Abstract
Purpose of review
Complement mediated hemolytic uremic syndrome (aHUS) accounts for a significant proportion of non-shiga toxin HUS. The purpose of this review is to outline the pathophysiology, clinical features and therapeutic options for aHUS.
Recent findings
In the last decade, strides have been made in identifying several new disease-causing mutations in complement-regulating proteins.
Summary
Complement mediated HUS (aHUS) has a worse prognosis compared with shiga toxin mediated HUS, often resulting in end stage renal disease. Early identification of aHUS is crucial so that plasma therapy can be initiated. After renal transplantation, there is very high risk of disease recurrence and graft loss. Eculizumab and combined liver–kidney transplantation offer promise for improved prognosis.
Keywords: factor H, factor I, membrane cofactor protein, plasma exchange
INTRODUCTION
Hemolytic uremic syndrome (HUS) is characterized by a triad of nonimmune hemolytic anemia, thrombocytopenia and renal failure. Thrombotic microangiopathy and endothelial injury is a common denominator in HUS. In children, about 90% of HUS is mediated by Shiga (verocytotoxin) toxin and is traditionally referred to as typical HUS. The remaining ~10% of cases are classified as atypical HUS (aHUS), which has a poorer prognosis compared with shiga toxin HUS. However, the term aHUS encompasses various causes, including but not limited to Streptococcus pneumoniae, complement mediated HUS, medications (calcineurin inhibitors, chemotherapy, antiplatelet agents, oral contraceptives), viral infections (HIV and influenza), autoimmune diseases (systemic lupus erythematosus, antiphospholipid antibody syndrome), pregnancy, and cobalamin deficiency. As we have learned more about the pathogenesis of HUS the term ‘aHUS’ is used to designate complement mediated HUS, and we will use aHUS to designate complement mediated HUS in this review. Several genetic mutations in complement regulatory proteins have been identified in patients with both familial and sporadic aHUS. Despite the advances in the field, ~30–40% of patients with aHUS do not have an identifiable abnormality in the complement system. Approximately 50% of patients with aHUS develop end stage renal disease (ESRD) and the mortality can be as high as 25%. The focus of this article is to review the complement system, pathophysiology of complement dysregulation, clinical features and current therapeutic options in aHUS.
COMPLEMENT CASCADE AND REGULATION
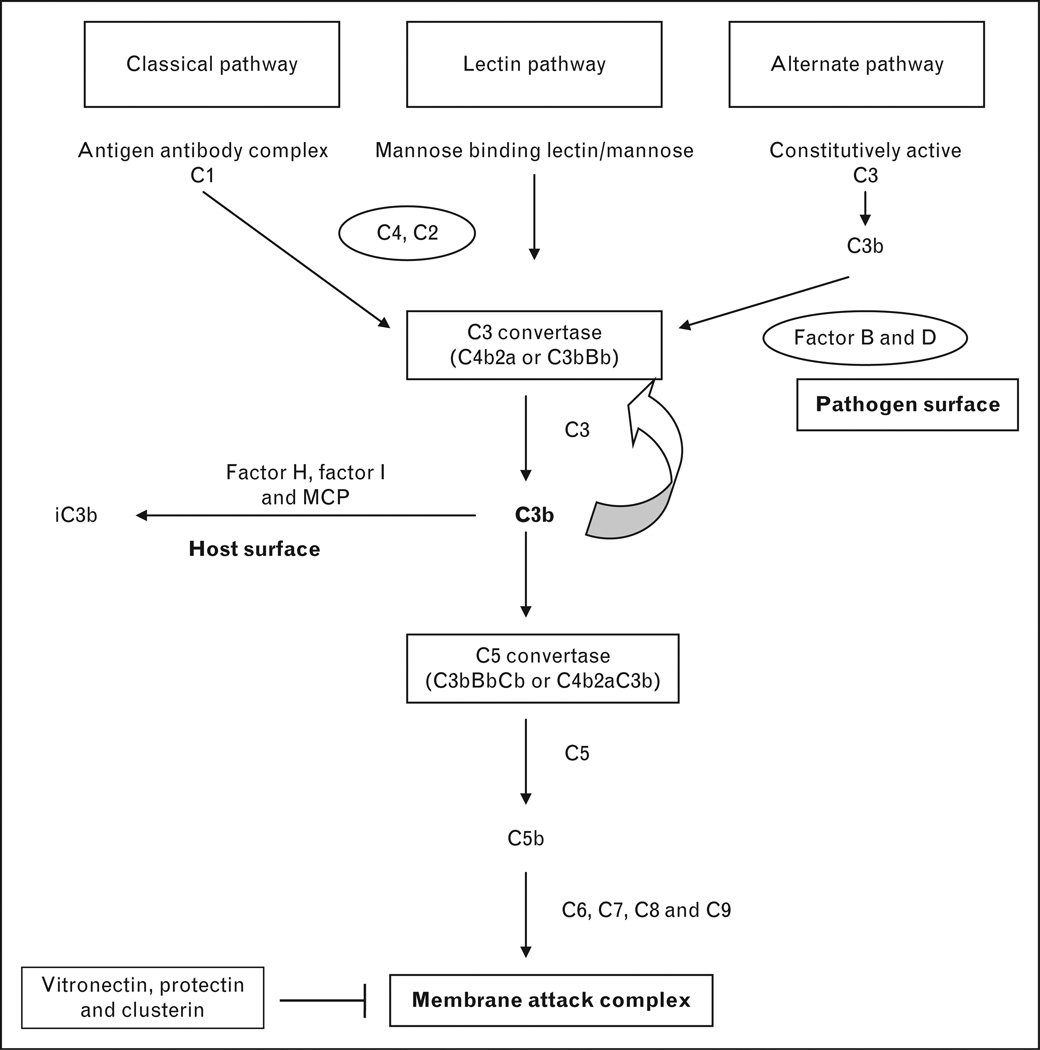
Various defense mechanisms exist in humans to protect from invading pathogens and the complement system is a key element of innate immunity that aids in rapid recognition and elimination of pathogens. The complement system consists of more than 30 different proteins, most of which are synthesized in the liver and distributed across plasma and cell surfaces [1]. The complement system has three primary functions; cellular lysis by opsonization, generation of inflammatory mediators and modulation of adaptive immune response [2]. Activation of the complement system is crucial and occurs by three different pathways and all of them converge at the formation of C3 convertase.
In the classical pathway, C1 complex (C1q, C1r and C1s) is activated by an antigen–antibody complex and sequential cleavage of C4 and C2 leads to the formation of C4bC2a, which is designated as C3 convertase [2]. In the lectin based pathway, mannose residues on microbial surfaces bind tomannose binding lectin and its associated proteases in plasma, and this complex cleaves C4 and C2 to generate C3 convertase (C4bC2a). C3 convertase then cleaves C3 to C3b, which can in turn bind to C3 convertase to form C5 convertase (C4bC2aC3b) [3].
The alternative pathway is unique because of low level continuous hydrolysis of C3 in the plasma to C3b. C3b, upon binding to a pathogen, enables binding of Factor B and this complex is cleaved by Factor D to C3bBb, the C3 convertase of the alternative complement pathway. C3b can then bind to C3 convertase to generate C5 convertase (C3bBbC3b) [2]. C5 convertase of both classical and alternative pathways activates C6, C7, C8 and C9 to form the membrane attack complex (MAC), which causes cell lysis [1,3]. The complement activation pathway is depicted in Fig. 1. More recently, plasmin, thrombin and kallikrein have been shown to activate the complement system but their physiological function in vivo is yet unclear [1,4].
FIGURE 1.
Normal complement activation cascade and its regulation. This figure illustrates the normal complement pathways and the factors that protect the host from injury. Complement can be activated by three different pathways: classical, lectin and alternate pathways. C3 convertase from the classical and lectin pathways is generated by antigen antibody complex and interaction of mannose residues on the microbial surface with mannose binding lectin, respectively, in the presence of C4 and C2. The alternate pathway is constitutively active at low levels generating C3b. C3b binds with complement factor B, which is cleaved by complement factor D to generate C3bBb; the alternate pathway C3 convertase. Thus, all three complement pathways converge at C3 convertase. Either of the C3 convertases can bind to C3b to generate C5 convertase (C3bBbC3b or C4b2aC3b). Either of the C5 convertases can cleave C5 to C5b, which then binds to C6, C7, C8 and C9 to generate membrane attack complex. The regulation of complement activation is critical to the host. The important complement regulatory proteins associated with aHUS are complement factor H, complement factor I and MCP. In the presence of complement factor H and MCP, complement factor I cleaves C3b to inactive C3b (iC3b). The carbohydrate content of the cell membrane determines whether complement factor B or H binds to C3b. Eukaryotes have polyanions on their cell surface and C3b in that instance will bind preferentially to complement factor H and C3b is inactivated. Prokaryotes (bacteria) lack polyanions and complement factor B binds C3b to generate C3 convertase and further activation of the complement system. The formation of membrane attack complex is inhibited by vitronectin, clusterin and protectin. MCP, membrane cofactor protein.
Regulation of the complement cascade is critical to limit self injury [3,4]. Complement regulatory proteins are broadly classified into fluid phase regulators or cell surface regulators based on their site of expression. These complement regulatory proteins act in the classical, lectin or alternate pathways to prevent uninhibited activation of the complement cascade. In the classical and lectin based pathways, the C1 esterase inhibitor plays an important role by inactivating proteases, including C1r and C1s. In the terminal complement pathway, complement Factor H related protein 1 (CFHR1) regulates C5 convertase [4]. The assembly of MAC is inhibited by CD59 (Protectin), vitronectin and clusterin [1].
As the alternative pathway contributes to 80–90% of total complement activity, its regulation is tightly controlled and complement factor H plays an important role [4]. Complement factor H and membrane cofactor protein (MCP) act as cofactors for complement factor I, a regulatory serine protease synthesized in the liver, which degrades C3b to inactive C3b (iC3b) [5]. Complement factor H acts on C3 convertase to accelerate its decay by competitively removing Bb from the C3 convertase (C3bBb) complex [3]. Degradation of C3b attenuates complement activity in all pathways. The cell surface carbohydrate content determines whether complement factor H or complement factor B binds to C3b. Specifically sialic acid, glycosaminoglycans and heparin that are present on the host cell surface promote the binding of complement factor H to C3b, thus attenuating the complement cascade. Prokaryotes lack these cell surface molecules and therefore complement factor B binds with higher affinity to C3b, initiating the complement cascade [4,6▪].
GENETICS OF AHUS AND PATHOGENESIS
The alternate pathway is continuously active, albeit at a low level. Alteration of function or deficiency of complement regulatory proteins of the alternate pathway leads to continuous generation of C3 and C5 convertases, resulting in the formation of the MAC. This uninhibited activation of the complement cascade at the site of vascular endothelium and platelets results in a prothrombic state, which leads to thrombotic microangiopathy, and certain vascular beds are more prone to injury than others [7▪▪].
Mutations in complement factor H (CFH), complement factor I (CFI), complement factor B (CFB), MCP/CD46 and C3 comprise about 50% of known mutations in patients with aHUS [8,9▪]. Another 10% of aHUS is due to autoantibodies to complement factor H. In about 10–12% of patients with aHUS, combined mutations of CFH, CFI, MCP, C3, CFB or thrombomodulin (THBD) may exist [7▪▪,10]. In the remaining 30–40% of patients with aHUS, there are no identified mutations. The majority of mutations in aHUS are heterozygous and familial occurrence is reported in up to 20% of the patients. aHUS can be inherited in an autosomal dominant or recessive fashion. Variable age of presentation and the presence of triggering factors (~80%) in patients with aHUS indicates that genetic mutations provide a predisposition to develop aHUS [11▪▪].
COMPLEMENT FACTOR H
Mutations of CFH are the most frequent genetic abnormality in aHUS, accounting for 20–30% of aHUS. CFH is a glycoprotein synthesized in the liver and is composed of 20 short consensus repeats (SCR); SCR 20 is the site for the majority of the identified mutations [12]. These mutations reduce the ability of CFH to bind to surface bound C3b, thus inhibiting the degradation of C3b and C3 convertase. More than 100 mutations in complement factor H have been described and approximately 75% are heterozygous mutations [10,13,14]. Although the majority of these mutations do not cause changes in plasma concentration of CFH, in about 30% of patients there is a quantitative decrease in CFH levels. Decreased C3 levels can be seen in 30–50% of patients with CFH mutations. Autoantibodies to CFH cause a functional deficiency of CFH and reduce binding of CFH to C3b, as seen in 6%–10% of children with aHUS [15,16]. Approximately 90% of patients who have autoantibodies to CFH have deletions of CFH-related proteins 1 (CFHR1) andCFH-related proteins 3 (CFHR3) [17,18].
MEMBRANE COFACTOR PROTEIN
Mutations in MCP account for about 10–15% of patients with aHUS and more than 40 mutations have been described [19]. MCP is a widely expressed transmembrane glycoprotein, which is critical in preventing C3 activation on host cells by serving as a cofactor for CFI [16]. Mutations in MCP are mostly heterozygous and cause decreased MCP activity, which leads to an uninhibited complement activation [7▪▪]. MCP mutations are associated with reduced expression of MCP on leucocytes in about 75% of cases and 30% of patients have low plasma C3 levels [8,16,20].
COMPLEMENT FACTOR I
Mutations of CFI comprise only 5–10% of patients with aHUS [21]. CFI is a serine protease that is synthesized in the liver, which helps in the degradation of C3b. Around 40 CFI mutations have been described and most of them are heterozygous [7▪▪]. Mutations in CFI result in either decreased activity of CFI or protein expression in the serum which causes impaired degradation of C3b. Plasma C3 and CFI concentrations are decreased in approximately 30% of patients with CFI mutations [22].
THROMBOMODULIN
THBD is an endothelial glycoprotein that plays an important role in the coagulation cascade. In the complement pathway, THBD also serves to accelerate CFI-mediated inactivation of C3b [16]. Mutations in THBD are mostly heterozygous and account for 3–5% cases of aHUS [23]. C3 levels are decreased in about 50% of patients with THBD mutations [7▪▪].
C3
Mutations in C3 account for about 2–10% of patients with aHUS and are usually heterozygous in nature [24]. Disease-causing mutations in C3 are a gain of function mutation as the mutant C3 contributes to increased formation of C3 convertase by binding to CFB [25]. The mutated C3 is not amenable to regulation by MCP, which normally serves to inactivate C3b. In approximately 80% of patients, C3 levels are decreased [7▪▪].
COMPLEMENT FACTOR B
Mutations in CFB account for 1–4% of patients with aHUS [26]. CFB mutations are a gain of function mutations in which the mutant CFB binds to C3b with increased affinity and stabilizes C3 convertase, making it resistant to degradation. Patients with CFB mutations always have low C3 levels due to continuous activation of the alternative pathway [7▪▪].
CLINICAL PRESENTATION
The age of onset of aHUS can be quite variable, but the majority of patients present during childhood [10]. In a cohort of 46 children with aHUS, Sellier-Leclerc et al. [22] demonstrated that 32 (70%) children had their first episode before the age of 2 years and eight of these children presented within the first 3 months of life. aHUS, especially due to mutations in CFH and CFI, can present as early as the neonatal period, whereas patients with MCP mutations are typically older than 1 year of age [7▪▪,12]. Patients with autoantibodies to CFH usually have onset of their disease between 7 and 11 years of age [17]. There does not appear to be a sex difference when the presentation is in childhood; however, in adults, aHUS predominantly affects women [10]. Thus, the index of suspicion for atypical HUS should be high in an infant who presents below the age of 6 months of age, as typically shiga toxin HUS presents between 1 and 5 years.
In the majority of children (80%), the onset of aHUS is triggered by an infectious process, such as an upper respiratory tract infection, fever or gastroenteritis [10,27]. Thus, diarrhea preceding the onset of HUS does not exclude aHUS. In adolescents and adults, pregnancy is a well-described inciting event [7▪▪,27]. Recently, reported triggers include vaccination, Hemophilus influenza or streptococcal infection [28▪]. The onset of aHUS can be sudden or insidious and children usually present with pallor, lethargy, and decreased urine output. The majority of the patients have severe hypertension [7▪▪,16,28▪]. In about 20% of children, extra-renal manifestations, including central nervous system or cardiac involvement, are present. In about 5% of patients, multivisceral involvement, including pancreatitis, hepatic dysfunction, pulmonary hemorrhage and gastrointestinal bleeding, is seen during the initial presentation of the disease [16].
In general, patients with aHUS have a poor prognosis. More than 60% of patients with mutations of CFH, CFI, C3, CFB and THBD either die or develop ESRD within1 year of presentation [16]. Patients with MCP have a better long-term outcome, with only 30% progressing to ESRD, though they tend to have a frequently relapsing course [20].
DIAGNOSTIC EVALUATION
Microangiopathic hemolytic anemia, thrombocytopenia and uremia are the components of the classic triad of HUS. Lactate dehydrogenase is elevated whereas haptoglobin is reduced due to intravascular hemolysis. An evaluation should be performed to identify other causes of HUS: Shiga toxin associated HUS, pneumococcal HUS or cobalamin deficiency. An ADAMTS13 activity should be measured in all patients suspected to have aHUS to exclude thrombotic thrombocytopenic purpura (TTP) [29▪]
Evaluation of aHUS should include serum C3 and C4 levels as well as serum concentrations of complement regulators, including CFH, CFI, CFB, anti-CFH antibodies and MCP expression on leucocytes. Blood should be drawn before therapy with plasma exchange as donor plasma can mask the deficiency [7▪▪,30]. Normal levels of C3, C4, CFH, CFI and CFB do not exclude aHUS. Patients should undergo genetic analysis of CFH, CFI, MCP, CFB, THBD, CFHR1–5 and C3 [7▪▪].
TREATMENT
Supportive care should be provided to all children with aHUS, which includes management of hypertension, renal replacement therapy when indicated, and correction of anemia and electrolyte abnormalities as well as providing adequate nutrition. Platelet transfusions are generally contraindicated as they worsen the thrombotic microangiopathy and anemia [31,32].
PLASMA THERAPY
Plasma exchange therapy is the first-line therapy for children with aHUS and should be initiated as soon as the diagnosis is suspected. Although plasma infusion replenishes deficient complement regulators, plasma exchange has the added benefit of removing mutant complement factors and/or autoantibodies [30]. The rate of remission after plasma exchange is variable (30–80%) depending on the genetic mutations. Plasma exchange is of little benefit in patients with a MCP mutation as MCP is not a circulating factor [8,10,16,22]. The duration, dose and frequency of plasma exchange should be adjusted on a case-by-case basis. Immunosuppressive agents are of no benefit in aHUS except in patients with complement factor H autoantibodies, in whom they can be used in conjunction with plasma exchange [16,33–35]. In the future, concentrated complement factor H (purified/recombinant) when available could be used to replenish deficient or mutated complement factor H [36▪].
ECULIZUMAB
The generation of terminal complement complex is quintessential for the pathogenesis of aHUS. Eculizumab is a monoclonal antibody to C5 that prevents cleavage of C5 to C5b, preventing the formation of terminal complement complex [36▪]. Recently, eculizumab has been used in patients with aHUS with good results [37,38]. There is an increased risk of developing Neisseria meningitis infection with the use of eculizumab and patients need to be vaccinated prior to initiation of therapy with eculizumab.
RENAL TRANSPLANTATION
The majority of children with aHUS will progress to ESRD and become candidates for renal transplantation. Kidney transplantation is associated with a high risk of recurrence (~50%) and approximately 80–90% of the kidney grafts that have recurrence will fail [16]. Recurrence of aHUS is dependent on the genetic mutation, with increased risk in patients with complement factor H, complement factor I and C3 mutations, whereas the risk is significantly less for patients with MCP mutations [7▪▪]. Thus, living donor kidney transplantation is contraindicated in children with mutations in CFH, CFB, CFI, C3 or THBD due to the increased risk of recurrence of aHUS. Moreover, there is an increased risk of the donor developing aHUS, as the donor might have disease-causing mutation with incomplete penetrance of the disease [16,39]. If living related kidney transplantation is the only option available to the family then the donor and recipient should undergo thorough genetic evaluation of the complement system and the donor should be counseled that even if the genetic testing is negative, the risk of developing aHUS is not completely eliminated. Eculizumab can be used in case of disease recurrence or as a preventive agent in patients with aHUS after renal transplantation [40–43]. Initial attempts at combined liver–kidney transplantation were disappointing due to complement activation and liver nonfunction; however, four patients who received peri-operative plasma exchange had a favorable outcome [44–46].
CONCLUSION
Complement-mediated HUS (aHUS) is significantly less common than shiga toxin HUS but has a worse prognosis with increased risk of ESRD. Identification of mutations in the complement regulatory proteins has advanced the field of diagnostics, therapeutics and the ability to predict outcome in patients with aHUS. Plasma therapy should be initiated as soon as the diagnosis of aHUS is suspected. There is high recurrence rate of aHUS even after renal transplantation. Combined liver–kidney transplantation and eculizumab are newer therapeutic options with good preliminary results.
Acknowledgements
This work was supported by NIH grants K08DK089295–01 (J.G.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 4.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavanagh D, Goodship TH. Membrane cofactor protein and factor I: mutations and transplantation. Semin Thromb Hemost. 2006;32:155–159. doi: 10.1055/s-2006-939771. [DOI] [PubMed] [Google Scholar]
- 6. Kerr H, Richards A. Complement-mediated injury and protection of endothelium: lessons from atypical haemolytic uraemic syndrome. Immunobiology. 2012;217:195–203. doi: 10.1016/j.imbio.2011.07.028. This article discusses in detail complement cascade activation and the different proteins that regulate the complement cascade.
- 7. Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. This is an outstanding and up-to-date review of the literature on aHUS. The authors outline a detailed approach to investigation of patients suspected to have aHUS. The treatment options are extensively discussed in this review article, including genetic counseling.
- 8.Caprioli J, Noris M, Brioschi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barbour T, Johnson S, Cohney S, Hughes P. Thrombotic microangiopathy and associated renal disorders. Nephrol Dial Transplant. 2012;27:2673–2685. doi: 10.1093/ndt/gfs279. This article describes the renal manifestations of thrombotic microangiopathy and discusses the disorders of complement activation in aHUS.
- 10.Loirat C, Noris M, Fremeaux-Bacchi V. Complement and the atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2008;23:1957–1972. doi: 10.1007/s00467-008-0872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noris M, Bresin E, Mele C, et al. Atypical hemolytic uremic syndrome. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews. Seattle: University of Washington; 2011. This is an excellent review of aHUS. This article describes in detail the clinical presentation, diagnostic work and therapeutic options for the different mutations in complement regulatory proteins.
- 12.Dragon-Durey MA, Fremeaux-Bacchi V. Atypical haemolytic uraemic syndrome and mutations in complement regulator genes. Springer Semin Immunopathol. 2005;27:359–374. doi: 10.1007/s00281-005-0003-2. [DOI] [PubMed] [Google Scholar]
- 13.Warwicker P, Goodship TH, Donne RL, et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53:836–844. doi: 10.1111/j.1523-1755.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RA, Winterborn MH. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin Exp Immunol. 1981;46:110–119. [PMC free article] [PubMed] [Google Scholar]
- 15.Dragon-Durey MA, Loirat C, Cloarec S, et al. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 16.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 17.Dragon-Durey MA, Sethi SK, Bagga A, et al. Clinical features of antifactor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2010;21:2180–2187. doi: 10.1681/ASN.2010030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore I, Strain L, Pappworth I, et al. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood. 2010;115:379–387. doi: 10.1182/blood-2009-05-221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards A, Kemp EJ, Liszewski MK, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2003;100:12966–12971. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, et al. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet. 2004;41:e84. doi: 10.1136/jmg.2004.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18:2392–2400. doi: 10.1681/ASN.2006080811. [DOI] [PubMed] [Google Scholar]
- 23.Delvaeye M, Noris M, De VA, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fremeaux-Bacchi V, Miller EC, Liszewski MK, et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roumenina LT, Frimat M, Miller EC, et al. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood. 2012;119:4182–4191. doi: 10.1182/blood-2011-10-383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goicoechea de JE, Harris CL, Esparza-Gordillo J, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavanagh D, Goodship TH. Atypical hemolytic uremic syndrome. Curr Opin Hematol. 2010;17:432–438. doi: 10.1097/MOH.0b013e32833cae86. [DOI] [PubMed] [Google Scholar]
- 28. Geerdink LM, Westra D, van Wijk JA, et al. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27:1283–1291. doi: 10.1007/s00467-012-2131-y. This is a retrospective study describing the mutations of complement regulatory proteins in Dutch and Belgian children. The authors compare the clinical course of aHUS in patients carrying the different mutations.
- 29. Kavanagh D, Goodship TH. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15–20. doi: 10.1182/asheducation-2011.1.15. This is an excellent review of the common genetic mutations in the complement regulatory proteins seen in aHUS and the clinical course of the patients who present with aHUS.
- 30.Ariceta G, Besbas N, Johnson S, et al. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol. 2009;24:687–696. doi: 10.1007/s00467-008-0964-1. [DOI] [PubMed] [Google Scholar]
- 31.Bitzan M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int Suppl. 2009;(112):S62–S66. doi: 10.1038/ki.2008.624. [DOI] [PubMed] [Google Scholar]
- 32.Bitzan M, Schaefer F, Reymond D. Treatment of typical (enteropathic) hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36:594–610. doi: 10.1055/s-0030-1262881. [DOI] [PubMed] [Google Scholar]
- 33.Kwon T, Dragon-Durey MA, Macher MA, et al. Successful pretransplant management of a patient with antifactor H autoantibodies-associated haemolytic uraemic syndrome. Nephrol Dial Transplant. 2008;23:2088–2090. doi: 10.1093/ndt/gfn063. [DOI] [PubMed] [Google Scholar]
- 34.Boyer O, Balzamo E, Charbit M, et al. Pulse cyclophosphamide therapy and clinical remission in atypical hemolytic uremic syndrome with anticomplement factor H autoantibodies. Am J Kidney Dis. 2010;55:923–927. doi: 10.1053/j.ajkd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Skerka C, Jozsi M, Zipfel PF, et al. Autoantibodies in haemolytic uraemic syndrome (HUS) Thromb Haemost. 2009;101:227–232. [PubMed] [Google Scholar]
- 36. Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011;26:41–57. doi: 10.1007/s00467-010-1556-4. This article discusses newer therapies for aHUS, including eculizumab.
- 37.Nurnberger J, Philipp T, Witzke O, et al. Eculizumab for atypical hemolyticuremic syndrome. N Engl J Med. 2009;360:542–544. doi: 10.1056/NEJMc0808527. [DOI] [PubMed] [Google Scholar]
- 38.Mache CJ, Acham-Roschitz B, Fremeaux-Bacchi V, et al. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:1312–1316. doi: 10.2215/CJN.01090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan MR, Thomas CP, Torrealba JR, et al. Recurrent atypical hemolytic uremic syndrome associated with factor I mutation in a living related renal transplant recipient. Am J Kidney Dis. 2009;53:321–326. doi: 10.1053/j.ajkd.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Akash SI, Almond PS, Savell VH, Jr, et al. Eculizumab induces long-term remission in recurrent posttransplant HUS associated with C3 gene mutation. Pediatr Nephrol. 2011;26:613–619. doi: 10.1007/s00467-010-1708-6. [DOI] [PubMed] [Google Scholar]
- 41.Nester C, Stewart Z, Myers D, et al. Preemptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2011;6:1488–1494. doi: 10.2215/CJN.10181110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weitz M, Amon O, Bassler D, et al. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic syndrome. Pediatr Nephrol. 2011;26:1325–1329. doi: 10.1007/s00467-011-1879-9. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerhackl LB, Hofer J, Cortina G, et al. Prophylactic eculizumab after renal transplantation in atypical hemolytic-uremic syndrome. N Engl J Med. 2010;362:1746–1748. doi: 10.1056/NEJMc1001060. [DOI] [PubMed] [Google Scholar]
- 44.Saland JM, Ruggenenti P, Remuzzi G. Liver-kidney transplantation to cure atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2009;20:940–949. doi: 10.1681/ASN.2008080906. [DOI] [PubMed] [Google Scholar]
- 45.Saland JM, Shneider BL, Bromberg JS, et al. Successful split liver-kidney transplant for factor H associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:201–206. doi: 10.2215/CJN.02170508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jalanko H, Peltonen S, Koskinen A, et al. Successful liver-kidney transplantation in two children with aHUS caused by a mutation in complement factor H. Am J Transplant. 2008;8:216–221. doi: 10.1111/j.1600-6143.2007.02029.x. [DOI] [PubMed] [Google Scholar]