Abstract
The neuronal subthreshold-operating A-type K+ current regulates electrical excitability, spike timing, and synaptic integration and plasticity. The Kv4 channels underlying this current have been implicated in epilepsy, regulation of dopamine release, and pain plasticity. However, the unitary conductance (γ) of neuronal somatodendritic A-type K+ channels composed of Kv4 pore-forming subunits is larger (∼7.5 pS) than that of Kv4 channels expressed singly in heterologous cells (∼4 pS). Here, we examined the putative novel contribution of the dipeptidyl-peptidase-like protein-6 DPP6-S to the γ of native [cerebellar granule neuron (CGN)] and reconstituted Kv4.2 channels. Coexpression of Kv4.2 proteins with DPP6-S was sufficient to match the γ of native CGN channels; and CGN Kv4 channels from dpp6 knock-out mice yielded a γ indistinguishable from that of Kv4.2 channels expressed singly. Moreover, suggesting electrostatic interactions, charge neutralization mutations of two N-terminal acidic residues in DPP6-S eliminated the increase in γ. Therefore, DPP6-S, as a membrane protein extrinsic to the pore domain, is necessary and sufficient to explain a fundamental difference between native and recombinant Kv4 channels. These observations may help to understand the molecular basis of neurological disorders correlated with recently identified human mutations in the dpp6 gene.
Introduction
The subthreshold-operating somatodendritic A-type K+ current in neurons (ISA) dampens action potential firing in a variety of ways and thereby critically influences synaptic integration and plasticity (Birnbaum et al., 2004; Jerng et al., 2004a; Covarrubias et al., 2008). The channels underlying this current (Kv4 channels) have been implicated in epilepsy, the regulation of dopamine release (Liss et al., 2001), and pain plasticity (Bernard et al., 2004; Hu et al., 2006). The physiological significance of these channels in health and disease underscores the importance of understanding their molecular composition and the determinants of different aspects of channel function. The Kv4 channel complex is thought to be composed of pore-forming (α) Kv4 subunits and at least two classes of auxiliary (β) subunits: K+-channel-interacting proteins (KChIPs) and dipeptidyl-peptidase-like (DPPL) proteins (Birnbaum et al., 2004; Jerng et al., 2004a; Covarrubias et al., 2008; Maffie and Rudy, 2008). Both β-subunits influence gating and surface expression profoundly and appear to be sufficient to recapitulate the kinetic and gating properties of ISA (Jerng et al., 2005; Amarillo et al., 2008). However, whether Kv4 β-subunits also help to determine the unitary conductance (γ) of the native Kv4 channel has remained unsolved. Under similar ionic and recording conditions, the γ of mammalian Kv4 α-subunits expressed alone in heterologous cells is 4–5 pS (Jerng et al., 1999; Beck et al., 2002; Holmqvist et al., 2002), while that of the native Kv4 channel in mammalian neurons is at least 1.5-fold to twofold larger (Hoffman et al., 1997; Bekkers, 2000; Korngreen and Sakmann, 2000; Chen and Johnston, 2004). Previous studies ruled out any significant contribution of KChIPs to the γ of the Kv4 channel complex (Beck et al., 2002; Holmqvist et al., 2002). KChIPs bind to the cytoplasmic N terminus and T1 domain of the channel and, therefore, may not directly influence the pore domain and access sites for K+ (Pioletti et al., 2006; Wang et al., 2007). In contrast, DPPL proteins are single-pass integral membrane proteins (Kin et al., 2001) that may interact with the permeation and gating modules of the channel directly. The favorable effects of two DPPL proteins (DPP6 and DPP10) on voltage-dependent gating and surface expression of Kv4 channels are well documented, and recent studies have gained insights into the mechanisms of action and physiological impact (Nadal et al., 2003, 2006; Jerng et al., 2004b; Zagha et al., 2005; Dougherty and Covarrubias, 2006; Amarillo et al., 2008; Barghaan et al., 2008; Kim et al., 2008; Soh and Goldstein, 2008). However, whether the favorable effect of DPPL proteins on the size of macroscopic currents may include increased γ is still unknown. This possibility is exceptional because most β-subunits of voltage-gated cationic channels influence gating and surface expression but have no impact on γ. We hypothesize that DPP6-S is a key molecular determinant of the native γ in Kv4 channel complexes.
To test this hypothesis, we investigated native Kv4 channels in cerebellar granule neurons (CGNs, rat and mouse) and heterologously expressed recombinant Kv4 isoforms in the absence or presence of auxiliary β-subunits. Kv4 channels in CGNs are likely to include DPP6-S as an auxiliary β-subunit (Nadal et al., 2006; Amarillo et al., 2008; Maffie and Rudy, 2008). The γ magnitudes of the CGN Kv4 channel, the Kv4.2+DPP6-S complex, and the ternary Kv4.2 complex (Kv4.2+KChIP1+DPP6-S) were indistinguishable (∼7.5 pS) and approximately twofold larger than that of the Kv4.2 channel expressed singly in tsA-201 cells (∼4 pS). Demonstrating the necessary contribution of DPP6-S to the native Kv4 channel γ, we found that the γ of CGN Kv4 channels from DPP6-S knock-out animals was ∼4 pS. Furthermore, charge neutralization mutations affecting two acidic residues (D18N and E20Q) at the cytoplasmic N terminus of DPP6-S eliminated the increase in γ. These results are compelling evidence of the key role of DPP6-S as a determinant of the native γ in the neuronal Kv4 channel complex. From a mechanistic perspective, this finding demonstrates how an extrinsic subunit can specifically determine the permeation properties of Kv4 channels. We will discuss the physiological and pathological implications in this action in the context of related studies.
Materials and Methods
Molecular biology reagents.
Rat Kv4.2 cDNA (gift from Dr. M. Sheng, Massachusetts Institute of Technology, Cambridge, MA) was in pRc-CMV (Invitrogen); rat Kv4.3 cDNA (gift from Dr. J. Nerbonne, Washington University) was in pBK-CMV (Stratagene); and rat DPP6-S cDNA (short splice variant; also known as DPPX-S) was in pSG5 (Stratagene). DPP6-S mutations were created with the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and confirmed by automated sequencing at the Nucleic Acid Facility of the Kimmel Cancer Center (Thomas Jefferson University, Philadelphia, PA).
Acute dissociation of cerebellar granule neurons.
The isolation of cerebellar granule neurons was performed as previously described by others (Oberdoerster, 2001) and according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Thomas Jefferson University. Briefly, rat or mouse pups [wild-type and dpp6 knock-out mice (Zagha et al., 2008)] were killed on postnatal day 7 or 8 and the brains removed. The cerebellum was separated from the brain and the meninges were detached. The cerebellum was minced and the tissue digested with trypsin (0.125%) and DNase I (0.2%) at 37°C for 30 min. Then, the tissue homogenate was suspended in complete DMEM and filtered through 70 and 40 μm nylon mesh. For electrophysiological recording, the cells were plated onto glass coverslips coated with poly-d-lysine.
Heterologous expression (tsA-201 cells).
The transfection of tsA-201 cells (gift from Dr. R. Horn, Thomas Jefferson University, Philadelphia, PA) with cDNAs encoding Kv4.2 and DPP6-S was accomplished by the calcium-phosphate method as reported before (Dougherty and Covarrubias, 2006; Amarillo et al., 2008); and a plasmid containing the CD8 cDNA (gift from Dr. R. Horn, Thomas Jefferson University, Philadelphia, PA) was also cotransfected to allow the identification of individually transfected cells by labeling them with beads bearing anti-CD8 antibody (Dynal Biotech).
Heterologous expression (Xenopus oocytes).
Female Xenopus laevis were obtained from Nasco and the oocytes were harvested according to a protocol approved by the IACUC of Thomas Jefferson University. Briefly, the frogs were anesthetized by immersing them in 0.2% 3-aminobenzoic acid ethyl ester (Sigma) for ∼30 min. Ovarian lobes (1–3) were removed by making a 1 cm abdominal incision. Then, the wound was sutured and the frog was allowed to recover. Before injection, oocytes were defolliculated by digestion with collagenase (2 mg · ml−1, Boehringer Mannheim) in calcium-free external solution. Capped cRNAs for expression in Xenopus laevis oocytes were produced by in vitro transcription using the Message Machine Kit (Ambion). The Kv4.3 and DPP6-S cRNAs were mixed (typically, 1:2 to 1:4 molar ratio) and immediately injected (total RNA injected ∼2.5–20 ng per oocyte) into defolliculated oocytes using a Nanoject microinjector (Drummond). Currents were recorded 2–3 d after injection.
Electrophysiology (CGNs and tsA-201 cells).
Kv4.2 single channels expressed in tsA-201 cells were measured in the tight-seal cell-attached configuration of the patch-clamp method with the following pipette solution: 2 mm KCl, 150 mm NaCl, 1.5 mm CaCl2, 1.5 mm MgCl2, and 10 mm HEPES, pH 7.4, adjusted with NaOH. To record unitary currents from CGN, 20 mm NaCl in the pipette solution (above) was replaced with 20 mm TEA-Cl (tetraethylammonium chloride). TEA-Cl does not affect Kv4 channels but was necessary to eliminate endogenous delayed rectifier K+ currents expressed in CGN. The external bath solution contained: 152 mm KCl, 1.5 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES, pH 7.4, adjusted with KOH. Elevated external KCl in this solution nulls the cell's resting membrane potential. Patch pipettes were made from thick-walled (0.32 mm) borosilicate glass capillaries (Sutter Instrument), and the tips were coated with Sylgard elastomer (Dow Corning) before fire polishing. In the bath solution, pipette resistance was in the range from 6 to 10 MΩ. Data were recorded at 5 or 10 kHz (−3 dB, eight-pole Bessel) and digitally filtered for analysis at 2 kHz (Data Analysis).
Electrophysiology (Xenopus oocytes).
To record whole-oocyte currents, the two-electrode voltage-clamp method was applied as described previously (Beck et al., 2002). The bath solution for these experiments had the composition of the external solution described below. Patch-clamp recording (cell-attached) was conducted as described previously (Beck et al., 2002) using an Axopatch 200B amplifier (Molecular Devices). Patch pipettes were constructed from Corning Glass 7052 or 7056 (Warner Instrument) and were coated with Sylgard elastomer before the experiment. The pipettes' tip resistance ranged between 5 and 10 MΩ in the bath solution (see below). The pipette solution (external) contained the following (in mm): 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES (pH 7.4, adjusted with NaOH). Under these conditions the Kv4.3 reversal potential is usually −95 to −100 mV. For cell-attached recordings, the resting membrane potential is clamped near zero by exposing the oocyte to an extracellular bath solution containing the following (in mm): 98 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES (pH 7.4, adjusted with KOH). Voltage-ramp protocols (−100 to +100 mV; 2.12 mV · ms−1) were also used to validate the estimates of the main unitary conductance in patches from Xenopus oocytes (supplemental material, available at www.jneurosci.org). The recordings were filtered at 1.5 kHz (−3 dB, 8-pole Bessel filter; Frequency Devices) and digitized at 5 kHz. All experiments were recorded at room temperature (23 ± 1°C).
Data analysis.
The pClamp suite (Clampfit, versions 9 and 10; Molecular Devices) was used for initial analysis and data reduction; and Origin 7.5 (OriginLab) was used for further analysis and curve fitting by nonlinear least-squares methods. The passive leak current and the capacitive transients were eliminated off-line by subtracting the average of null sweeps (no opening events) within a record (typically, 10–30% of the acquired sweeps). At the beginning of the depolarizing steps, when the open probability is high, most recordings in the analyzed datasets did not display superimposed opening events. The recordings from Kv4.2-ternary channels were the exception (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). Frequently, the level of expression of these channels in cell-attached patches produced macroscopic currents. The activity was reduced to the level of single channels by applying relatively depolarized holding membrane potentials (−80 to −60 mV), which induces steady-state inactivation. To measure the mean unitary amplitudes, we constructed traditional all-point amplitude (APA) histograms from individual sweeps digitally filtered at 2 kHz (bin size = 0.02 pA). Only sweeps with significant unitary activity were analyzed in detail (e.g., see Fig. 1), and the length of the sweep included in the analysis was adjusted to sample sufficient background points (closed level) without overwhelming the number of apparent opening points. The APA histograms from individual sweeps were described by assuming a sum of Gaussian terms. Between 1 and 10 kHz, the variance of the background was indistinguishable from the variance of the open level (see Fig. 3). Therefore, for a given APA histogram, the variances of all components (main open level and sublevels) in the Gaussian sum were fixed at the background's level and the mean amplitudes were estimated from the best fit (see Fig. 2). To test the accuracy of the unitary mean amplitude estimates obtained from the multi-Gaussian fits, we applied a bootstrap resampling plan (Efron, 1982) and examined >30 randomized APA histograms/patch from selected patches. The outcome of this analysis is summarized in supplemental Table S1 (available at www.jneurosci.org as supplemental material).
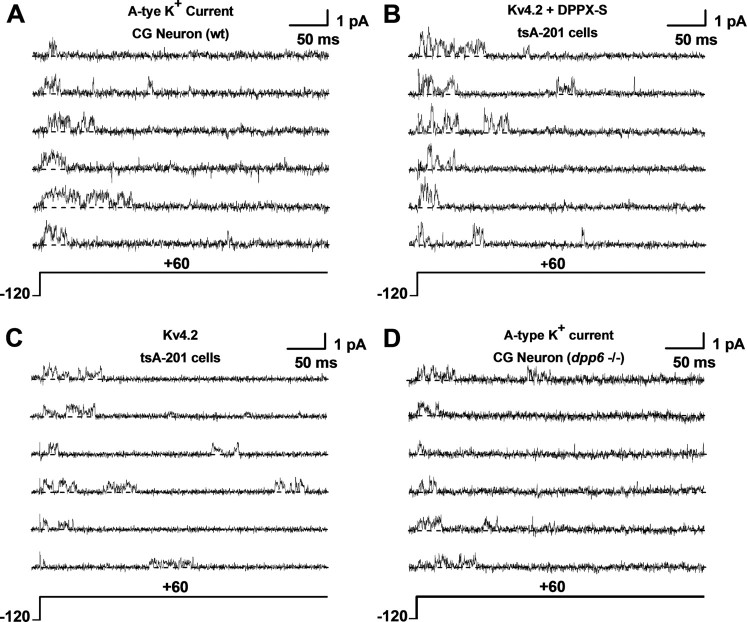
Figure 1.
Unitary currents mediated by native and recombinant Kv4.2 channels. A–D, Unitary current traces evoked by 400 ms step depolarizations from −120 to +60 mV. The holding potential was −120 mV and the start-to-start interval was 2 s, which allows complete recovery from inactivation between pulses. All currents were acquired in the cell-attached configuration of the patch-clamp method (see Materials and Methods). Kv4.2 and Kv4.2+DPP6-S represent currents from tsA-201 cells transfected with the corresponding cDNAs; CG Neuron wt and CG Neuron dpp6−/− correspond to native A-type K+ currents expressed in cerebellar granule neurons from wild-type (rat) and knock-out (mouse) animals, respectively (see Materials and Methods). Each set includes six traces from the same patch. The currents were originally recorded at 10 kHz (−3 dB, low-pass Bessel filter) and digitized at 20 μs intervals. For display and amplitude analysis, the current records were digitally filtered at 2 kHz (Clampfit's Gaussian filter; see Materials and Methods). The pipette solution contained physiological salt concentrations, and the bath solution contained elevated K+ to null the resting membrane potential (see Materials and Methods). The dashed line accompanying each trace marks the zero current (closed) level.
Figure 3.
Kv4.2 unitary currents do not exhibit fast flickering. A, Overlay of unitary current traces displayed at two different bandwidths, 5 and 2 kHz (gray and black, respectively) (−3 dB, Gaussian filter; see Materials and Methods). The currents were evoked by the pulse protocol indicated at the bottom of this panel. The dashed white line depicts the zero current (closed) level. B, Magnified overlay of a current trace low-pass filtered at 10 and 0.5 kHz (gray and black, respectively). Dashed line and arrow mark the zero current level. By comparing these traces, opening events were identified by eye before evaluating the variances of the closed (background) and open levels. C, APA histograms of the closed (left) and main-open (right) levels generated from current traces acquired at 10 kHz (−3 dB, low-pass Bessel filter) and digitized at 20 μs intervals. The solid lines superimposed on the histograms are best-fit Gaussians. The best-fit mean (i) and variance (σ2) are indicated in the graphs. D, APA histograms of the closed (left) and main-open (right) levels generated from current traces acquired as described above and digitally filtered at 2 kHz. Additional aspects of these graphs are as explained for C above. Note that the variances of the closed and open levels at a given bandwidth are indistinguishable and that the mean amplitude of the main-open level is insensitive to bandwidth between 2 and 10 kHz. Excessive flickering due to relatively fast gating between the open and closed levels is therefore unlikely.
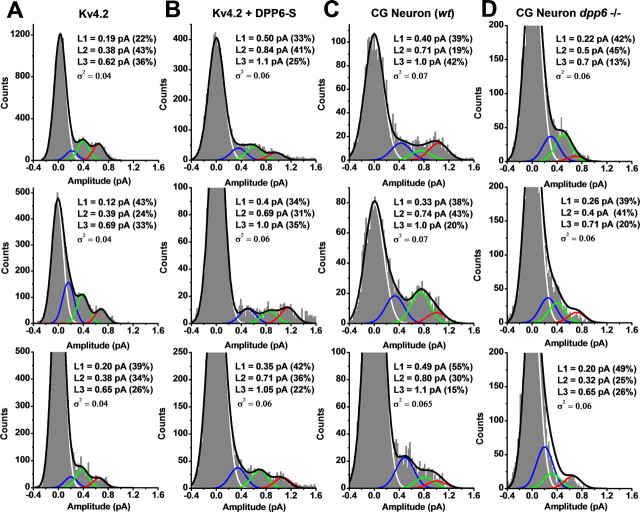
Figure 2.
Amplitude analysis of Kv4.2 unitary currents. A–D, Representative all-point amplitude (APA) histograms from unitary currents evoked by step depolarizations to +60 mV. The bin size in all cases is 0.02 pA. These APA histograms were derived from selected traces displayed in Figure 1: Kv4.2, traces 5, 3, and 4; Kv4.2+DPP6-S, traces 3, 5, and 1; CGN wt, traces 3, 5, and 6; CGN dpp6−/−, traces 5, 6, and 4. These traces are numbered from the top in each set from Figure 1. A sum of four Gaussian terms was used to describe the histogram profiles (black line; see Materials and Methods), and the individual distributions corresponding to the terms of the Gaussian sum are color coded and plotted separately to indicate the closed level (white) and three open levels (L1, L2, and L3 in blue, green, and red, respectively). L1 and L2 are sublevels and L3 is the main level of the unitary current. For each APA histogram, the best-fit sum of Gaussian terms assumes that the variance of the open levels was identical to that of the background (closed level) (Fig. 3 and Materials and Methods). The best-fit parameters are displayed in an inset for each graph. In some instances, zooming on the small peaks corresponding to the open levels caused the apparent truncation of the peak corresponding to the closed level (baseline).
To verify the traditional analysis method, we constructed Patlak's mean–variance plots from individual sweeps and calculated running averages to extract the mean amplitudes of the open levels (Dempster, 1993; Patlak, 1993) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Briefly, running averages selectively exclude points close to and during transitions between states. To minimize histogram distortion due to smoothing of transitions between states, the exclusion criterion is based on determining the variance within the chosen averaging window. Relative to the background noise, the variance is excessive when the chosen window spans a transition between states. Therefore, this window is excluded from the analysis. Otherwise, the variance of sample windows completely contained within an open or closed state is near the minimum variance of the background, and therefore, the corresponding data points are included in the average. The net effect of this procedure is to decrease the variance of the amplitude distributions, and consequently, it sharpens the histogram peaks of the resolved current levels; however, it is biased toward long open and closed events. In general, we found a good agreement between the results generated by this method and the traditional analysis of APA histograms (above). For additional verification in Xenopus oocytes, we estimated γ from voltage-ramp experiments (above). All results are expressed as mean ± SEM of n independent measurements (patches). Differences were evaluated by applying a one-way ANOVA test and were considered significant at p < 0.05.
Results
The unitary currents from native and recombinant Kv4 channels
In the cell-attached configuration, we recorded Kv4 unitary currents mediated by native channels [cerebellar granule neurons (CGNs)] and recombinant Kv4.2 channels expressed in mammalian tsA-201 cells (see Materials and Methods). In all instances, the unitary currents were evoked by step depolarizations (from −120 mV to different test potentials), and were typically observed with high probability within the first 100 ms of the step's duration (Fig. 1). Inactivation of single-channel activity is consistent with the transient A-type behavior of Kv4 channels; and moreover, these unitary currents were insensitive to 20 mm tetraethylammonium (TEA, see Materials and Methods), which is characteristic of A-type K+ currents mediated by Kv4 channels (Jerng et al., 2004a; Amarillo et al., 2008). Also, as observed previously, these unitary currents exhibited complex kinetics and frequent subconducting levels (Jerng et al., 1999; Beck et al., 2002; Holmqvist et al., 2002) (Fig. 1; see Fig. 5; supplemental material, available at www.jneurosci.org). To survey the amplitudes of the unitary currents, we constructed all-point-amplitude (APA) histograms from individual sweeps and fitted them with a sum of Gaussian terms to extract the mean values (see Materials and Methods) (Fig. 2). In all cases, this analysis revealed at least three distinct open levels (L1, L2, and L3), which typically occurred within bursts of single-channel activity (Figs. 1, 2; Table 1). At +60 mV, the mean amplitudes of the open levels of rat CGN channels were as follows (in pA): 0.43 ± 0.05, 0.81 ± 0.06, and 0.96 ± 0.03 (n = 3) (Fig. 2C, Table 1). CGN channels from mice exhibited similar amplitudes (data not shown), which are nearly identical to those observed in recordings from recombinant Kv4.2 channels coexpressed with DPP6-S (Fig. 2B, Table 1). In contrast, the mean unitary amplitudes from recombinant Kv4.2 channels expressed alone were significantly smaller (Fig. 2A, Table 1). Suggesting the specific influence of DPP6-S on unitary amplitudes, the relative contributions of the distinct open levels appeared mostly unchanged (Table 1). The results from multi-Gaussian fits were validated by applying a bootstrap resampling plan to assess the accuracy of the unitary mean amplitude estimates (see Materials and Methods; supplemental Table S1, available at www.jneurosci.org as supplemental material). In addition, distinct open levels and their amplitudes were confirmed unambiguously from mean–variance plots and histograms of running averages (Dempster, 1993; Patlak, 1993) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). These results suggest strongly that DPP6-S in native and recombinant Kv4 complexes is responsible for the differences in the unitary current amplitudes. To support this explanation more firmly, we examined unitary currents mediated by CGN channels from DPP6-S knock-out (dpp6−/−) mice. As expected, the mean unitary currents of Kv4.2 channels expressed singly in tsA-201 cells and CGN channels from dpp6−/− mice were similar (Figs. 1C,D, 2A,D).
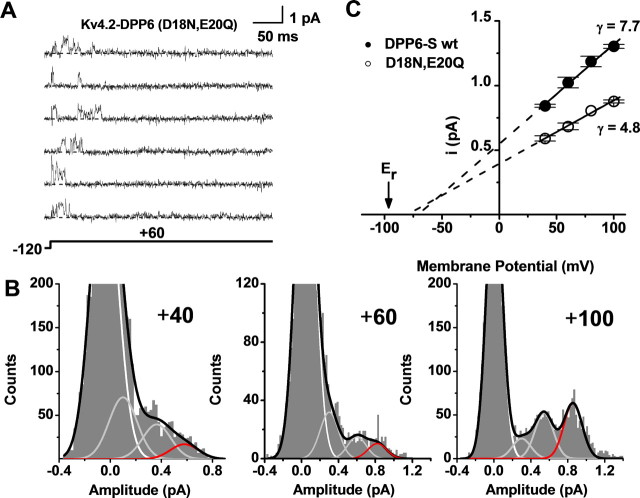
Figure 5.
Acidic N-terminal residues determine the effect of DPP6-S on Kv4 unitary conductance. A, Unitary currents induced by the expression of the Kv4.2+DPP6-S (D18N, E20Q) complex in tsA-201 cells. These currents were evoked by step depolarizations from −120 to +60 mV. The dashed line accompanying each trace marks the zero current (closed) level. Other details were as explained in Figure 1 legend. B, APA histograms generated, analyzed, and displayed as explained in Figures 2 and 4. Data from currents recorded at three different voltages are shown (+40, +60, and +100 mV). Zooming on the small peaks corresponding to the open levels caused the apparent truncation of the peak corresponding to the closed level (baseline). C, Unitary current–voltage relations from the Kv4.2+DPP6-S channel complex (wild type and mutant). Other aspects of this graph are as explained in Figure 4 legend. The control data are replotted from Figure 4. Note that the DPP6-S (D18N, E20Q) mutant loses its ability to increase the main unitary slope conductance of the Kv4.2 channel. All symbols with error bars in the unitary current–voltage plots represent means ± SEM (n = 3).
Table 1.
Mean unitary current amplitudes at +60 mV
| L1 (pA) | L2 (pA) | L3 (pA) | |
|---|---|---|---|
| Kv4.2 | 0.198 ± 0.016 | 0.421 ± 0.02 | 0.635 ± 0.024 |
| (42.6 ± 8.7%) | (43.1 ± 6.9%) | (14.3 ± 7.5%) | |
| Kv4.2+DPP6-S | 0.345 ± 0.03 | 0.712 ± 0.05 | 1.023 ± 0.03 |
| (36.8 ± 7.4%) | (45.7 ± 8.7%) | (17.5 ± 8.8%) | |
| CGN (rat) | 0.432 ± 0.05 | 0.809 ± 0.06 | 0.96 ± 0.03 |
| (45.9 ± 9.2%) | (40.63 ± 8.9%) | (13.6 ± 9.1%) |
All values are means ± SEM (n = 3). Numbers in parentheses indicate the percentage of histogram area in different open levels (excluding the area corresponding to closed level). The mean unitary current of the Kv4.2 channel was significantly different from those of Kv4.2+DPP6-S and CGN (rat) channels at p < 0.001 (one-way ANOVA).
The mean amplitudes of Kv4.2 unitary currents are independent of recording bandwidth
DPP6-S may have produced an apparent increase in the unitary current by reducing fast flickering between the open and closed states of the channel. If Kv4.2 channels expressed alone exhibit fast flickering, the recording bandwidth should have a significant effect on the mean unitary amplitude of opening events. At broader bandwidths the amplitude of the openings would be closer to the true value; and narrower bandwidths would attenuate the amplitude of the opening events by filtering out fast flickering. To investigate this possibility, we examined Kv4.2 channels expressed alone and compared the mean unitary current between recordings low-pass filtered at 10 and 2 kHz (evoked by a step to +80 mV) (Fig. 3). Analysis of the corresponding APA histograms showed that the mean open amplitudes were 0.56 ± 1.49 pA and 0.55 ± 0.3 pA (mean ± SD) at 10 and 2 kHz, respectively (Fig. 3C,D). The apparent variances of the open and closed levels at 10 kHz were also indistinguishable, which is consistent with the absence of very fast flickering. The lack of an effect of recording bandwidth on mean unitary amplitude of the open channel suggests strongly that Kv4.2 channels do not exhibit fast flickering that could confound the results of this study. Therefore, the apparent increase in the unitary current induced by DPP6-S could not have resulted from filtering fast flickering of the unitary currents observed in the absence of DPP6-S.
DPP6-S is necessary and sufficient to explain the larger unitary conductance of neuronal Kv4 channels
To characterize the unitary current–voltage plots from native and recombinant Kv4.2 channels, we determined the slope conductances between +40 and +100 mV (Fig. 4). Homomeric Kv4.2 channels yielded 2 ± 0.01, 3.5 ± 0.02, and 4.1 ± 0.25 pS corresponding to levels L1, L2, and L3, respectively (Fig. 4C). The channels resulting from coexpressing Kv4.2 and DPP6-S displayed increased conductances: 5.5 ± 0.02, 7.4 ± 0.02, and 7.7 ± 0.3 pS for L1, L2, and L3, respectively (n = 3) (Fig. 4D). The anomalous extrapolated reversal potentials obtained from the slopes suggested exacerbated outward rectification and/or reduced K+ selectivity in the presence of DPP6-S (see Discussion). However, macroscopic current measurements have demonstrated no evidence of altered ionic selectivity of Kv4 channels associated with DPP6-S (Nadal et al., 2003, 2006; Dougherty and Covarrubias, 2006; Amarillo et al., 2008). To determine the contribution of DPP6-S to the ion permeation properties of native and recombinant Kv4.2 channels, we focused on the main unitary conductance (γm) corresponding to the L3 level (Fig. 4E, Table 2). The γm of Kv4.2 channels expressed alone was close to previous estimates (4.1 ± 0.25 pS, n = 3); and as suspected, DPP6-S increased it by ∼90% (7.7 ± 0.3 pS, n = 3). This increase recapitulates the γm of the native CGN channels (7.3 ± 0.4 pS, n = 3). Moreover, the γm of CGN channels from dpp6−/− mice was indistinguishable from that of Kv4.2 channels (4.2 ± 0.3 pS, n = 3). Thus, DPP6-S is necessary and sufficient to increase the unitary conductance of Kv4 channels, and thereby recapitulate the unitary conductance of the neuronal Kv4 channel in CGNs.
Figure 4.
DPP6-S is sufficient and necessary to recapitulate the unitary of conductance of neuronal Kv4 channels. A, B, APA histograms generated and analyzed as described in Figure 2 legend. The top, middle, and bottom histograms are derived from unitary current records evoked by pulses to +40, +80, and +100 mV, respectively. Other aspects of the recordings are as explained in Figure 1 legend. The black line represents the best-fit sum of four Gaussian terms. The white, gray, and red lines depict the theoretical distributions corresponding to background, sublevels, and main level, respectively. Zooming on the small peaks corresponding to the open levels caused the apparent truncation of the peak corresponding to the closed level (baseline). C, Unitary current–voltage relations from the recombinant homomeric Kv4.2 channel. The plots correspond to the amplitudes of the sublevels (squares and triangles) and the main level (circle), and the solid lines are the best linear regressions that estimate the slope conductances shown on the right-hand side of the graph. The dashed lines indicate the extrapolations to the voltage axis. Er is the reversal potential estimated experimentally from macroscopic tail-current measurements. D, Unitary current–voltage relations from the recombinant Kv4.2+DPP6-S channel complex. Other aspects of this graph are as explained for C above. Note that DPP6-S increases the slope conductances, and that in this condition the extrapolations to the voltage axis deviate significantly from the estimated Er. The latter suggests significant outward rectification of the unitary currents (text). E, Unitary current–voltage relations corresponding to the main open level (L3) of native and recombinant Kv4.2 channels. Note that the presence of DPP6-S in the native and recombinant cases confers a larger unitary conductance to the main open level. All symbols with error bars in the unitary current–voltage plots represent means ± SEM (n = 3; the CGN wt mouse is the exception, n = 2).
Table 2.
Main unitary conductance of recombinant and native Kv4 channels
| Kv4 channel | γm (pS) | n |
|---|---|---|
| Kv4.2 | 4.1 ± 0.3 | 3 |
| Kv4.3 | 4.4 ± 0.3 | 5 |
| Kv4.2:DPP6-S | 7.7 ± 0.3 | 3 |
| Kv4.3:DPP6-S | 6.9 ± 0.3 | 5 |
| Kv4.2 ternary | 7.8 ± 0.4 | 3 |
| CGN, rat | 7.3 ± 0.4 | 3 |
| CGN, mouse | 7.4, 7.8 | 2 |
| CGN, dpp6−/− | 4.2 ± 0.3 | 3 |
| Kv4.2:DPP6-S (D18N, E20Q) | 4.8 ± 0.2 | 3 |
| Kv4.3:DPP6-S (D18N, E20Q) | 4.9 ± 0.1 | 4 |
All values are means ± SEM from n cell-attached patches (third column). For CGN (mouse), two independent estimates are shown. The γm values were determined from the slope conductances of the unitary current–voltage relations corresponding to the L3 level in each condition (Figs. 2, 4). The γm of the Kv4.2 channel was significantly different from those of Kv4.2+DPP6-S and CGN (rat) channels at p < 0.001 (one-way ANOVA).
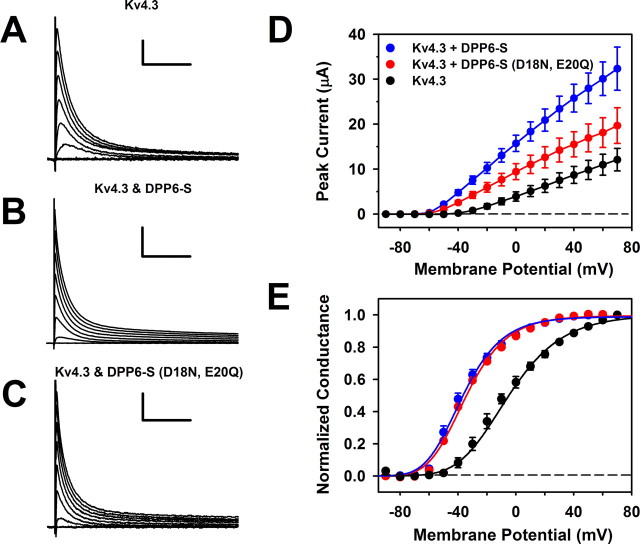
The favorable effect of DPP6-S on the γm of Kv4 channels was confirmed by examining Kv4.3 channels expressed in Xenopus oocytes, either alone or coexpressed with DPP6-S (supplemental Figs. S2–S4, available at www.jneurosci.org as supplemental material). As expected, the γm values of Kv4.3 and Kv4.2 channels are similar in the absence of auxiliary subunits (4.4 ± 0.3 pS, n = 5) (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). However, as for Kv4.2 coexpressed with DPP6-S in tsA-201 cells (Figs. 2–4), coexpression of Kv4.3 and DPP6-S in Xenopus oocytes resulted in significantly increased γm (6.9 ± 0.3 pS, n = 5) (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). The expression of DPP6-S was confirmed by demonstrating nearly complete inactivation of the Kv4.3 channels when the membrane potential was held relatively depolarized (−70 mV) (legends of supplemental Figs. S2, S4, available at www.jneurosci.org as supplemental material). This inactivation is expected because DPP6-S shifts the voltage dependence of steady-state inactivation in the hyperpolarized direction (Nadal et al., 2003; Dougherty and Covarrubias, 2006).
Any possible influence of KChIPs was tested by recording unitary currents from the Kv4.2 ternary complex (Kv4.2:KChIP1:DPP6-S) in tsA-201 cells. Although the kinetics of the Kv4.2 ternary complexes and the Kv4.2:DPP6-S complex are clearly different (Amarillo et al., 2008), we found no difference between the magnitudes of the γm (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). This observation confirms that KChIP1 does not influence the unitary conductance of neuronal Kv4 channels (Beck et al., 2002). Overall, the results strongly support the view of DPP6-S as an integral subunit of neuronal Kv4 channels. Strengthening this conclusion further, unitary current recordings from binary (Kv4.2 or Kv4.3 plus DPP6-S), ternary (including KChIP1), or native (CGN) channels did not exhibit transitions between high- and low-conductance phenotypes over the duration of the experiments.
Cytoplasmic N-terminal acidic residues in DPP6-S are responsible for the increase in unitary conductance
We hypothesized that negatively charged amino acid side chains (D18, E20) in the juxtamembrane cytoplasmic N-terminal region of DPP6-S could be responsible for the favorable effect of DPP6-S on the unitary conductance of Kv4 channels. To test this hypothesis, we created the double mutant DPP6-S (D18N, E20Q) and coexpressed it with Kv4.2 (in tsA-201 cells). The importance of testing this hypothesis is twofold: (1) it may shed light on the mechanism underlying the main observations of this study; and (2) it may help dissociate the specific role of these residues from the gating effects of the DPP6-S transmembrane and extracellular regions. The γm of Kv4.2 channels coexpressed with the DPP6-S double mutant was reduced to the level of that of Kv4.2 channels expressed alone (Fig. 5, Table 2).
The lack of effect of DPP6-S (D18N, E20Q) on the γm of Kv4 channels was confirmed in Xenopus oocytes (supplemental Fig. S6, available at www.jneurosci.org as supplemental material). The γm of Kv4.3 channels expressed alone or coexpressed with DPP6-S (D18N, E20Q) was virtually identical, suggesting that the mutation could have suppressed DPP6-S expression or destroyed the interaction with the channel. Contrary to these possibilities, however, the charge neutralization mutations did not affect the ability of DPP6-S to shift voltage-dependent gating in the hyperpolarized direction and accelerate current kinetics (Fig. 6). Macroscopically, the main effect of the DPP6-S mutations was to reduce the upregulation of the peak current, which is consistent with a reduced γm. Therefore, without influencing gating, N-terminal acidic amino acids in DPP6-S play a specific role in ion permeation in Kv4 channels. The DPP6-S double mutant results suggest an electrostatic action of acidic amino acids, which directly influences the Kv4 channel unitary conductance (see Discussion).
Figure 6.
Functional expression and gating properties of Kv4.3 channels coexpressed with DPP6-S (D18N, E20Q) in Xenopus oocytes. A–C, Whole-oocyte currents evoked by step depolarizations to command voltages ranging between −90 and +70 mV. Pulses were delivered from a holding potential of −100 mV at 10 mV intervals (for display, every other trace is shown). The start-to-start interval between pulses was ≥3 s. The scale bars correspond to 1 μA/100 ms, 5 μA/100 ms, and 1 μA/100 ms in A–C, respectively. D, Peak current–voltage relations of the currents represented in A–C. Symbols are mean ± SEM (n ≥ 7). Note that the double mutant DPP6-S (D18N, E20Q) exhibits a reduced ability to upregulate the peak current. E, Peak conductance–voltage relations corresponding to the experiments depicted in D. The solid lines are best-fit fourth-order Boltzmann functions with the following best-fit parameters (midpoint voltage and slope factor): V1/2 (Kv4.3) = −6 mV; k (Kv4.3) = 23 mV; V1/2 (Kv4.3+DPP6-s) = −36.7 mV; k (Kv4.3+DPP6-S) = 17 mV; V1/2 [Kv4.3+DPP6-S (D18N, E20Q)] = −34.7 mV; k [Kv4.3+DPP6-S (D18N, E20Q)] = 17 mV. Note that both wild-type and double mutant DPP6-S induce nearly identical leftward shifts (ΔV1/2 ≈ −30 mV).
Discussion
To explain a significant discrepancy between native and reconstituted Kv4 channels, we investigated the contribution of DPP6-S to γ. The conditions examined (Table 2) included Kv4.2 or Kv4.3 channels expressed in tsA-201 cells or Xenopus oocytes; either alone or coexpressed with DPP6-S (wild type and mutant). In addition, we measured the γ of native CGN channels from rat and mouse (wild type and dpp6−/−). Last, to gain insights into the structure–function relation, we tested the role of negatively charged amino acids at the juxtamembrane cytoplasmic region of DPP6-S. Overall, the results provide compelling evidence of a new role of DPP6-S as the Kv4 channel auxiliary β-subunit that dictates the γ of somatodendritic A-type K+ channels in CGN and possibly other DPP6-expressing neurons having Kv4 channels with similar γ.
Except for one study that found a unitary conductance of 13 pS (Korngreen and Sakmann, 2000), under similar ionic conditions, the reported γ of somatodendritic A-type K+ channels in the mammalian CNS ranges between 6 and 8.5 pS (Hoffman et al., 1997; Bekkers, 2000; Chen and Johnston, 2004). This range is in contrast to the significantly smaller unitary conductance of heterologously expressed homomeric Kv4 channels (Jerng et al., 1999; Beck et al., 2002; Holmqvist et al., 2002). Coexpressing Kv4.2 with DPP6-S yielded channels with a γm virtually identical to that of native Kv4 channels in CGN (∼7.5 pS). Furthermore, the γm of native Kv4 channels in CGN from mice lacking DPP6 was virtually identical to that of recombinant homomeric Kv4 channels (∼4 pS). It is therefore clear that DPP6-S is necessary and sufficient to recapitulate the physiological ion permeation properties of native Kv4 channels in neurons. Interestingly, the γ of Kv4 channels underlying the ITO in cardiac myocytes is 4 pS (Campbell et al., 1993). This might be related to the lack of expression of DPP6 in these cells. The dpp6 gene is not expressed in the heart from rat, mouse, or rabbit (Radicke et al., 2005) (A. Ozaita, W. Coetzee, and B. Rudy, unpublished observations).
Generally, auxiliary β-subunits of voltage-gated cationic channels affect gating kinetics and trafficking to the cell surface (Hanlon and Wallace, 2002; McCrossan and Abbott, 2004; Torres et al., 2007), and may confer redox modulation (Pan et al., 2008). However, the effects of these β-subunits on ion permeation properties are much less common and poorly understood. DPP6-S is a salient case because not only does it promote surface expression and facilitate gating and inactivation of Kv4 channels, it also enhances their γ. Comparatively, KCNE β-subunits are especially interesting because they interact promiscuously with Kv channels, affect gating (Abbott et al., 2001), and appear to increase the γ of KCNQ (Kv7) channels (Sesti and Goldstein, 1998; Yang and Sigworth, 1998) by unknown mechanisms. Uncertainties in this finding resulted from rapid unitary flickering and a significant bandwidth dependence of relatively small unitary currents. The estimations of γ were thus based on fluctuation analysis, which included untested assumptions about channel gating (Yang and Sigworth, 1998). Similar mechanisms may not explain the apparent similarities between the effects of KCNE β-subunits and DPP6-S on γ. Although both KCNE β-subunits and DPP6-S are single-pass membrane proteins, they are not genetically related, and the KCNE β-subunits' topology (cytoplasmic C terminus) is opposite to that of DPPL proteins. Moreover, the stoichiometry of the KCNQ+KCNE complex is 4:2 (Morin and Kobertz, 2008), whereas that of the Kv4.2+DPP6 complex is 4:4 (Soh and Goldstein, 2008); and KCNE may contribute to the permeation pathway in the pore domain directly (Tapper and George, 2001; McCrossan and Abbott, 2004; Kang et al., 2008). In contrast, DPP6-S may act through an electrostatic mechanism involving its cytoplasmic N-terminal region (see below).
Recently, the β2 subunit of the large-conductance calcium-activated K+ (KvCa or BK) channel was also identified as a regulator of ion permeation (Chen et al., 2008). BK channels are distantly related to Kv channels and are concomitantly gated by membrane potential depolarization and intracellular Ca2+. In contrast to DPP6-S and KCNEs, the BK channel β2 subunit introduces outward rectification by modestly decreasing the inward unitary currents under symmetrical ionic conditions. A lysine-rich extracellular loop connecting the two transmembrane segments of the β2 subunit appears responsible for the outward rectification through electrostatic repulsion (Chen et al., 2008). A more distant relationship to our study comes from the cytoplasmic β4-subunit of L-type voltage-gated Ca2+ channels, which increases the γ slightly (∼10%), but nothing is known about the underlying mechanism (Schjött et al., 2003).
Eliminating the increase in γ by conservative point mutations that neutralize cytoplasmic negative charges at the N terminus of DPP6-S is mechanistically informative. Critically, these mutations did not change the ability of DPP6-S to favorably influence voltage-dependent gating and kinetics (Fig. 6). Thus, the DPP6-S mutations do not obliterate expression or alter the protein's structure globally. Moreover, these results suggest that the membrane-spanning region and the cytoplasmic N terminus of DPP6-S are independent functional modules: the former may regulate gating through a direct transmembrane interactions with the channel's voltage sensing domain (Dougherty and Covarrubias, 2006); and the latter may regulate γ by an electrostatic mechanism. In BK channels, their characteristically large γ is in part determined by a ring of cytoplasmic acidic amino acids in the pore-forming α-subunit (Brelidze et al., 2003). The net negative charge conferred by these residues helps to concentrate K+ near the inner mouth of the channel. Analogously, we hypothesize that acidic amino acids in DPP6-S are ideally located in the intracellular juxtamembrane region of the protein to play a similar role. If the Kv4:DPP6-S stoichiometry is 4:4 in a fourfold symmetrical complex (Soh and Goldstein, 2008), a ring of eight negative charges may help to electrofocus and concentrate K+ at the inner mouth of Kv4 channels. Consequently, there is an increase in the channel's γ. This working hypothesis predicts outward rectification because the excess of internal negative charges would influence outward currents preferentially. Accordingly, exacerbated outward rectification was particularly apparent in the current–voltage plots of sublevels observed in the presence of DPP6-S (see Results) (Fig. 4D). Since the homologous DPP10a lacks one of the two juxtamembrane negative charges responsible for the conductance increase, we predict that neurons that use DPP10a instead of DPP6, such as cerebellar Purkinje cells and hippocampal GABAergic interneurons (citations), may have a smaller unitary conductance than neurons that have DPP6, such as CGNs and hippocampal CA1 pyramidal neurons (Amarillo et al., 2008; Kim et al., 2008).
DPP6-S has favorable synergistic effects on crucial aspects of Kv4 channel function. It promotes trafficking to the plasma membrane, enhances γ, accelerates recovery from inactivation, and reduces the energetic cost of gating by shifting the voltage dependence of activation toward negative membrane potentials. Namely, ISA upregulation by DPP6-S results from promoting channel trafficking to the plasma membrane and increasing γ. In Xenopus oocytes, the latter effect actually accounts for >50% of the increase in total Kv4 current induced by DPP6 in the absence of KChIPs (Fig. 6D). Since both KChIPs and DPP6-S promote channel trafficking, DPP6-S may account for all of the additional increase in macroscopic current when both auxiliary proteins are present, as is the case in neurons (Maffie and Rudy, 2008). It is therefore noteworthy that the total ISA is reduced to less than half in CGNs of DPP6 knock-out mice (Ozaita, Coetzee, and Rudy, unpublished observations). Given that KChIPs are still present in neurons from these mice, it is likely that the change in γ is substantially responsible for the effect of DPP6 ablation on ISA. Demonstrating that DPP6-S is necessary and sufficient to reconstitute the γ of the neuronal Kv4 channel complex is additionally important because it provides compelling evidence for the integral contribution of DPP6-S to the molecular assembly that underlies ISA.
The macroscopic Kv4 conductance in neurons is optimized by DPP6-S to oppose hyperexcitability and sharpen signaling (Kim et al., 2008). Rapid repriming upon hyperpolarization and operation over a relatively hyperpolarized range of membrane potentials permits effective regulation of excitability in the subthreshold range of membrane potentials. Therefore, the evolution of DPP6-S as an integral partner of neuronal Kv4 channels underlying ISA was probably driven by the advantageous gains in speed, efficiency, and ability to dampen excitability. Accordingly, we have observed interesting changes resulting from DPP6 ablation in knock-out mice, including effects on long-term potentiation, learning, and social behavior (Zagha et al., 2008). Dissecting how different effects of DPP6 contribute to functional or behavioral changes in knock-out mice will be challenging, as will be the potential contribution of DPP6 mutations to disease states. Interestingly, recent reports correlate human mutations in dpp6 genes with susceptibilities to amyotrophic lateral sclerosis (ALS) and autism (van Es et al., 2008; Marshall et al., 2008). These findings highlight the importance of understanding how DPP6 affects Kv4 channel function.
Footnotes
This work was supported by grants from the National Institutes of Health (R01 NS032337-13 to M.C. and NS045217 and NS30989 to B.R.). We thank Dianna Reineck for the creation and functional expression of the DPP6-S (D18N, E20Q) mutant. Also, we thank Dr. Richard Horn for his critical reading of this manuscript and help with the bootstrap resampling of histograms and the Covarrubias laboratory for comments and feedback throughout the realization of the work. Particularly, we thank Aditya Bhattacharji for excellent technical assistance.
References
- Abbott et al., 2001.Abbott GW, Goldstein SA, Sesti F. Do all voltage-gated potassium channels use MiRPs? Circ Res. 2001;88:981–983. doi: 10.1161/hh1001.091869. [DOI] [PubMed] [Google Scholar]
- Amarillo et al., 2008.Amarillo Y, De Santiago-Castillo JA, Dougherty K, Maffie J, Kwon E, Covarrubias M, Rudy B. Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons. J Physiol. 2008;586:2093–2106. doi: 10.1113/jphysiol.2007.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghaan et al., 2008.Barghaan J, Tozakidou M, Ehmke H, Bähring R. Role of N-terminal domain and accessory subunits in controlling deactivation-inactivation coupling of Kv4.2 channels. Biophys J. 2008;94:1276–1294. doi: 10.1529/biophysj.107.111344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck et al., 2002.Beck EJ, Bowlby M, An WF, Rhodes KJ, Covarrubias M. Remodelling inactivation gating of Kv4 channels by KChIP-1, a small-molecular-weight calcium binding protein. J Physiol. 2002;538:691–706. doi: 10.1113/jphysiol.2001.013127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers, 2000.Bekkers JM. Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol. 2000;525:611–620. doi: 10.1111/j.1469-7793.2000.t01-2-00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard et al., 2004.Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Birnbaum et al., 2004.Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Brelidze et al., 2003.Brelidze TI, Niu X, Magleby KL. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc Natl Acad Sci U S A. 2003;100:9017–9022. doi: 10.1073/pnas.1532257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell et al., 1993.Campbell DL, Rasmusson RL, Qu Y, Strauss HC. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol. 1993;101:571–601. doi: 10.1085/jgp.101.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2008.Chen M, Gan G, Wu Y, Wang L, Wu Y, Ding J. Lysine-rich extracellular rings formed by hbeta2 subunits confer the outward rectification of BK channels. PLoS ONE. 2008;3:e2114. doi: 10.1371/journal.pone.0002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen and Johnston, 2004.Chen X, Johnston D. Properties of single voltage-dependent K+ channels in dendrites of CA1 pyramidal neurones of rat hippocampus. J Physiol. 2004;559:187–203. doi: 10.1113/jphysiol.2004.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias et al., 2008.Covarrubias M, Bhattacharji A, De Santiago-Castillo JA, Dougherty K, Kaulin YA, Na-Phuket TR, Wang G. The neuronal Kv4 channel complex. Neurochem Res. 2008;33:1558–1567. doi: 10.1007/s11064-008-9650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster, 1993.Dempster J. Computer analysis of electrophysiological signals. San Diego: Academic; 1993. [Google Scholar]
- Dougherty and Covarrubias, 2006.Dougherty K, Covarrubias M. A dipeptidyl aminopeptidase-like protein remodels gating charge dynamics in Kv4.2 channels. J Gen Physiol. 2006;128:745–753. doi: 10.1085/jgp.200609668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron, 1982.Efron B. The jackknife, the bootstrap and other resampling plans. Bristol, UK: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- Hanlon and Wallace, 2002.Hanlon MR, Wallace BA. Structure and function of voltage-dependent ion channel regulatory beta subunits. Biochemistry. 2002;41:2886–2894. doi: 10.1021/bi0119565. [DOI] [PubMed] [Google Scholar]
- Hoffman et al., 1997.Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons [see comments] Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Holmqvist et al., 2002.Holmqvist MH, Cao J, Hernandez-Pineda R, Jacobson MD, Carroll KI, Sung MA, Betty M, Ge P, Gilbride KJ, Brown ME, Jurman ME, Lawson D, Silos-Santiago I, Xie Y, Covarrubias M, Rhodes KJ, Distefano PS, An WF. Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc Natl Acad Sci U S A. 2002;99:1035–1040. doi: 10.1073/pnas.022509299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al., 2006.Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW., 4th The Kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jerng et al., 1999.Jerng HH, Shahidullah M, Covarrubias M. Inactivation gating of Kv4 potassium channels: molecular interactions involving the inner vestibule of the pore. J Gen Physiol. 1999;113:641–660. doi: 10.1085/jgp.113.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng et al., 2004a.Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004a;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jerng et al., 2004b.Jerng HH, Qian Y, Pfaffinger PJ. Modulation of Kv4.2 channel expression and gating by dipeptidyl peptidase 10 (DPP10) Biophys J. 2004b;87:2380–2396. doi: 10.1529/biophysj.104.042358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng et al., 2005.Jerng HH, Kunjilwar K, Pfaffinger PJ. Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J Physiol. 2005;568:767–788. doi: 10.1113/jphysiol.2005.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang et al., 2008.Kang C, Tian C, Sönnichsen FD, Smith JA, Meiler J, George AL, Jr, Vanoye CG, Kim HJ, Sanders CR. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2008.Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, Rudy B, Hoffman DA. The Kv4 accessory protein DPPX is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100:1835–1847. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin et al., 2001.Kin Y, Misumi Y, Ikehara Y. Biosynthesis and characterization of the brain-specific membrane protein DPPX, a dipeptidyl peptidase IV-related protein. J Biochem. 2001;129:289–295. doi: 10.1093/oxfordjournals.jbchem.a002856. [DOI] [PubMed] [Google Scholar]
- Korngreen and Sakmann, 2000.Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol. 2000;525:621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss et al., 2001.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. EMBO J. 2001;20:5715–5724. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffie and Rudy, 2008.Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol. 2008;586:5609–5623. doi: 10.1113/jphysiol.2008.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall et al., 2008.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrossan and Abbott, 2004.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Morin and Kobertz, 2008.Morin TJ, Kobertz WR. Counting membrane-embedded KCNE beta-subunits in functioning K+ channel complexes. Proc Natl Acad Sci U S A. 2008;105:1478–1482. doi: 10.1073/pnas.0710366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal et al., 2003.Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- Nadal et al., 2006.Nadal MS, Amarillo Y, Vega-Saenz de Miera E, Rudy B. Differential characterization of three alternative spliced isoforms of DPPX. Brain Res. 2006;1094:1–12. doi: 10.1016/j.brainres.2006.03.106. [DOI] [PubMed] [Google Scholar]
- Oberdoerster, 2001.Oberdoerster J. Isolation of cerebellar granule cells from neonatal rats. Curr Protoc Toxicol. 2001:12.7.1–12.7.10. doi: 10.1002/0471140856.tx1207s09. [DOI] [PubMed] [Google Scholar]
- Pan et al., 2008.Pan Y, Weng J, Cao Y, Bhosle RC, Zhou M. Functional coupling between the Kv1.1 channel and aldoketoreductase Kvbeta1. J Biol Chem. 2008;283:8634–8642. doi: 10.1074/jbc.M709304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak, 1993.Patlak JB. Measuring kinetics of complex single ion channel data using mean-variance histograms. Biophys J. 1993;65:29–42. doi: 10.1016/S0006-3495(93)81041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioletti et al., 2006.Pioletti M, Findeisen F, Hura GL, Minor DL., Jr Three-dimensional structure of the KChIP1-Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol. 2006;13:987–995. doi: 10.1038/nsmb1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicke et al., 2005.Radicke S, Cotella D, Graf EM, Ravens U, Wettwer E. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative beta-subunit of human cardiac transient outward current encoded by Kv4.3. J Physiol. 2005;565:751–756. doi: 10.1113/jphysiol.2005.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjött et al., 2003.Schjött JM, Hsu SC, Plummer MR. The neuronal beta 4 subunit increases the unitary conductance of L-type voltage-gated calcium channels in PC12 cells. J Biol Chem. 2003;278:33936–33942. doi: 10.1074/jbc.M302059200. [DOI] [PubMed] [Google Scholar]
- Sesti and Goldstein, 1998.Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKS channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112:651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh and Goldstein, 2008.Soh H, Goldstein SA. I SA channel complexes include four subunits each of DPP6 and Kv4.2. J Biol Chem. 2008;283:15072–15077. doi: 10.1074/jbc.M706964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper and George, 2001.Tapper AR, George AL., Jr Location and orientation of minK within the I(Ks) potassium channel complex. J Biol Chem. 2001;276:38249–38254. doi: 10.1074/jbc.M103956200. [DOI] [PubMed] [Google Scholar]
- Torres et al., 2007.Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: beta-subunits and voltage-dependent K+ channels. J Biol Chem. 2007;282:24485–24489. doi: 10.1074/jbc.R700022200. [DOI] [PubMed] [Google Scholar]
- van Es et al., 2008.van Es MA, van Vught PW, Blauw HM, Franke L, Saris CG, Van den Bosch L, de Jong SW, de Jong V, Baas F, van't Slot R, Lemmens R, Schelhaas HJ, Birve A, Sleegers K, Van Broeckhoven C, Schymick JC, Traynor BJ, Wokke JH, Wijmenga C, et al. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat Genet. 2008;40:29–31. doi: 10.1038/ng.2007.52. [DOI] [PubMed] [Google Scholar]
- Wang et al., 2007.Wang H, Yan Y, Liu Q, Huang Y, Shen Y, Chen L, Chen Y, Yang Q, Hao Q, Wang K, Chai J. Structural basis for modulation of Kv4 K+ channels by auxiliary KChIP subunits. Nat Neurosci. 2007;10:32–39. doi: 10.1038/nn1822. [DOI] [PubMed] [Google Scholar]
- Yang and Sigworth, 1998.Yang Y, Sigworth FJ. Single-channel properties of IKS potassium channels. J Gen Physiol. 1998;112:665–678. doi: 10.1085/jgp.112.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha et al., 2005.Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, McCormack T, Akinsanya KO, Qi SY, Rudy B. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem. 2005;280:18853–18861. doi: 10.1074/jbc.M410613200. [DOI] [PubMed] [Google Scholar]
- Zagha et al., 2008.Zagha EW, Maffie JK, Jeong H-Y, Nadal M, Clark B, Kwon E, Goldberg EM, Rudy B. Electrophysiological and behavioral characterization of DPPX (DPP6) knock-out mice. Soc Neurosci Abstr. 2008;34:234, 13. [Google Scholar]