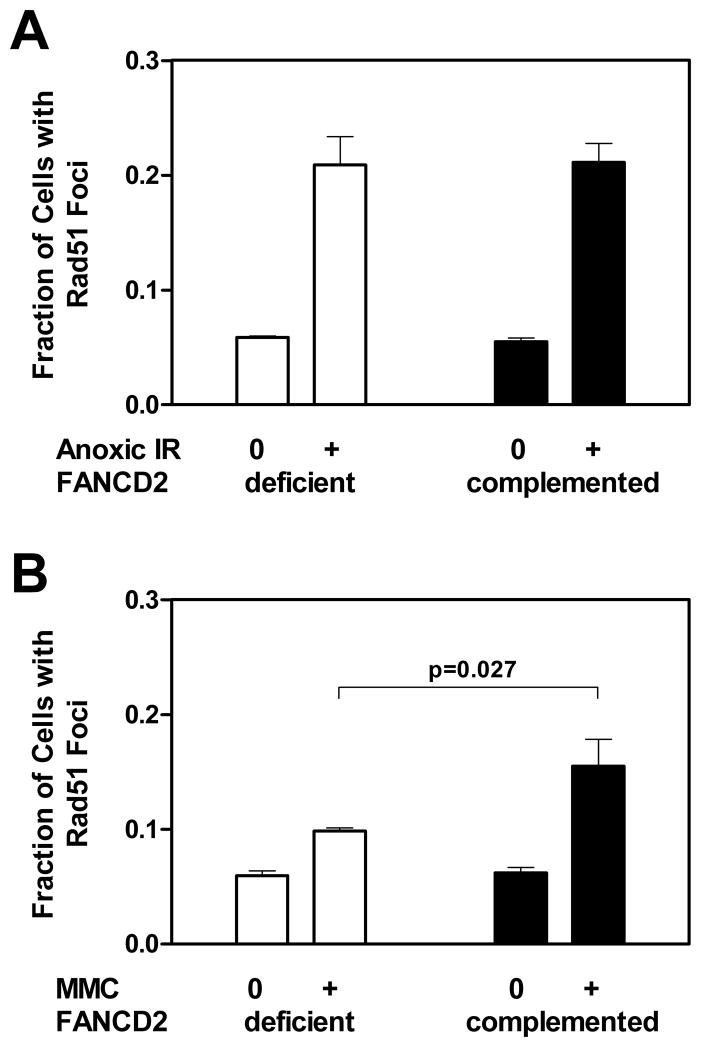
We also considered that ICL typically require HR for removal. We thus assayed the formation of RAD51 foci as an established surrogate marker of HR activity. Approximately isoeffective doses of anoxic radiation and MMC as a control were used (yielding ~ 10% cell survival). Figure 3A demonstrates the ability of PD20 and PD20-wtD2 cells to promote the formation of RAD51 foci after exposure to radiation under anoxia. PD20 cells mounted an obvious RAD51 foci response upon irradiation under anoxic conditions, i.e., a 3.6-fold foci induction above the background levels in untreated cells, and this was virtually identical to the foci induction seen in the wild-type cells. By comparison, PD20 cells showed impaired MMC-induced RAD51 foci formation, i.e., an only 1.7-fold induction of foci over background, which was lower than in PD20-wtD2 cells (p=0.027) and consistent with a separate study (Willers et al. 2008). In summary, this observation is consistent with a reduced ability of FANCD2-deficient cells to repair MMC-induced ICL via HR. In contrast, the HR response of PD20 cells to anoxic radiation appeared normal, arguing against the presence of a large amount of ICL requiring HR for repair.
Figure 3.
Formation of RAD51 foci in FANCD2-deficient (PD20) and wild-type complemented (PD20-wtD2) fibroblasts. (A) The fraction of cells with at least 5 foci is plotted for untreated cells (0) versus cells exposed to 16 Gy ionizing radiation (IR) at 0% oxygen (+) after 5 hours. The relative induction of foci was 3.6-fold for FANCD2-deficient cells. (B) Foci formation analogous to panel A 5 hours following mock-treatment (0) or treatment with mitomycin C (MMC) at 0.5 μg/ml (+). The induction was 1.7-fold for FANCD2-deficient cells. The relative induction of foci above untreated levels between the two cell lines was compared using the unpaired t-test (two-sided p-value). Bars represent means with standard error based on three independent repeat experiments.
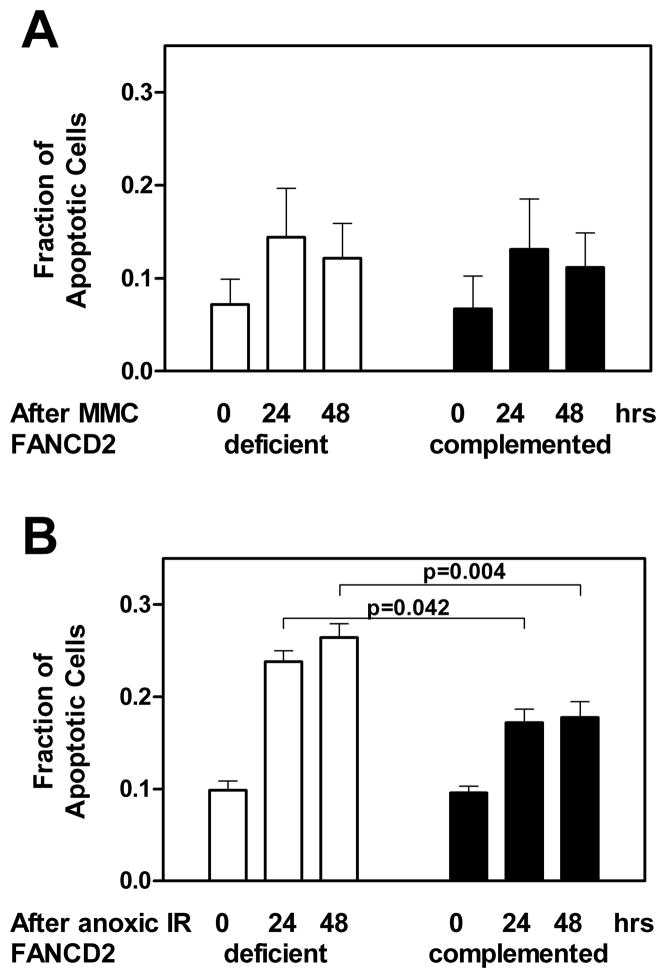
In addition, we considered that FA fibroblasts typically die due to lethal chromatid-type aberrations resulting from replication forks collapsing at ICL (Willers et al. 2008) but not due to apoptosis (Ridet et al. 1997). Accordingly, we found no difference in the fraction of apoptotic cells upon treatment with MMC, with apoptotic levels of approximately 11–12% in both cell lines at 48 hours (Figure 4A). Similarly, after normoxic irradiation, apoptotic levels were 9.6% and 12.5% for PD20-wtD2 and PD20 cells (data not shown). However, following anoxic irradiation, FANCD2-deficient PD20 cells were significantly more prone to undergo apoptosis than the wild-type complemented cells, i.e., 26.4% versus 17.7% at 48 hours, respectively (p<0.05) (Figure 4B). Together, our data suggest that the anoxic radiosensitivity of FANCD2-deficient cells is the consequence of a yet to be identified mechanism that leads to apoptotic cell death.
Figure 4.
Apoptotic cell death of FANCD2-deficient (PD20) and wild-type complemented (PD20-wtD2) fibroblasts. (A) The relative fraction of apoptotic cells is given at the indicated time after treatment with 0.5 μg/ml mitomycin C (MMC) treatment. (B) Analogous to panel A for treatment with 16 Gy ionizing radiation (IR) under anoxic conditions. The relative induction of apoptotic cell death above untreated levels between the two cell lines was compared using the unpaired t-test (two-sided p-value). Bars represent means with standard error based on three independent repeat experiments.
Discussion
Here, we report for the first time that FANCD2-deficient human fibroblasts show hypersensitivity to ionizing radiation compared to wild-type cells when irradiated under conditions of 0% or 3% oxygen, which is not seen when cells are irradiated in air. Our data provide an important confirmation of interesting recent findings by Begg and colleagues who described a pronounced radiosensitivity of FANCC- and FANCG-mutant fibroblasts compared to normal cells at < 0.1% oxygen (Sprong et al. 2006). Notably, in that study the comparison was between strains from different individuals and based on large variations in the hypoxic survival readout (Figure 5 in (Sprong et al. 2006)). The current study expands on the data by Begg and colleagues in two significant ways.
First, our data indicate that the radiosensitivity of FANCD2-deficient cells is not correlated with an impaired ability to promote RAD51-dependent HR but with increased apoptosis, which was not investigated by Begg and colleagues (Sprong et al. 2006). We caution, however, that the association of impaired RAD51 foci formation with a FA defect is not consistent in the literature (Godthelp et al. 2006, Houghtaling et al. 2003, Tebbs et al. 2005, X. Wang et al. 2004, Digweed et al. 2002, Willers et al. 2008). Importantly, the alkaline comet assay did not suggest the presence of a large amount of ICL after anoxic irradiation. Furthermore, radiosensitization of FANCD2 deficient cells was more pronounced when maintaining the cells under anoxia for an additional hour following irradiation (Figure 2C). This observation is not consistent with the idea that changes in the type or amount of radiation-induced DNA damage upon lowering the oxygen concentration were the cause of the radiosensitizing effect. Rather, the data suggest that the anoxic conditions impaired the ability of PD20 cells to respond to or repair radiation damage to DNA. Interestingly, several groups have observed increased amounts of DNA damage or chromosomal aberrations in irradiated FA cells compared to normal controls (Bigelow et al. 1979, Djuzenova et al. 2004, Garcia-Higuera et al. 2001, Parshad et al. 1983). Yet, such damage has not translated into reduced cell survival. It is tempting to speculate that FA cells may have evolved tolerance mechanisms under conditions of high oxygen tension in-vitro that allow them to survive radiation damage and that can be reversed under acute anoxia. This notion is consistent with the observation of increased apoptosis of FANCD2 deficient cells when switching from normoxic to anoxic conditions (Figure 4B).
Second, we observed radiosensitization not only under anoxia but also at 3% oxygen (Figure 2C). What is the significance of this finding? Most human tissues including skin are not exposed to the artificial 20% oxygen concentration that is commonly used in cell culture experiments, but typically are in an environment of approximately 3% oxygen (Davies 2000). This is of potential relevance for the described discrepancy between clinical and cellular FA radiosensitivity (Marcou et al. 2001, Alter 2002, Kalb et al. 2004). In one example, skin fibroblasts from a patient with FA group A who experienced pronounced normal tissue toxicity from radiation therapy for an oropharyngeal squamous cell carcinoma showed normal clonogenic survival in vitro (Marcou et al. 2001). It will be interesting to determine whether repeating the in vitro survival assay under hypoxic conditions would unmask the radiosensitivity phenotype of that patient’s fibroblasts. Conceivably, the observed small difference in radiosensitivity after a 1-hour incubation at 3% oxygen (Figure 2D) may be enhanced in a physiological setting of long-term 3% oxygen exposure or during a course of fractionated radiation treatment, and this will be an interesting question for follow-up studies.
Our findings do not contradict the observations by Kalb et al. who used cell culture conditions of 3% oxygen concentration but did not detect a difference in radiosensitivity between FA and normal cells (Kalb et al. 2004). In that study, cells were handled at room air for irradiation while our data suggest that the hypersensitivity of FA cells to radiation is only revealed if cells are maintained at 0–3% oxygen at the time of irradiation and immediately thereafter. Of note, in another report, PD20 cells irradiated at room air demonstrated a small difference in radiation survival at doses of 6–8 Gy (Taniguchi et al. 2002). In contrast, under our assay conditions, there was clearly no suggestion of increased radiosensitivity over the entire dose range up to 8 Gy (Figure 1A). The reason for this small discrepancy between the two laboratories is unknown. Similar to our results, another study reported that the clonogenic survival of primary fibroblasts derived from a patient with FA complementation group D2 was comparable to the survival of control strains from healthy donors over a dose range of 1–8 Gy (Kalb et al. 2004).
In conclusion, assaying cells from FA patients for their radiosensitivity under conditions of reduced oxygen concentration may serve as a predictor of clinical radiation hypersensitivity when using radiation in conditioning regimens prior to bone marrow transplantation or as part of the treatment of solid tumors. Our data are also consistent with the idea that FA proteins are not exclusively involved in the repair of replication fork-blocking DNA lesions (reviewed in (Bagby and Alter 2006, Pagano et al. 2005)). The mechanisms underlying the improved growth of FA cells under conditions of low oxygen concentration and the associated hypersensitivity to ionizing radiation constitute an important arena for future investigations.
Acknowledgments
The excellent technical assistance of Chake Tokadjian is acknowledged. This research was supported in part by grants from Susan G. Komen for the Cure (to LAK and HW) and NIH P01 CA095227 (to KDH).
References
- Alter BP. Radiosensitivity in Fanconi’s anemia patients. Radiotherapy and Oncology. 2002;62:345–7. doi: 10.1016/s0167-8140(01)00474-1. [DOI] [PubMed] [Google Scholar]
- Arlett CF, Harcourt SA. Survey of radiosensitivity in a variety of human cell strains. Cancer Research. 1980;40:926–32. [PubMed] [Google Scholar]
- Bagby GC, Alter BP. Fanconi anemia. Seminars in Hematology. 2006;43:147–56. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Bigelow SB, Rary JM, Bender MA. G2 chromosomal radiosensitivity in Fanconi’s anemia. Mutation Research. 1979;63:189–99. doi: 10.1016/0027-5107(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Bremer M, Schindler D, Gross M, Dork T, Morlot S, Karstens JH. Fanconi’s anemia and clinical radiosensitivity report on two adult patients with locally advanced solid tumors treated by radiotherapy. Strahlentherapie und Onkologie. 2003;179:748–53. doi: 10.1007/s00066-003-1099-8. [DOI] [PubMed] [Google Scholar]
- Dahm-Daphi J, Hubbe P, Horvath F, El-Awady RA, Bouffard KE, Powell SN, Willers H. Nonhomologous end-joining of site-specific but not of radiation-induced DNA double-strand breaks is reduced in the presence of wild-type p53. Oncogene. 2005;24:1663–72. doi: 10.1038/sj.onc.1208396. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–89. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- Digweed M, Rothe S, Demuth I, Scholz R, Schindler D, Stumm M, Grompe M, Jordan A, Sperling K. Attenuation of the formation of DNA-repair foci containing RAD51 in Fanconi anaemia. Carcinogenesis. 2002;23:1121–6. doi: 10.1093/carcin/23.7.1121. [DOI] [PubMed] [Google Scholar]
- Djuzenova C, Flentje M, Plowman PN. Radiation response in vitro of fibroblasts from a fanconi anemia patient with marked clinical radiosensitivity. Strahlentherapie und Onkologie. 2004;180:789–97. doi: 10.1007/s00066-004-1250-1. [DOI] [PubMed] [Google Scholar]
- Duckworth-Rysiecki G, Taylor AM. Effects of ionizing radiation on cells from Fanconi’s anemia patients. Cancer Research. 1985;45:416–20. [PubMed] [Google Scholar]
- Fornace AJ, Jr, Little JB, Weichselbaum RR. DNA repair in a Fanconi’s anemia fibroblast cell strain. Biochimica et Biophysica Acta. 1979;561:99–109. doi: 10.1016/0005-2787(79)90494-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D’Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Molecular Cell. 2001;7:249–62. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- Gluckman E. Radiosensitivity in Fanconi anemia: application to the conditioning for bone marrow transplantation. Radiotherapy and Oncology. 1990;18(Suppl 1):88–93. doi: 10.1016/0167-8140(90)90182-v. [DOI] [PubMed] [Google Scholar]
- Godthelp BC, Wiegant WW, Waisfisz Q, Medhurst AL, Arwert F, Joenje H, Zdzienicka MZ. Inducibility of nuclear Rad51 foci after DNA damage distinguishes all Fanconi anemia complementation groups from D1/BRCA2. Mutation Research. 2006;594:39–48. doi: 10.1016/j.mrfmmm.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Held KD. Interactions of radioprotectors and oxygen in cultured mammalian cells. I. Dithiothreitol effects on radiation-induced cell killing. Radiation Research. 1985;101:424–33. [PubMed] [Google Scholar]
- Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, Grompe M. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes and Development. 2003;17:2021–35. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, Ikeda H, Fox EA, D’Andrea AD. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–9. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- Hows JM, Chapple M, Marsh JC, Durrant S, Yin JL, Swirsky D, Gordon-Smith EC. Bone marrow transplantation for Fanconi’s anaemia: the Hammersmith experience 1977–89. Bone Marrow Transplantation. 1989;4:629–34. [PubMed] [Google Scholar]
- Jakobs PM, Sahaayaruban P, Saito H, Reifsteck C, Olson S, Joenje H, Moses RE, Grompe M. Immortalization of four new Fanconi anemia fibroblast cell lines by an improved procedure. Somatic Cell and Molecular Genetics. 1996;22:151–7. doi: 10.1007/BF02369905. [DOI] [PubMed] [Google Scholar]
- Joenje H, Arwert F, Eriksson AW, de Koning H, Oostra AB. Oxygen-dependence of chromosomal aberrations in Fanconi’s anaemia. Nature. 1981;290:142–3. doi: 10.1038/290142a0. [DOI] [PubMed] [Google Scholar]
- Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nature Review in Genetics. 2001;2:446–57. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- Kalb R, Duerr M, Wagner M, Herterich S, Gross M, Digweed M, Joenje H, Hoehn H, Schindler D. Lack of sensitivity of primary Fanconi’s anemia fibroblasts to UV and ionizing radiation. Radiation Research. 2004;161:318–25. doi: 10.1667/rr3138. [DOI] [PubMed] [Google Scholar]
- Kennedy RD, D’Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes and Development. 2005;19:2925–40. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Research. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyakhovich A, Surralles J. Disruption of the Fanconi anemia/BRCA pathway in sporadic cancer. Cancer Letters. 2006;232:99–106. doi: 10.1016/j.canlet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Marcou Y, D’Andrea A, Jeggo PA, Plowman PN. Normal cellular radiosensitivity in an adult Fanconi anaemia patient with marked clinical radiosensitivity. Radiotherapy and Oncology. 2001;60:75–9. doi: 10.1016/s0167-8140(01)00370-x. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–8. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Pagano G, Degan P, d’Ischia M, Kelly FJ, Nobili B, Pallardo FV, Youssoufian H, Zatterale A. Oxidative stress as a multiple effector in Fanconi anaemia clinical phenotype. European Journal of Haematology. 2005;75:93–100. doi: 10.1111/j.1600-0609.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- Palcic B, Skarsgard LD. Reduced oxygen enhancement ratio at low doses of ionizing radiation. Radiation Research. 1984;100:328–39. [PubMed] [Google Scholar]
- Parshad R, Sanford KK, Jones GM. Chromatid damage after G2 phase x-irradiation of cells from cancer-prone individuals implicates deficiency in DNA repair. Proceedings of the National Academy of Science U S A. 1983;80:5612–6. doi: 10.1073/pnas.80.18.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purschke M, Kasten-Pisula U, Brammer I, Dikomey E. Human and rodent cell lines showing no differences in the induction but differing in the repair kinetics of radiation-induced DNA base damage. International Journal of Radiation Biology. 2004;80:29–38. doi: 10.1080/09553000310001642885. [DOI] [PubMed] [Google Scholar]
- Ridet A, Guillouf C, Duchaud E, Cundari E, Fiore M, Moustacchi E, Rosselli F. Deregulated apoptosis is a hallmark of the Fanconi anemia syndrome. Cancer Research. 1997;57:1722–30. [PubMed] [Google Scholar]
- Rothfuss A, Grompe M. Repair kinetics of genomic interstrand DNA cross-links: evidence for DNA double-strand break-dependent activation of the Fanconi anemia/BRCA pathway. Molecular and Cellular Biology. 2004;24:123–34. doi: 10.1128/MCB.24.1.123-134.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Hammond AT, Moses RE. Hypersensitivity to oxygen is a uniform and secondary defect in Fanconi anemia cells. Mutation Research. 1993;294:255–62. doi: 10.1016/0921-8777(93)90008-5. [DOI] [PubMed] [Google Scholar]
- Saleh-Gohari N, Bryant HE, Schultz N, Parker KM, Cassel TN, Helleday T. Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks. Molecular and Cellular Biology. 2005;25:7158–69. doi: 10.1128/MCB.25.16.7158-7169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D, Hoehn H. Fanconi anemia mutation causes cellular susceptibility to ambient oxygen. American Journal of Human Genetics. 1988;43:429–35. [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, Matsuoka S, Vinciguerra P, McDonald ER, 3rd, Hurov KE, Luo J, Ballif BA, Gygi SP, Hofmann K, D’Andrea AD, Elledge SJ. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong D, Janssen HL, Vens C, Begg AC. Resistance of hypoxic cells to ionizing radiation is influenced by homologous recombination status. International Journal of Radiation Oncology Biology Physics. 2006;64:562–72. doi: 10.1016/j.ijrobp.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Garcia-Higuera I, Xu B, Andreassen PR, Gregory RC, Kim ST, Lane WS, Kastan MB, D’Andrea AD. Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways. Cell. 2002;109:459–72. doi: 10.1016/s0092-8674(02)00747-x. [DOI] [PubMed] [Google Scholar]
- Tebbs RS, Hinz JM, Yamada NA, Wilson JB, Salazar EP, Thomas CB, Jones IM, Jones NJ, Thompson LH. New insights into the Fanconi anemia pathway from an isogenic FancG hamster CHO mutant. DNA Repair (Amst) 2005;4:11–22. doi: 10.1016/j.dnarep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Thompson LH, Hinz JM, Yamada NA, Jones NJ. How Fanconi anemia proteins promote the four Rs: replication, recombination, repair, and recovery. Environmental and Molecular Mutagenesis. 2005;45:128–42. doi: 10.1002/em.20109. [DOI] [PubMed] [Google Scholar]
- Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nature Review in Genetics. 2007;8:735–48. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- Wang X, Andreassen PR, D’Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Molecular and Cellular Biology. 2004;24:5850–62. doi: 10.1128/MCB.24.13.5850-5862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbaum RR, Nove J, Little JB. X-ray sensitivity of fifty-three human diploid fibroblast cell strains from patients with characterized genetic disorders. Cancer Research. 1980;40:920–5. [PubMed] [Google Scholar]
- Willers H, Kachnic LA, Luo CM, Li L, Purschke M, Borgmann K, Held KD, Powell SN. Biomarkers and mechanisms of FANCD2 function. Journal of Biomedicine and Biotechnology. 2008;2008:821529. doi: 10.1155/2008/821529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JB, Johnson MA, Stuckert AP, Trueman KL, May S, Bryant PE, Meyn RE, D’Andrea AD, Jones NJ. The Chinese hamster FANCG/XRCC9 mutant NM3 fails to express the monoubiquitinated form of the FANCD2 protein, is hypersensitive to a range of DNA damaging agents and exhibits a normal level of spontaneous sister chromatid exchange. Carcinogenesis. 2001;22:1939–46. doi: 10.1093/carcin/22.12.1939. [DOI] [PubMed] [Google Scholar]
- Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, Chung JH, Powell SN, Xia F. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Molecular and Cellular Biology. 2004;24:708–18. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]