Abstract
Background
Emerging evidence demonstrates that targeting the tumor proteasome is a promising strategy for cancer therapy.
Objective
This review summarizes recent results from cancer clinical trials using specific proteasome inhibitors or some natural compounds that have proteasome-inhibitory effects.
Methods
A literature search was carried out using PubMed. Results about the clinical application of specific proteasome inhibitors and natural products with proteasome-inhibitory activity for cancer prevention or therapy were reviewed.
Results/conclusion
Bortezomib, the reversible proteasome inhibitor that first entered clinical trials, has been studied extensively as a single agent and in combination with glucocorticoids, cytotoxic agents, immunomodulatory drugs and radiation as treatment for multiple myeloma and other hematological malignancies. The results in some cases have been impressive. There is less evidence of bortezomib's efficacy in solid tumors. Novel irreversible proteasome inhibitors, NPI-0052 and carfilzomib, have also been developed and clinical trials are underway. Natural products with proteasome-inhibitory effects, such as green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG), soy isoflavone genistein, and the spice turmeric compound curcumin, have been studied alone and in combination with traditional chemotherapy and radiotherapy against various cancers. There is also interest in developing these natural compounds as potential chemopreventive agents.
Keywords: clinical trials, combinational therapy, hematological malignancies, natural products, proteasome inhibitors, solid tumors
1. Introduction
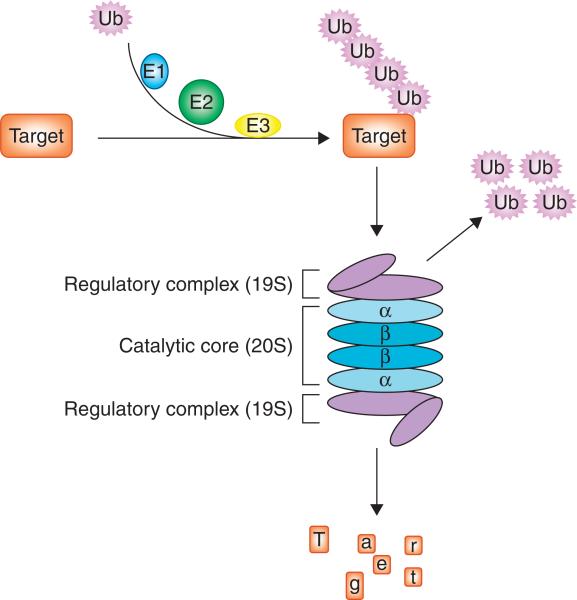
Protein synthesis and degradation is a tightly regulated process that is essential for normal cellular homeostasis. Most intracellular proteins are degraded by the proteasome, which is a multicatalytic enzyme complex containing one catalytic core, the 20S proteasome, and two 19S regulatory complexes (Figure 1). The proteolytic activity of the proteasome resides in the 20S proteasomal subunits, β1, β2 and β5, which are responsible for caspase-like (or peptidyl-glutamyl peptide-hydrolyzing-like, PGPH-like), trypsin-like, and chymotrypsin-like activities, respectively (Figure 2). Many proteasome target proteins, such as cyclins [1-4], tumor suppressor protein p53 [5], pro-apoptotic protein Bax [6], the cyclin-dependent kinase inhibitor (CKI) p27 [7], and the inhibitor of NF-κB, IκB-α [8,9], are involved in important processes of carcinogenesis and cancer survival. Proteasome inhibition in cancer cells leads to accumulation of pro-apoptotic target proteins and induction of cell death. The clinical efficacy of bortezomib in multiple myeloma and other hematologic malignancies lends credence to the concept that targeting the proteasome is a promising strategy for cancer treatment. In this review, we will discuss the development of bortezomib and other agents that target the tumor proteasome.
Figure 1. The ubiquitin-proteasome pathway.
Prior to proteasome degradation, target proteins are flagged with polyubiquitin chain transferred by ubiquitination system through interaction of the ubiquitin-activating enzyme E1, the ubiquitin-conjugating enzyme E2, and the ubiquitin ligase E3. Target proteins with polyubiquitin tag could be recognized by the 19S regulatory complex of the proteasome and fed into the 20S catalytic core, which has four stacked rings in αββα order. Each of the inner β rings contains seven β subunits with catalytic site facing the central cavity. Each of the outer α rings contains seven α subunits that serve as a gate for protein entry into the inner catalytic site.
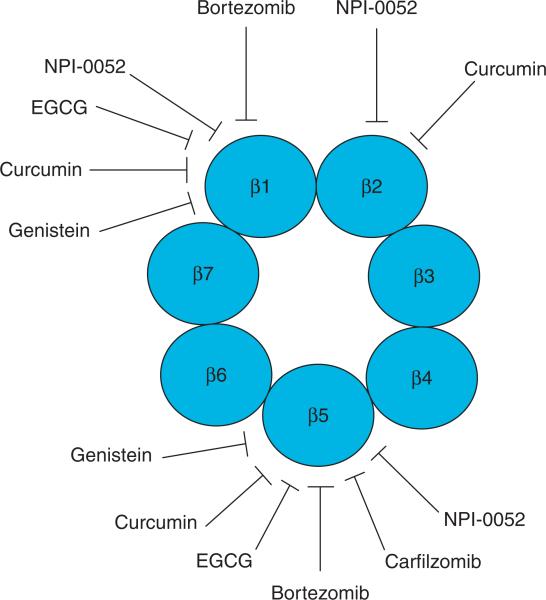
Figure 2. Proteasome inhibition by several compounds used in clinical trials.
β subunits of the proteasome have three catalytic sites: β1 (associated with caspase-like activity), β2 (associated with trypsin-like activity), and β5 (associated with chymotrypsin-like activity). Compounds in clinical trials could preferentially inhibit β5 chymotryptic site of the proteasome.
2. Proteasome inhibitor bortezomib in clinical trials
The first proteasome inhibitor approved by the US FDA for clinical use is bortezomib (Velcade, PS-341; Figure 3), a dipeptide boronic acid analogue developed by Millennium Pharmaceuticals and Johnson & Johnson Pharmaceutical Research & Development. This reversible proteasome inhibitor preferentially inhibits the β5-mediated chymotrypsin-like and β1-mediated PGPH-like activities of the proteasome (Figure 2) through an interaction of boronic acid at the C-terminus of bortezomib with the active N-terminus threo-nine of β5 or β1 subunit [10,11]. It has been demonstrated that the ratio of β2 subunit to (β1 + β5)-subunits varies in leukemia cells and influences their inherent sensitivity to the effects of bortezomib [12].
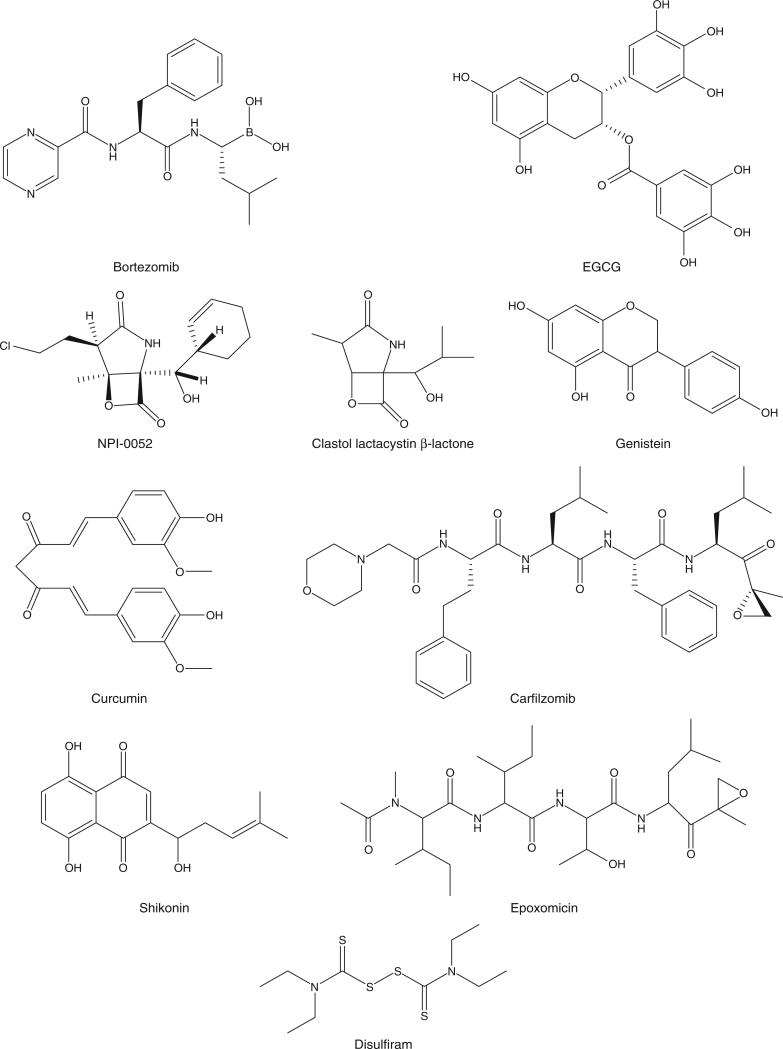
Figure 3.
Chemical structures of proteasome inhibitors and some natural products with proteasome-inhibitory effects.
2.1 Bortezomib clinical trials in multiple myeloma and other hematological malignancies
Patients with multiple myeloma (MM) have elevated circulating proteasome [13]. Clinical studies have demonstrated that circulating proteasome levels could serve as an independent prognostic factor for survival of MM, supporting the idea of targeting proteasome therapy in MM [13]. After a proof-of-concept study demonstrated the correlation of proteasome inhibition by bortezomib with tumor cell killing [14], clinical trials evaluating bortezomib as treatment for MM were initiated. Now approved by the FDA for the treatment of MM, the efficacy of bortezomib as a single agent or in combination with other chemotherapeutic drugs or radiation has been extensively investigated in MM as well as other hematological malignancies [15]. The remarkable single-agent efficacy of bortezomib in MM was demonstrated in Phase I trials that enrolled patients with various hematologic malignancies [16]. Following this, bortezomib was evaluated further in relapsed/refractory myeloma in two Phase II trials, the Clinical Response and Efficacy Study of Bortezomib in the Treatment of Relapsing Multiple Myeloma (CREST) and the larger Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition Therapy (SUMMIT).
The CREST trial compared two doses of bortezomib (1.3 mg/m2 and 1.0 mg/m2) and permitted the addition of dexamethasone in cases of inadequate response after two to four cycles of therapy. The response rate was slightly higher in the 1.3 mg/m2 group (50 vs 37%) [17]. Prominent side effects of bortezomib included myelosuppression, particularly thrombocytopenia, gastrointestinal symptoms, and peripheral sensory neuropathy. Recently, an extended follow-up report of the CREST trial reported a 5-year overall survival of 45% in the 1.3 mg/m2 group and 32% in the 1.0 mg/m2 group [18]. In the SUMMIT trial, 202 patients with relapsed, refractory myeloma were treated with bortezomib at a dose of 1.3 mg/m2, with dexamethasone added if needed, as in the CREST trial. The response rate in this trial was 35%, remarkable in this heavily pretreated patient population [19]. Subsequently, the APEX (Assessment of Proteasome Inhibition for Extending Remissions) trial, a large Phase III study comparing bortezomib with high-dose dexamethasone in previously treated myeloma, demonstrated bortezomib's superior activity compared with dexamethasone [20]. A study update confirmed that after a median 22 months’ follow-up, there was a 6-month overall survival benefit for the patients initially randomized to the bortezomib arm, despite substantial subsequent crossover from dexamethasone to bortezomib. It also demonstrated that both the overall response rate and complete response rate continued to improve compared with what was reported in the initial analysis with ongoing treatment [21]. Although the overall incidence of infections was similar in the bortezomib and dexamethasone arms of the APEX trial, the incidence of varicella zoster virus (VZV) reactivation was higher in the bortezomib arm (13 vs 5%, p = 0.0002) [22], prompting the recommendation that VZV prophylaxis be considered in all patients receiving bortezomib-based therapy.
Bortezomib-containing combination regimens have been explored extensively in MM. The most important of these is the combination of bortezomib with melphalan and prednisone (MP). This Phase III VISTA trial (Velcade as Initial Standard Therapy in Multiple Myeloma: Assessment with Melphalan and Prednisone) compared bortezomib-MP with MP in 682 patients with newly diagnosed myeloma. The trial was stopped early after it was found that bortezomib-MP was statistically superior to MP in terms of overall response rate (71 vs 35%), complete response rate (30 vs 4%), time to progression (24 vs 16.6 months), and overall survival [23]. Patients treated with bortezomib-MP had more treatment-related side effects, including neuropathy; but the incidence of the most severe complications, including treatment-related deaths, was similar in both arms. The combination of bortezomib plus pegylated liposomal doxorubicin has been shown to be more active than bortezomib alone in relapsed refractory myeloma [24], a finding of particular significance because both drugs can be used safely in patients with renal insufficiency, a frequent complication of myeloma [25]. In the up-front setting, the combination of bortezomib, doxorubicin, and dexamethasone induces responses in > 90% of myeloma patients [26].
Another highly effective bortezomib-containing combination is bortezomib-thalidomide-dexamethasone, with reported response rates in the range of 53 – 65% in patients with relapsed-refractory myeloma [27,28]. A Phase I/II trial of a triple drug regimen incorporating bortezomib with cyclophosphamide and prednisone in relapsed/refractory patients with MM produced an unprecedented effectiveness with 95% overall response, including complete responses (in > 50% of patients) plus partial and minimal response and encouraging 1-year survival [29]. Perhaps the most eagerly anticipated bortezomib-containing combination regimen is bortezomib plus the immunomodulatory drug, lenalidomide. The overall response rate to this combination in a large Phase I/II study in newly diagnosed myeloma was reported to be almost 100% [30]. Other investigators have begun exploring more complex bortezomib-containing regimens as up-front myeloma therapy – including prior to autologous stem cell transplant – and it is expected that the therapeutic landscape in myeloma with respect to bortezomib will continue to evolve rapidly [31,32].
Although preclinical studies demonstrated that bortezomib enhanced radiosensitivity in MM [33], responses of MM patients to the combinational treatment of bortezomib and radiation were different. While a woman with MM receiving spinal radiation with concurrent bortezomib developed severe, acute enteritis [34], concurrent bortezomib and radiation in a male MM patient showed an acceptable GI tolerance [35]. Now a Phase I study (NCT00398762; The University of Michigan Comprehensive Cancer Center) to test the combination of bortezomib and radioimmunotherapy, a targeted form of radiation in treating patients with relapsed mantle cell or aggressive lymphoma, is recruiting patients.
The adherence of abnormal myeloma cells in the bone marrow leads to increased osteoclast function and decreased osteoblast activity, resulting in a microenvironment for MM cells thriving but osteolytic bone lesions for MM patients [36]. Treatment with bortezomib could significantly increase serum levels osteoblast markers, osteocalcin and bone-specific alkaline phosphatase (BAP) in MM patients [37]. In addition, bortezomib inhibited receptor activator of nuclear factor kappa B ligand (RANKL)-mediated activation of NF-κB and AP-1, and therefore in turn inhibited NF-κB and AP-1-related osteoclast [38]. Recently, detailed dissection of the interactions of MM cells and host microenvironment suggested that combined therapy targeting both MM cells and the host microenvironment might be possible to achieve complete eradication of MM [39].
One important area of research is to identify molecular mechanisms of bortezomib resistance in myeloma and other malignancies. Perifosine, an Akt inhibitor that prevents bortezomib-induced upregulation of survivin, has been shown to have synergistic antimyeloma activity with bortezomib [40], and is one of several new agents currently under clinical investigation. Bortezomib has also been shown to have activity in other hematologic malignancies. In the same Phase I trial mentioned previously [16], one patient with refractory mantle cell lymphoma (MCL) and another with follicular center cell lymphoma (FCCL) responded to treatment with single-agent bortezomib. In a Phase II clinical trial of patients with relapsed refractory B-cell non-Hodgkin's lymphoma (NHL) using bortezomib at a dose of 1.5 mg/m2 on days 1, 4, 8, and 11 of repeating 21-day cycles, 41% of 33 patients with MCL responded to therapy. The response rate in the other NHL patients in this trial, most of whom had diffuse large B-cell lymphoma, was less impressive [41].
Another Phase II study involving MCL patients and other patients with indolent NHL showed responses in patients with FCCL [42]. Two additional Phase II studies have since reconfirmed the activity of bortezomib in MCL [43,44], leading to its FDA approval for treatment of this condition. The experience using bortezomib as treatment of Hodgkin's lymphoma is much more limited, but the drug appears to have minimal activity in this setting [45]. The response rate observed when bortezomib was combined with gemcitabine for the treatment of relapsed Hodgkin's lymphoma was not higher than that which might be expected from gemcitabine alone, and this combination was associated with significant liver toxicity [46].
2.2 Bortezomib clinical trials in solid tumors
Although clinical studies with bortezomib have demonstrated marked efficacy in the treatment of hematological malignancies, its efficacy in solid tumors appears limited. Bortezomib was tolerated, but no responses were observed in patients with aggressive metastatic breast cancers when bortezomib was used as a single agent [47,48]. A Phase I trial of bortezomib combined with weekly paclitaxel was conducted in patients with advanced breast, ovarian and prostate cancers. The lack of a clear clinical advantage in terms of efficacy of bortezomib with weekly paclitaxel over single-agent taxanes, and the cumulative neurological toxicity of this regimen, preclude its further clinical development [49]. In another trial, the combination of bortezomib and capecitabine was better tolerated, but the moderate antitumor activity in heavily pretreated metastatic breast cancer patients compared with that which might be expected from capecitabine alone was disappointing [50]. Likewise, when the combination of bortezomib plus docetaxel was explored in anthracycline-pretreated advanced/metastatic breast cancer patients, the 29% overall response rate and 5.4-month median time to progression [51] was similar to what has been previously reported for single-agent docetaxel in this setting (response rate: 32%; median time to progression: 5.7 months) [52].
Single-agent bortezomib exhibited only modest efficacy against advanced androgen-independent prostate cancer [53]. A Phase I/II dose escalation study show that bortezomib/docetaxel combination therapy demonstrated antitumor activity in patients with androgen-independent prostate cancer; however, its efficacy was similar to that of docetaxel treatment alone [54]. Similarly, treatment with combination of weekly bortezomib and docetaxel did not show improved efficacy versus previous results with docetaxel alone in the first-line treatment of patients with hormone-refractory prostate cancer [55]. Bortezomib single treatment or combination treatment with prednisone did not show significant antitumor effects in patients with castration-resistant metastatic prostate cancer [56].
Various studies in which bortezomib was added to standard therapies as treatment of advanced solid tumors have proven disappointing. It does not appear that bortezomib improves the efficacy of permetrexed in non-small cell lung cancer [57], gemcitabine in pancreatic cancer [58], irinotecan in various advanced solid tumors [59], or carboplatin in ovarian cancer [60,61]. Additionally, bortezomib as a single agent did not induce any objective responses in patients with metastatic neuroendocrine tumors [62].
Bortezomib in combination with radiotherapy in various solid tumors is being tested in trials. Bortezomib was given concurrently with re-irradiation to patients with recurrent head and neck squamous cell carcinoma (HNSCC). Inhibition of the proteasome activity along with inhibition of NF-κB activation and induction of cell death after treatment were observed compared with pretreatment [63]. In addition, two Phase I trials studying the effectiveness, side effects and best dose of bortezomib combining with radiation therapy (NCT00016003) or when given together with cetuximab and radiation therapy with or without cisplatin (NCT00629226) in treating patients with advanced head and neck cancer are recruiting patients at multiple cancer centers.
A further three trials are also recruiting patients: a Phase I study of bortezomib in combination with 5-fluorouracil and radiotherapy in treating patients with locally advanced and metastatic rectal cancer (NCT00280176, Lineberger Comprehensive Cancer Center and Vanderbilt-Ingram Cancer Center); a Phase I/II trial studying the side effects and best dose of bortezomib, paclitaxel, and carboplatin when given with radiation therapy for treatment in patients with advanced non-small cell lung cancer (NCT00093756); and a Phase I open-label, dose-escalation, safety study of the combination of bortezomib and chemoradiation for the treatment of patients with malignancies of the brain, head and neck, and cervix (NCT00329589, Thomas Jefferson University).
Taken together, although bortezomib plays a significant role in the treatment of hematological malignancies, unfortunately it has not demonstrated a similar clinical benefit in patients with solid tumors. Preclinical studies have indicated that proteasome inhibitors such as bortezomib activate antiapoptotic and mitogenic signaling pathways, including EGFR, extracellular signal-regulated kinase (ERK) and PI3K/Akt in pancreatic cancer, which might contribute to its lack of efficacy against solid organ malignancies [64]. Similarly, it has been shown that bortezomib activated the Akt pathway in prostate cancer cells (as has been demonstrated in myeloma), thus there has been some interest in targeting this pathway to overcome inherent bortezomib resistance in prostate cancer [65].
2.3 Toxicity of bortezomib
The most frequent toxic side effects associated with bortezomib treatment are nausea, fatigue, and diarrhea [17,19]; more serious adverse events include thrombocytopenia, peripheral neuropathy, neutropenia, and lymphopenia. Patients who started bortezomib treatment with low baseline platelet counts were at the greatest risk of developing clinically significant thrombocytopenia [19]. Similarly, the risk of developing neuropathy was highest in those patients with baseline neuropathy at the start of bortezomib therapy. In myeloma patients, neuropathy improved or resolved in the majority of patients with prompt dose reduction or discontinuation of bortezomib [19].
3. New proteasome inhibitors NPI-0052 and carfilzomib in clinical trials
3.1 NPI-0052
NPI-0052 (Figure 3), also known as salinosporamide A, is developed by Nereus Pharmaceuticals, Inc. (San Diego, CA, USA) [66]. Its structure is similar to clasto-lactacystin β-lactone (Figure 3), the active form of the first identified proteasome inhibitor lactacystin [67]. Different from bortezomib, NPI-0052 inhibits all the three activities of the proteasome in an irreversible manner with preference to inhibit chymotryptic activity and trypsin-like activity (Figure 2) [68,69]. Phase I dose-finding studies of NPI-0052 against advanced solid tumors or refractory lymphomas [70] and MM [71] have been conducted. Preclinical evidence of synergistic activity with lenalidomide provides a rationale for exploring this combination clinically in patients with MM [72]. Other combination studies, such as a Phase Ib open-label study to evaluate NPI-0052 in combination with vorinostat in patients with advanced non-small cell lung cancer, have also been initiated (NCT00667082, Nereus Pharmaceuticals).
3.2 Carfilzomib
Another novel proteasome inhibitor that has entered the clinic is carfilzomib (PR-171) (Figure 3), developed by Proteolix, Inc. (South San Francisco, CA, USA). Structurally it is related to epoxomicin (Figure 3). Carfilzomib is an irreversible proteasome inhibitor, with increased specificity for the chymotryptic activity of the proteasome over caspase-like or trypsin-like activities (Figure 2) [73]. Carfilzomib is more specific to target the chymotryptic site of the proteasome than bortezomib and NPI-0052. Furthermore, carfilzomib is more potent than bortezomib and could overcome bortezomib resistance [73]. Two Phase II trials demonstrate response rates in the range of 25 – 54% in patients with previously treated myeloma [74,75]. A clinical trial evaluating carfilzomib with lenalidomide and dexamethasone for safety and activity in relapsed MM is currently underway (NCT00603447, Proteolix).
4. Natural compounds with proteasome-inhibitory effects in clinical trials
4.1 Green tea polyphenols
Tea is widely consumed throughout the world, especially in Asian countries; (−)-epigallocatechin-3-gallate (EGCG) (Figure 3) is the most abundant and most active constituent of green tea. We previously reported that EGCG was a potent inhibitor of purified proteasome (Figure 2) [76]. The carbon of the ester bond of EGCG is essential for targeting, thereby inhibiting the proteasome in cancer cells. EGCG could also inhibit tumor cellular proteasome in vitro and in vivo [76,77]. EGCG or a green tea extract can be administrated orally, which provides some advantages in clinical trials. The efficacy of green tea on chemoprevention and chemotherapy has been widely investigated.
It has been reported that green tea consumption produced promising effects against development of various types of cancers without inducing major toxicities. In a pilot study to investigate the effects of green tea on premalignant lesions before prostate cancer development, 1-year daily treatment with 600 mg green tea capsules in 60 patients with high-grade prostate intraepithelial neoplasia significantly reduced the incidence of prostate cancer, showing 3% incidence among the 30 green tea-treated subjects compared with 30% incidence among the 30 placebo-treated subjects. No significant side effects or adverse effects were documented [78]. Similarly, risk of prostate cancer declined with increasing frequency, duration, and quantity of green tea consumption in a case-control study conducted in southeast China [79]. The effect of green tea against development of colon cancer was also observed. After the ingestion of a single dose of green tea, basal levels of prostaglandin E2 (PGE2), a biomarker of colorectal carcinogenesis, was reduced, suggesting green tea as a colorectal chemopreventive agent [80]. However, a large Phase II trial was set up to investigate the chemopreventive effects of decaffeinated green tea (DGT) on esophageal squamous carcinogenesis in China. Symptom-free patients with varying severity of esophageal precancerous lesions were enrolled in this study. An 11-year follow-up suggested that 1-year DGT intervention was not sufficient to alleviate the esophageal precancerous lesions [81].
Data obtained from chemotherapeutic application of green tea extracts as treatment of established solid tumors are not impressive, with limited activity observed in prostate cancer trials. In a Phase II clinical trial in patients without symptoms of prostate cancer but with biopsy-proven malignancy and clinical evidence of androgen-independent disease, green tea showed minimal antineoplastic activity [82]. One out of 42 patients showed tumor response as defined by the decline of serum prostate specific antigen (PSA) after administration of green tea powder 6 g/day for 6 months. Radiographic or physical examination indicated no biochemical tumor response [82].
The effect of green tea consumption on progressive hormone refractory prostate cancer has also been evaluated in a clinical trial [83]. The end point of the study was disease progression measured by either a relative PSA rise > 25% or radiological progression within a 2-month period. Only one out of 15 patients demonstrated a modest decrease of PSA, but this patient developed progressive nodal disease. Apparent slowing of disease progression was observed in six out of 15 patients in the first 1 – 4 months of the therapy, but the disease progressed within 3 – 5 months in these patients. The authors concluded that green tea produced minimal effects against hormone-refractory prostate cancers [83]. Similarly, no response was observed in patients with advanced lung cancer in a Phase I trial of green tea extract. All the enrolled 17 patients developed progressive disease within 4 months [84]. In contrast, clinicians at the Mayo Clinic reported clinical regression of chronic low-grade B-cell malignancies in four patients who had started taking green tea supplements without any other antineoplastic therapy [85], prompting interest in exploring the potential of green tea polyphenols in chronic lymphocytic leukemia and other hematologic malignancies.
One side effect of radiotherapy is the development of secondary tumors that depend on neovascularization. Annabi and colleagues reported that pretreatment of the human umbilical vein endothelial cell (HUVEC) with EGCG prevented ionizing radiation-stimulated cell migration, in vitro tubulogenesis and cell adhesion [86]. It is therefore important to test whether tea polyphenols consumption could sensitize human cancer cells to radiation.
In summary, data from available trials indicate that green tea polyphenols provide a rationale for exploring these compounds further in hematologic malignancies and as chemoprevention in prostate cancer, but not as therapy for established solid tumors. In vitro studies demonstrated that green tea polyphenol EGCG could sensitize tumor cells to chemotherapy and radiotherapy. For example, EGCG increased the sensitivity of prostate cancer cells to TRAIL (tumor necrosis factor related apoptosis-inducing ligand) [87] and enhanced the susceptibility of ovarian cancer cells to cisplatin [88]. To further investigate whether these bench results could be translated to bedside effects will be interesting.
It should be noted that very recently, Golden and co-workers reported that (−)-EGCG reacted directly with bortezomib, thus antagonizing the cell-killing efficacy of bortezomib in MM. The authors suggest that consumption of green tea products may be contraindicated during therapy with bortezomib [89]. While investigations on the combinational therapies with natural compounds are important, potential nutrition–drug interactions should also be kept in mind.
4.2 Genistein
Genistein (Figure 3) is the most active isoflavone present in soy. Antitumor effects of genistein may lie in its inhibition of cell proliferation, activation of apoptosis and inhibition of angiogenesis. Multiple molecular targets of genistein have been explored that are involved in these processes [90]. In silico computational docking predicted the possibility that genistein acts as a proteasome inhibitor (Figure 2) [91]. Consistently, around 30% of the chymotrypsin-like activity of a purified 20S proteasome could be inhibited by 1 μM of genistein [91]. It should be noted that plasma levels of genistein are in the range of 0.5 – 2.5 μM, and the concentrations of genistein vary in different tissues and organs [92,93]. Additionally, numerous studies have observed downregulation of NF-κB activity, a downstream event of proteasome inhibition in tumor cells in the presence of genistein [94]. These findings indicate the possibility that a partial inhibition of proteasome activity by genistein at physiological concentrations might contribute to its reported cancer-preventive and anticancer effects.
Genistein is regarded as a phytoestrogen because it is structurally similar to hormone and capable of binding to hormone receptors [95]. Therefore, genistein is proposed to be beneficial for hormone-related cancers, such as prostate and breast cancers. Epidemiological studies demonstrate that a soy-rich diet is associated with low risk of breast and prostate cancer, which is consistent with estradiol and androgen regulation [96]. The relationship between plasma isoflavones and fibrocystic breast cancer has been investigated in a large number of the Chinese population, showing that isoflavone exposure was inversely associated with fibrocystic breast conditions and breast cancer [97].
Chemotherapeutically applied genistein is usually given in a soy isoflavone formula containing genistein, daidzein, and glycitin at various ratios. The clinical characteristics and pharmacokinetic parameters of soy isoflavones have been extensively examined in healthy people as well as in prostate cancer patients. Genistein doses of 1, 2, 4, 8, or 16 mg/kg body weight were tested in 30 healthy men in a Phase I clinical trial. No clinical toxicities were observed, although lipoprotein lipase and hypophosphate were elevated, which may have been related to the treatment [98]. A double-blind clinical trial was conducted to evaluate the effects of 900 mg/day oral administration of soy isoflavones for 84 days to healthy post-menopausal women. Very few adverse events occurred, and the only drug-related adverse events were mild or grade I in severity [99]. Soy isoflavones (delivering approximately 300 or 600 mg/day genistein and half this much daidzein for > 3 months) were well tolerated in patients with stage B, C, or D adenocarcinoma of the prostate [100]. In an effort to determine the DNA-damaging effects of genistein, it was given daily to 20 prostate cancer patients at around 4 mg/kg body weight for 28 days and 8 mg/kg for additional 56 days. No significant genotoxicity was observed in these patients [101]. All these results indicate that soy isoflavones are safe in humans highly exceeding normal dietary intake. Using urinary concentrations of phytoestrogens as indicators, association of phytoestrogens with the rate of disease progression in men with untreated, localized prostate cancer was investigated in 191 British patients. However, the results indicate that phytoestrogen intake could not prevent disease progression [102].
A Phase II randomized, double-blinded, placebo-controlled trial was conducted to evaluate modulation of purified isoflavones on steroid hormones in men diagnosed with localized prostate cancer. Isoflavones were increased in serum, but failed to produce a corresponding, significant modulation of steroid hormone levels [103]. In an open-label Phase II nonrandomized trial, patients with relapse clinically localized prostate cancer after prior local therapy were treated with 141 mg/day isoflavone 12 months. Compared to that before study, the slope of PSA after study entry was found significant lower in 6 out of 20 patients, demonstrating some intervention effect on recurrent prostate cancer in a fraction of patients [104].
The dietary cancer-preventive agent genistein is expected to potentiate antitumor activities of common chemotherapeutics. In a Phase II clinical trial, the efficacy of lycopene alone or in combination with soy isoflavones on serum PSA levels in men with prostate cancer was investigated at Karmanos Cancer Institute. The activity of soy isoflavones against prostate cancer progression was observed; there may not be an additive effect between the two compounds when taken together [105]. Two other Phase II clinical trials are also underway, including a Phase II clinical trial to investigate combined therapy of genistein and gemcitabine hydrochloride for the treatment of patients with stage IV breast cancer (NCT00244933; Karmanos Cancer Institute) and a Phase II study to evaluate the use of genistein together with gemcitabine and erlotinib to treat patients with locally advanced or metastatic pancreatic cancer (NCT00376948; Karmanos Cacner Institute and MD Anderson Cancer Center at University of Texas).
Soy isoflavone was reported to sensitize non-small cell lung cancer (NSCLC) cells with nonfunctional p53 against radiation [106]. A treatment with genistein made both radiosensitive MO54 and radioresistant T98 human malignant glioma cells more sensitive to ionizing radiation [107]. Soy isoflavone was also found to inhibit prostate cancer cell growth and potentiates radiation-induced cell killing in vitro and in an ortho-topic model by downregulation of radiation induced APE1/Ref-1 and NF-κB expression [108]. Soy isoflavone was also used in patients with a rising PSA after radiotherapy treatment, and shown to delay PSA progression [104,109]. A randomized Phase II trial studying the effects of isoflavone together with radiotherapy in patients with localized prostate cancer (NCT00243048; Barbara Ann Karmanos Cancer Institute) has been completed; no publication is yet available. Another randomized study to evaluate the effectiveness and direct effect of a commercial soy supplement on prostate cancer prior to radiation or other treatment is currently recruiting patients (NCT00255125, VA Medical Center, Kansas City MO).
4.3 Curcumin
Curcumin (diferuloylmethane) (Figure 3) is derived from the spice turmeric. Curcumin inhibits cell proliferation, invasion, angiogenesis, metastasis, and osteoclastogenesis, which are involved in multiple cellular targets, such as NF-κB and COX-2 [110,111]. Curcumin possesses the ability to inhibit all three proteasomal activities, but with the highest potency to the chymotrypsin-like activity (Figure 2) [112]. Inhibition of the proteasome activity by curcumin was tightly associated with cell death induction in human colon cancer cultures and tumors grown in nude mice [112].
Pharmacological studies revealed that curcumin was poorly available after oral administration with low nanomolar levels of the parent compound and large amount of its glucuronide and sulfate conjugates found in the peripheral or portal circulation [113,114]. Despite its poor availability, the chemopreventive effects of curcumin have been reported. Daily administration of 8 g curcumin for 3 months demonstrated histological improvement of various precancerous lesions [115]. Inhibition of COX-2 activity and PGE2 production is associated with the chemopreventive efficacy of curcumin [116].
Chemotherapeutic effects of curcumin in colon cancer have been reported. A dose-escalation study of curcuma extract containing 36 – 180 mg curcumin was performed in 15 patients with advanced colorectal cancer refractory to standard chemotherapies. Oral curcuma extract was well tolerated, and dose-limiting toxicity was not observed. Neither curcumin nor its metabolites were detected in blood or urine, but curcumin was recovered from feces. Radiologically stable disease was demonstrated in five patients over 2 – 4 months of treatment [117]. A daily dose of 3.6 g curcumin could significantly decrease levels of inducible PGE2 production and DNA adduct (M(1)G) formed by malondialdehyde, confirming that curcumin could be achieved at pharmacologically active levels in the colorectum of humans [118,119].
Garcea and colleagues conducted a pilot trial to investigate whether oral administration of curcumin could reach concentrations that are sufficient to elicit pharmacological activity of the agent in liver tissue. Patients with hepatic metastasis from colon cancer were treated with 450 – 3600 mg/day curcumin for 1 week prior to surgery. Results indicated that curcumin at these doses could not be detected in liver tissues. The authors concluded that clinical trials of curcumin should focus on the prevention of colorectal tumors, but not tumors distant from the locus of absorption such as the liver [113]. In a Phase II clinical trial of curcumin in advanced pancreatic cancer, one patient had stable disease for > 18 months. No toxicities were observed for curcumin administration at 8 g/day. Downregulation of NF-κB and COX-2 was detected after post-treatment with curcumin [114].
Trials currently underway include an evaluation of colon cancer patients treated with gemcitabine in combination with curcumin and celecoxib (NCT00295035; Tel-Aviv Sourasky Medical Center) and pancreatic cancer patients treated with curcumin combined with gemcitabine (NCT00192842; Rambam Health Care Campus).
As a radiosensitizer, curcumin pretreatment enhanced radiotherapy in various cancers, including cervical, prostate and squamous cell carcinoma, in preclinical studies [120-123]. A clinical trial to evaluate the efficacy combining a curcumin preparation with standard radiation therapy and chemotherapy (capecitabine) in locally advanced rectal cancer is underway (NCT00745134; MD Anderson Cancer Center).
4.4 Shikonin
Shikonin (Figure 3) is an active naphthoquinone that is mainly isolated from the traditional Chinese medicine Zi Cao (gromwell). Several molecular targets have been explored related to cell-killing effect of shikonin. The proteasome was identified as one of its targets [124]. Shikonin inhibited the purified proteasome as well as cellular proteasome in cultured tumor cells and tumor tissues [124]. So far, only one clinical trial using shikonin-containing mixture is available. In 19 cases of late-stage lung cancer that were not suitable for surgery and irradiation, a shikonin-containing mixture reduced tumor diameter radiographically in 63.3% of patients, with a reported remission rate of 36.9%, and 1-year survival rate of 47.3% [125]. Furthermore, administration of the shikonin-containing mixture increased the body weight and appetite of patients. No harmful effects on the peripheral system, heart, kidney and liver were observed after shikonin treatment [125].
4.5 Disulfiram
Disulfiram (Figure 3) has been approved by the FDA for the treatment of alcoholism for over five decades. Disulfiram is a member of the dithiocarbamate family with metal-binding properties [126]. One of the potential targets of disulfiram is superoxide dismutase, inhibition of which could lead to inhibition of the angiogenic potential [127]. Disulfiram alone had little effect, but in the presence of Cu(II), it was converted back to the two-electron oxidized form of diethyldithiocarbamate, which is the active form in inducing apoptosis [128]. We have reported that disulfiram could bind to cellular copper to form a complex that has a proteasome-inhibitory effect, which might contribute to its apoptosis-inducing effect [129]. In addition to copper, disulfiram could complex with zinc. Brar and colleagues reported that a combination of oral zinc gluconate and disulfiram at currently approved doses for alcoholism could induce > 50% reduction in hepatic metastases, and produced clinical remission in a patient with stage IV metastatic ocular melanoma. The patient has continued on oral zinc gluconate and disulfiram therapy for 53 continuous months, with negligible side effects [126].
A Phase I/II trial to evaluate disulfiram in metastatic melanoma has been completed (NCT00256230; Chao Family Comprehensive Cancer Center); however, the results are not yet available. Also, a Phase I study to determine the safety and toxicity profile of disulfiram and copper gluconate co-treatment in refractory malignancies with liver metastasis is currently recruiting patients (NCT00742911; Huntsman Cancer Institute).
4.6 Other natural proteasome inhibitors
In addition to the above-mentioned natural products, several other natural proteasome inhibitors have been identified and tested for cancer therapy in vitro or in vivo in animal models. Some of them are identified from microorganism, including lactacystin and epoxomicin found in Streptomyces, and belactosin, gliotoxin and TMC-95A found in the culture broth of microorganism. Lactacystin inhibits all three enzymatic activities [67] and the other compounds primarily inhibit the chymotryptic activity of the proteasome [130,131]. Among them, lactacystin, epoxomicin and gliotoxin have been investigated for antitumor property in vivo, showing effectiveness against gliosarcoma, melanoma and breast cancer. In addition to monotherapy, lactacystin was found to sensitize refractory colon cancer HT-29 cells to etoposide and doxorubicin through restoration of topo IIα expression. We apologize for not being able to refer all the original publications on this topic because of the limited space.
Dietary flavonoids such as apigenin and quercetin are also shown to inhibit the proteasome activity [132]. Some studies demonstrated that dietary intake of flavonoids including apigenin and quercetin reduced the risk of cancer, while others did not support such an association. Apigenin alone was shown to inhibit tumor growth and metastasis in human breast and ovarian cancer, as well as melanoma cells, in vivo. Apigenin in combination with gemcitabine or cisplatin was shown to enhance tumor growth inhibition in human pancreatic cancer and melanoma in vivo. Quercetin was also effective against various cancers in single or combination treatment.
Finally, medicinal compounds such as celastrol and withaferin A isolated from medicinal plants has been reported to inhibit purified and cellular proteasome, as well as proteasome in tumor tissue [133,134]. Significant antitumor effect and antiangiogenic activity of using celastrol or withaferin A were observed in human prostate and breast cancer as well as melanoma. The radiosensitizing effect of withaferin A was reported in various mouse tumors.
To our best knowledge, except for one Phase I trial to test dose escalation, toxicity and efficacy of quercetin [135], there is no other available publication in relation with the clinical application using these natural compounds for cancer treatment. However, the efficacy in preclinical studies suggests their potential clinical use for cancer therapy. Again, because of the limited space, we could not review these proteasome inhibitors in detail.
5. Conclusion
Proteasome inhibitors represent a novel class of anticancer drugs that have a proven track record in conferring significant clinical benefit. So far bortezomib is the most widely investigated proteasome inhibitor in clinical trials. It has demonstrated remarkable efficacy against hematological malignancies, in both single and combination regimens, and is FDA-approved for treatment of MM and MCL. However, bortezomib has failed to show significant efficacy for the treatment of solid tumors. The toxicity of bortezomib is predictable and manageable, requiring the clinician to be familiar with managing the drug's side-effects. Researchers continue to investigate new proteasome inhibitors with increased potency and decreased side effects. The new proteasome inhibitors, NPI-0052 and carfilzomib, have been developed and studies are ongoing to evaluate their clinical characteristics. Furthermore, several natural compounds such as EGCG, genistein, curcumin and shikonin have been proven to inhibit the tumor proteasomal activity in vitro and in animal models. However, whether these compounds could also inhibit the tumor proteasome activity in a clinical setting should be investigated. Clinical studies using the natural products with proteasome-inhibitory activity were performed. Most of them suggest chemopreventive, but not chemotherapeutic, efficacy. Furthermore several trials suggest their chemo- or radiosensitizing activity, although this should be confirmed by more clinical studies.
6. Expert opinion
The association of proteasome inhibition with induction of apoptosis led to the clinical translation of proteasome inhibitors. Since being approved by the FDA for treatment of patients with MM and MCL, the proteasome inhibitor bortezomib has been well investigated in clinical trials. It demonstrates promising efficacy against hematological malignancies as a single treatment, or synergistic effects in combination with other chemotherapeutics or radiation. However, bortezomib alone showed minimal effects in the treatment of solid tumors, and bortezomib in combination could not improve patients’ response to currently used chemotherapy or radiation in solid tumors. Bortezomib resistance in solid tumors further encouraged researchers to develop novel proteasome inhibitors that act differently from bortezomib and novel natural compounds with proteasome-inhibitory activity as chemo/radiosensitizers. Recently, NPI-0052 and carfilzomib, the two representatives of a new generation of proteasome inhibitors, have been developed; they are currently being evaluated in clinical trials.
In addition to the above proteasome inhibitors, several natural compounds isolated from dietary products, medicinal plants and microorganisms have been shown to inhibit tumor proteasome activity, both in vitro and in animal models. However, whether these compounds could also inhibit tumor proteasome activity in a clinical setting should be investigated. Furthermore, natural compounds with a proteasome-inhibitory effect were also investigated in various clinical trials, showing some promising effect on chemo-/radiosensitization and chemoprevention, but limited efficacy on chemotherapy. Few of the clinical results supported application of natural products as a single treatment for established tumors, because of their lack of efficacy. Future clinical trials should further confirm natural proteasome inhibitors’ effect of sensitizing cancer cells to chemotherapy and radiotherapy.
Acknowledgements
This research is partially supported by Karmanos Cancer Institute of Wayne State University (to QP Dou), Department of Defense Breast Cancer Research Program Awards (W81XWH-04-1-0688 and DAMD17-03-1-0175 to QP Dou), National Cancer Institute/NIH (1R01CA120009; 5R03CA112625 to QP Dou), and the National Cancer Institute/NIH Cancer Center Support Grant (to Karmanos Cancer Institute). The authors thank Di Chen, Michael Frezza, and Carol Maconochie for critical reading of the manuscript.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–8. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 2.Won KA, Reed SI. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 1996;15:4182–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–72. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Lee J, Cho SY, Fine HA. Proteasome-mediated destruction of the cyclin a/cyclin-dependent kinase 2 complex suppresses tumor cell growth in vitro and in vivo. Cancer Res. 2004;64:3949–57. doi: 10.1158/0008-5472.CAN-03-3906. [DOI] [PubMed] [Google Scholar]
- 5.Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci USA. 2000;97:3850–5. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagano M, Tam SW, Theodoras AM, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Hagler J, Palombella VJ, et al. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–97. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 9.Alkalay I, Yaron A, Hatzubai A, et al. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–21. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 11.Dou QP, Goldfarb RH. Bortezomib (Millennium Pharmaceuticals). IDrugs. 2002;5:828–34. [PubMed] [Google Scholar]
- 12.Kraus M, Ruckrich T, Reich M, et al. Activity patterns of proteasome subunits reflect bortezomib sensitivity of hematologic malignancies and are variable in primary human leukemia cells. Leukemia. 2007;21:84–92. doi: 10.1038/sj.leu.2404414. [DOI] [PubMed] [Google Scholar]
- 13.Jakob C, Egerer K, Liebisch P, et al. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109:2100–5. doi: 10.1182/blood-2006-04-016360. [DOI] [PubMed] [Google Scholar]
- 14.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–22. [PubMed] [Google Scholar]
- 15.Sterz J, von Metzler I, Hahne JC, et al. The potential of proteasome inhibitors in cancer therapy. Expert Opin Investig Drugs. 2008;17:879–95. doi: 10.1517/13543784.17.6.879. [DOI] [PubMed] [Google Scholar]
- 16.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–7. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 17.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–72. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 18.Jagannath S, Barlogie B, Berenson JR, et al. Updated survival analyses after prolonged follow-up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma. Br J Haematol. 2008;143:537–40. doi: 10.1111/j.1365-2141.2008.07359.x. [DOI] [PubMed] [Google Scholar]
- 19.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 20•.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [Bortezomib efficacy was shown superior to high-dose dexamethasone for the treatment of relapsed MM in a large Phase III study.] [DOI] [PubMed] [Google Scholar]
- 21.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–60. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 22.Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the Phase III APEX study. J Clin Oncol. 2008;26:4784–90. doi: 10.1200/JCO.2007.14.9641. [DOI] [PubMed] [Google Scholar]
- 23•.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [Effective strategy using bortezomib combination as initial treatment for MM.] [DOI] [PubMed] [Google Scholar]
- 24.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized Phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 25.Blade J, Sonneveld P, San Miguel JF, et al. Pegylated liposomal doxorubicin plus bortezomib in relapsed or refractory multiple myeloma: efficacy and safety in patients with renal function impairment. Clin Lymphoma Myeloma. 2008;8:352–5. doi: 10.3816/CLM.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 26.Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129:755–62. doi: 10.1111/j.1365-2141.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- 27.Pineda-Roman M, Zangari M, van Rhee F, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22:1419–27. doi: 10.1038/leu.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciolli S, Leoni F, Gigli F, et al. Low dose Velcade, thalidomide and dexamethasone (LD-VTD): an effective regimen for relapsed and refractory multiple myeloma patients. Leuk Lymphoma. 2006;47:171–3. doi: 10.1080/10428190500272721. [DOI] [PubMed] [Google Scholar]
- 29•.Reece DE, Rodriguez GP, Chen C, et al. Phase I-II trial of bortezomib plus oral cyclophosphamide and prednisone in relapsed and refractory multiple myeloma. J Clin Oncol. 2008;26:4777–83. doi: 10.1200/JCO.2007.14.2372. [Highly effective bortezomib combination strategy as treatment for relapsed MM.] [DOI] [PubMed] [Google Scholar]
- 30.Richardson P, Lonial S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma: encouraging efficacy in high risk groups with updated results of a Phase I/II Study [abstract 92]. Blood. 2008;112(Suppl 1) [Google Scholar]
- 31.Kumar S, Flinn IW, Noga SJ, et al. Safety and Efficacy of novel combination therapy with bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in newly diagnosed multiple myeloma: initial results from the Phase I/II multi-center EVOLUTION study [abstract 93]. Blood. 2008;112(Suppl 1) [Google Scholar]
- 32.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–85. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 33.Goel A, Dispenzieri A, Greipp PR, et al. PS-341-mediated selective targeting of multiple myeloma cells by synergistic increase in ionizing radiation-induced apoptosis. Exp Hematol. 2005;33:784–95. doi: 10.1016/j.exphem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Mohiuddin MM, Harmon DC, Delaney TF. Severe acute enteritis in a multiple myeloma patient receiving bortezomib and spinal radiotherapy: case report. J Chemother. 2005;17:343–6. doi: 10.1179/joc.2005.17.3.343. [DOI] [PubMed] [Google Scholar]
- 35.Berges O, Decaudin D, Servois V, Kirova YM. Concurrent radiation therapy and bortezomib in myeloma patient. Radiother Oncol. 2008;86:290–2. doi: 10.1016/j.radonc.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Terpos E, Dimopoulos MA, Sezer O. The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia. 2007;21:1875–84. doi: 10.1038/sj.leu.2404843. [DOI] [PubMed] [Google Scholar]
- 37.Heider U, Kaiser M, Muller C, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77:233–8. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 38.von Metzler I, Krebbel H, Hecht M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21:2025–34. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 39.Anderson KC. Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol. 2007;35:155–62. doi: 10.1016/j.exphem.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Hideshima T, Catley L, Raje N, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138:783–91. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- 41.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:667–75. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–84. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 43.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter Phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–74. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 44.Belch A, Kouroukis CT, Crump M, et al. A Phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol. 2007;18:116–21. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 45.Younes A, Pro B, Fayad L. Experience with bortezomib for the treatment of patients with relapsed classical Hodgkin lymphoma. Blood. 2006;107:1731–2. doi: 10.1182/blood-2005-09-3731. [DOI] [PubMed] [Google Scholar]
- 46.Mendler JH, Kelly J, Voci S, et al. Bortezomib and gemcitabine in relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2008;19:1759–64. [Google Scholar]
- 47.Yang CH, Gonzalez-Angulo AM, Reuben JM, et al. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006;17:813–7. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- 48.Engel RH, Brown JA, Von Roenn JH, et al. A Phase II study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Invest. 2007;25:733–7. doi: 10.1080/07357900701506573. [DOI] [PubMed] [Google Scholar]
- 49.Cresta S, Sessa C, Catapano CV, et al. Phase I study of bortezomib with weekly paclitaxel in patients with advanced solid tumours. Eur J Cancer. 2008;44:1829–34. doi: 10.1016/j.ejca.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Schmid P, Kuhnhardt D, Kiewe P, et al. A Phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol. 2008;19:871–6. doi: 10.1093/annonc/mdm569. [DOI] [PubMed] [Google Scholar]
- 51.Awada A, Albanell J, Canney PA, et al. Bortezomib/docetaxel combination therapy in patients with anthracycline-pretreated advanced/metastatic breast cancer: a Phase I/II dose-escalation study. Br J Cancer. 2008;98:1500–7. doi: 10.1038/sj.bjc.6604347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones SE, Erban J, Overmoyer B, et al. Randomized Phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–51. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 53.Papandreou CN, Daliani DD, Nix D, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 54.Dreicer R, Petrylak D, Agus D, et al. Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Cancer Res. 2007;13:1208–15. doi: 10.1158/1078-0432.CCR-06-2046. [DOI] [PubMed] [Google Scholar]
- 55.Hainsworth JD, Meluch AA, Spigel DR, et al. Weekly docetaxel and bortezomib as first-line treatment for patients with hormone-refractory prostate cancer: a Minnie Pearl Cancer Research Network Phase II trial. Clin Genitourin Cancer. 2007;5:278–83. doi: 10.3816/CGC.2007.n.004. [DOI] [PubMed] [Google Scholar]
- 56.Morris MJ, Kelly WK, Slovin S, et al. A Phase II trial of bortezomib and prednisone for castration resistant metastatic prostate cancer. J Urol. 2007;178:2378–83. doi: 10.1016/j.juro.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Davies AM, Ho C, Metzger AS, et al. Phase I study of two different schedules of bortezomib and pemetrexed in advanced solid tumors with emphasis on non-small cell lung cancer. J Thorac Oncol. 2007;2:1112–6. doi: 10.1097/JTO.0b013e31815ba7d0. [DOI] [PubMed] [Google Scholar]
- 58.Alberts SR, Foster NR, Morton RF, et al. PS-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group (NCCTG) randomized Phase II study. Ann Oncol. 2005;16:1654–61. doi: 10.1093/annonc/mdi324. [DOI] [PubMed] [Google Scholar]
- 59.Ryan DP, O'Neil BH, Supko JG, et al. A Phase I study of bortezomib plus irinotecan in patients with advanced solid tumors. Cancer. 2006;107:2688–97. doi: 10.1002/cncr.22280. [DOI] [PubMed] [Google Scholar]
- 60.Aghajanian C, Dizon DS, Sabbatini P, et al. Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J Clin Oncol. 2005;23:5943–9. doi: 10.1200/JCO.2005.16.006. [DOI] [PubMed] [Google Scholar]
- 61.Ramirez PT, Landen CN, Jr, Coleman RL, et al. Phase I trial of the proteasome inhibitor bortezomib in combination with carboplatin in patients with platinum- and taxane-resistant ovarian cancer. Gynecol Oncol. 2008;108:68–71. doi: 10.1016/j.ygyno.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 62.Shah MH, Young D, Kindler HL, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;10:6111–8. doi: 10.1158/1078-0432.CCR-04-0422. [DOI] [PubMed] [Google Scholar]
- 63.Van Waes C, Chang AA, Lebowitz PF, et al. Inhibition of nuclear factor-kappaB and target genes during combined therapy with proteasome inhibitor bortezomib and reirradiation in patients with recurrent head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:1400–12. doi: 10.1016/j.ijrobp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Sloss CM, Wang F, Liu R, et al. Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin Cancer Res. 2008;14:5116–23. doi: 10.1158/1078-0432.CCR-07-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayala G, Yan J, Li R, et al. Bortezomib-mediated inhibition of steroid receptor coactivator-3 degradation leads to activated Akt. Clin Cancer Res. 2008;14:7511–8. doi: 10.1158/1078-0432.CCR-08-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feling RH, Buchanan GO, Mincer TJ, et al. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angew Chem Int Ed Engl. 2003;42:355–7. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- 67.Fenteany G, Standaert RF, Lane WS, et al. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–31. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 68.Groll M, Huber R, Potts BC. Crystal structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J Am Chem Soc. 2006;128:5136–41. doi: 10.1021/ja058320b. [DOI] [PubMed] [Google Scholar]
- 69.Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–19. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 70.Hamlin PA, Aghajanian C, Hong D, et al. First-in-human phase 1 dose escalation study of NPI-0052, a novel proteasome inhibitor, in patients with lymphoma and solid tumor [abstract 4939]. Blood. 2008;112(Suppl 1) [Google Scholar]
- 71.Richardson P, Hofmeister CC, Zimmerman TM, et al. Phase 1 clinical trial of NPI-0052, a novel proteasome inhibitor in patients with multiple myeloma [abstract 2770]. Blood. 2008;112(Suppl 1) [Google Scholar]
- 72.Chauhan D, Singh AV, Brahmandam M, et al. Combination of a novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vivo synergistic cytotoxicity in multiple myeloma [abstract 3662]. Blood. 2008;112(Suppl 1) doi: 10.1182/blood-2009-03-213009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–90. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jagannath S, Vij R, Stewart AK, et al. Initial results of PX-171-003, an open-label, single-arm, Phase II studyof carfilzomib (CFZ) in patients with relapsed and refractory multiple myeloma (MM) [abstract 864]. Blood. 2008;112(Suppl 1) [Google Scholar]
- 75.Vij R, Wang M, Orlowski R, et al. Initial results of PX-171-004, an open-label, single-arm, Phase II study of carfilzomib (CFZ) in patients with relapsed myeloma (MM) [abstract 865]. Blood. 2008;112(Suppl 1) [Google Scholar]
- 76.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 77.Landis-Piwowar KR, Huo C, Chen D, et al. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67:4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 78•.Bettuzzi S, Brausi M, Rizzi F, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [Chemopreventive effect of green tea was demonstrated on prostate cancer development.] [DOI] [PubMed] [Google Scholar]
- 79.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–5. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 80.August DA, Landau J, Caputo D, et al. Ingestion of green tea rapidly decreases prostaglandin E2 levels in rectal mucosa in humans. Cancer Epidemiol Biomarkers Prev. 1999;8:709–13. [PubMed] [Google Scholar]
- 81.Wang LD, Zhou Q, Feng CW, et al. Intervention and follow-up on human esophageal precancerous lesions in Henan, northern China, a high-incidence area for esophageal cancer. Gan To Kagaku Ryoho. 2002;29:159–72. [PubMed] [Google Scholar]
- 82.Jatoi A, Ellison N, Burch PA, et al. A Phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–6. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 83.Choan E, Segal R, Jonker D, et al. A prospective clinical trial of green tea for hormone refractory prostate cancer: an evaluation of the complementary/alternative therapy approach. Urol Oncol. 2005;23:108–13. doi: 10.1016/j.urolonc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 84.Laurie SA, Miller VA, Grant SC, et al. Phase I study of green tea extract in patients with advanced lung cancer. Cancer Chemother Pharmacol. 2005;55:33–8. doi: 10.1007/s00280-004-0859-1. [DOI] [PubMed] [Google Scholar]
- 85.Shanafelt TD, Lee YK, Call TG, et al. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30:707–12. doi: 10.1016/j.leukres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 86.Annabi B, Lee YT, Martel C, et al. Radiation induced-tubulogenesis in endothelial cells is antagonized by the antiangiogenic properties of green tea polyphenol (−) epigallocatechin-3-gallate. Cancer Biol Ther. 2003;2:642–9. [PubMed] [Google Scholar]
- 87.Siddiqui IA, Malik A, Adhami VM, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–63. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 88.Chan MM, Soprano KJ, Weinstein K, Fong D. Epigallocatechin-3-gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J Cell Physiol. 2006;207:389–96. doi: 10.1002/jcp.20569. [DOI] [PubMed] [Google Scholar]
- 89.Golden EB, Lam PY, Kardosh A, et al. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009 doi: 10.1182/blood-2008-07-171389. [In Press]; available online: doi:10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 90.Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. 2002;21:265–80. doi: 10.1023/a:1021210910821. [DOI] [PubMed] [Google Scholar]
- 91.Kazi A, Daniel KG, Smith DM, et al. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965–76. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe S, Yamaguchi M, Sobue T, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J Nutr. 1998;128:1710–5. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 93.Uehar M, Arai Y, Watanabe S, Adlercreutz H. Comparison of plasma and urinary phytoestrogens in Japanese and Finnish women by time-resolved fluoroimmunoassay. Biofactors. 2000;12:217–25. doi: 10.1002/biof.5520120134. [DOI] [PubMed] [Google Scholar]
- 94.Sarkar FH, Adsule S, Padhye S, et al. The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Rev Med Chem. 2006;6:401–7. doi: 10.2174/138955706776361439. [DOI] [PubMed] [Google Scholar]
- 95.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 96.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–42. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lampe JW, Nishino Y, Ray RM, et al. Plasma isoflavones and fibrocystic breast conditions and breast cancer among women in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2007;16:2579–86. doi: 10.1158/1055-9965.EPI-07-0368. [DOI] [PubMed] [Google Scholar]
- 98.Busby MG, Jeffcoat AR, Bloedon LT, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75:126–36. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 99.Pop EA, Fischer LM, Coan AD, et al. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: a Phase I clinical trial. Menopause. 2008;15:684–92. doi: 10.1097/gme.0b013e318167b8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fischer L, Mahoney C, Jeffcoat AR, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48:160–70. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- 101.Miltyk W, Craciunescu CN, Fischer L, et al. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77:875–82. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- 102.Venkitaraman R, Thomas K, Grace P, et al. Baseline urinary phytoestrogen levels and the natural history of untreated, localised prostate cancer in a British population. Int J Biol Markers. 2008;23:192–7. doi: 10.1177/172460080802300310. [DOI] [PubMed] [Google Scholar]
- 103.Kumar NB, Krischer JP, Allen K, et al. A Phase II randomized, placebo-controlled clinical trial of purified isoflavones in modulating steroid hormones in men diagnosed with localized prostate cancer. Nutr Cancer. 2007;59:163–8. doi: 10.1080/01635580701432678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104•.Pendleton JM, Tan WW, Anai S, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [Dietary intervention with isoflavone supplementation in a fraction of recurrent prostate cancer patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaishampayan U, Hussain M, Banerjee M, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 106.Hermann RM, Fest J, Christiansen H, et al. Radiosensitization dependent on p53 function in bronchial carcinoma cells by the isoflavone genistein and estradiol in vitro. Strahlenther Onkol. 2007;183:195–202. doi: 10.1007/s00066-007-1561-0. [DOI] [PubMed] [Google Scholar]
- 107.Honda N, Yagi K, Ding GR, Miyakoshi J. Radiosensitization by overexpression of the nonphosphorylation form of IkappaB-alpha in human glioma cells. J Radiat Res. 2002;43:283–92. doi: 10.1269/jrr.43.283. [DOI] [PubMed] [Google Scholar]
- 108.Raffoul JJ, Banerjee S, Singh-Gupta V, et al. Down-regulation of apurinic/ apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67:2141–9. doi: 10.1158/0008-5472.CAN-06-2147. [DOI] [PubMed] [Google Scholar]
- 109.Schroder FH, Roobol MJ, Boeve ER, et al. Randomized, double-blind, placebo controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur Urol. 2005;48:922–30. doi: 10.1016/j.eururo.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 110.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 111.von Metzler I, Krebbel H, Kuckelkorn U, et al. Curcumin diminishes human osteoclastogenesis by inhibition of the signalosome-associated I kappaB kinase. J Cancer Res Clin Oncol. 2009;135:173–9. doi: 10.1007/s00432-008-0461-8. [DOI] [PubMed] [Google Scholar]
- 112.Milacic V, Banerjee S, Landis-Piwowar KR, et al. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–92. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garcea G, Jones DJ, Singh R, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 115.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 116.Plummer SM, Hill KA, Festing MF, et al. Clinical development of leukocyte cyclooxygenase 2 activity as a systemic biomarker for cancer chemopreventive agents. Cancer Epidemiol Biomarkers Prev. 2001;10:1295–9. [PubMed] [Google Scholar]
- 117•.Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–900. [Effectiveness of oral administration of curcumin-containing extract against colon cancer.] [PubMed] [Google Scholar]
- 118.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 119.Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–5. [PubMed] [Google Scholar]
- 120.Li M, Zhang Z, Hill DL, et al. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–96. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 121.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chendil D, Ranga RS, Meigooni D, et al. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- 123.Khafif A, Hurst R, Kyker K, et al. Curcumin: a new radio-sensitizer of squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:317–21. doi: 10.1016/j.otohns.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 124.Yang H, Zhou P, Huang H, et al. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int J Cancer. 2009;124:2450–9. doi: 10.1002/ijc.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo XP, Zhang XY, Zhang SD. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Zhong Xi Yi Jie He Za Zhi. 1991;11:598–9, 80. [PubMed] [Google Scholar]
- 126.Brar SS, Grigg C, Wilson KS, et al. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Mol Cancer Ther. 2004;3:1049–60. [PubMed] [Google Scholar]
- 127.Marikovsky M, Nevo N, Vadai E, Harris-Cerruti C. Cu/Zn superoxide dismutase plays a role in angiogenesis. Int J Cancer. 2002;97:34–41. doi: 10.1002/ijc.1565. [DOI] [PubMed] [Google Scholar]
- 128.Cen D, Brayton D, Shahandeh B, et al. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47:6914–20. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 129.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–33. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 130.Meng L, Mohan R, Kwok BH, et al. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA. 1999;96:10403–8. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsukamoto S, Yokosawa H. Natural products inhibiting the ubiquitin-proteasome proteolytic pathway, a target for drug development. Curr Med Chem. 2006;13:745–54. doi: 10.2174/092986706776055571. [DOI] [PubMed] [Google Scholar]
- 132.Chen D, Daniel KG, Chen MS, et al. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–32. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 133.Yang H, Shi G, Dou QP. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from ‘Indian winter cherry’. Mol Pharmacol. 2007;71:426–37. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 134.Yang H, Chen D, Cui QC, et al. Celastrol, a triterpene extracted from the Chinese ‘Thunder of God Vine,’ is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–65. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 135.Ferry DR, Smith A, Malkhandi J, et al. Phase I clinical trial of the flavonoid quercetin:pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2:659–68. [PubMed] [Google Scholar]