Abstract
MicroRNAs (miRNAs) are endogenous, single-stranded, noncoding RNAs of 21 to 23 nucleotides that regulate gene expression, typically by binding the 3′ untranslated regions of target messenger RNAs. It is estimated that miRNAs are involved in the regulation of 30% of all genes and almost every genetic pathway. Recently, the misregulation of miRNAs has been linked to various human diseases including cancer and viral infections, identifying miRNAs as potential targets for drug discovery. Thus, small-molecule modifiers of miRNAs could serve as lead structures for the development of new therapeutic agents and be useful tools in the elucidation of detailed mechanisms of miRNA function. As a result, we have developed a high-throughput screen for potential small-molecule regulators of the liver-specific microRNA miR-122, which is involved in hepatocellular carcinoma development and hepatitis C virus infection. Our small-molecule screen employs a Huh7 human hepatoma cell line stably transfected with a Renilla luciferase sensor for endogenous miR-122. The assay was optimized and validated using an miR-122 antisense agent and a previously identified small-molecule miR-122 inhibitor. The described reporter assay will enable the high-throughput screening of small-molecule miR-122 inhibitors and can be readily extended to other miRNAs.
Keywords: high-throughput assay, cell-based assay, luciferase, microRNA, small-molecule inhibitor
Introduction
MicroRNAs (miRNAs) are endogenous, single-stranded, noncoding RNAs of 21 to 23 nucleotides that regulate gene expression in a sequence-specific fashion by binding the 3′-UTR of target mRNAs.1 miRNAs down-regulate gene function by either inhibiting translation or accelerating the degradation of the mRNA.1 They are transcribed from the genome and undergo several posttranscriptional processing steps via a dedicated miRNA pathway. Almost 2000 miRNAs from humans are currently listed in miRBase,2,3 and it is estimated that they control more than 30% of all genes and thus are involved in almost every genetic pathway.4 Recently, miRNAs have been linked to a variety of human diseases, including cancer, heart disease, and viral infections, and their connection to cancer development and progression is now well established.5 Small-molecule modifiers of miRNAs6,7 could serve as lead structures for the development of new therapeutic agents and could be useful tools in the elucidation of detailed mechanisms of miRNA function.
miRNA miR-122 is involved in the regulation of lipid and cholesterol metabolism and is the most abundant miRNA in the liver.8 Recently, it was discovered that miR-122 is down-regulated in hepatocellular carcinoma (HCC), a primary cancer of the liver. Several cellular targets of miR-122 in primary liver carcinomas and the HCC cell lines HepG2 and Heg3B have been identified, including cyclin-G1 (CCNG1) and Bcl-w, an antiapoptotic Bcl-2 family member.9,10 Many cases of HCC result from chronic infection with the hepatitis C virus (HCV), and it was discovered that miR-122 is necessary for HCV replication and infectious virus production through interaction with the viral genome.11 The HCV genome contains two miR-122 target sites in the 5′ noncoding region of the virus, and miR-122 binding results in the upregulation of viral RNA production.12 It was reported that knockdown of miR-122 with antisense agents resulted in a decrease of HCV RNA replication in human liver cells11 and reduced HCV levels in chronically infected primates.13 These promising results suggest that miR-122 could be a viable target for antiviral therapy using small-molecule miR-122 inhibitors. However, the identification of a small molecule with optimal miR-122 inhibitory activity and specificity remains challenging and is most appropriately addressed by a high-throughput screening of hundreds of thousands of compounds.
Recently, a small-molecule modifier screen for miR-122 based on the psiCHECK-2 (Promega, Madison, WI) reporter plasmid was developed, and a pilot screen was conducted.14 The psiCHECK-2 vector was selected because it contains both a Renilla luciferase and an independently transcribed firefly luciferase reporter gene, which can be used for normalization purposes to account for variation in transfection efficiency and cell viability. The complementary sequence of miR-122 was inserted downstream of the Renilla luciferase gene, between the PmeI and SgfI restriction sites. Thus, the presence of mature miR-122 will lead to a decrease in the Renilla luciferase signal (Fig. 1), enabling the detection of endogenous miR-122 levels. In the presence of a small-molecule inhibitor of miR-122, the Renilla luciferase expression will be restored, leading to an increased luciferase signal, enabling the identification of small-molecule inhibitors of miR-122 function. Using a reporter system that results in increased luciferase signal in the presence of an active inhibitor rules out false-positives due to compound toxicity, which can occur in an assay based on a decreased reporter signal. However, compounds identified using this screening approach could still have off-target effects and need to be validated using secondary assays. The ability of the reporter to detect endogenous miR-122 was validated by transiently transfecting the generated psiCHECK-miR122 construct into Huh7 human hepatoma cells.14 The assay was validated by cotransfection with an miR-122 antagomir antisense agent as a positive control.
Figure 1.
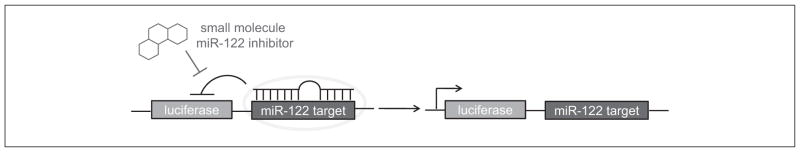
Design of the microRNA miR-122 assay. The developed luciferase reporter can detect the presence of a functional mature miR-122 through repression of the Renilla luciferase signal. In the presence of a small-molecule inhibitor of miR-122 or a miR-122 antagomir, the luciferase expression is restored.
Using Huh7 cells transiently transfected with the psiCHECK-miR122 reporter, a small pilot screen of 1364 compounds in a 96-well format was conducted, and a small-molecule inhibitor of miR-122 was discovered (Fig. 2). Compound 1 displayed specificity for miR-122 and induced a reduction in both mature miR-122 and primary miR-122 levels.14 This pilot screen validates the ability to discover small-molecule inhibitors of miR-122 function.
Figure 2.
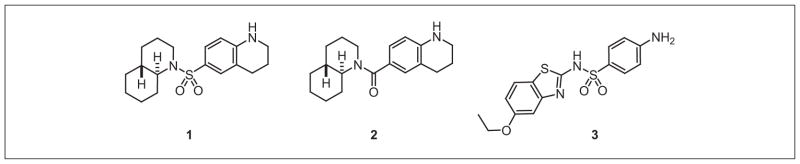
Small-molecule inhibitors of miR-122 discovered through a pilot screen using the developed miR-122 reporter assay and subsequent structure-activity relationship studies.
The next step is the screening of substantially larger small-molecule libraries of 105 to 106 compounds to identify hit structures that can be further optimized through structure-activity relationship (SAR) studies and validated using secondary assays to provide potent and specific miR-122 inhibitors. The previously developed assay based on the transient transfection of the psiCHECK-miR122 reporter will not be sufficient for high-throughput screening because of the high cost of transfection reagents, the extensive transfection procedures, and the variations between different plates and different days associated with transient transfections. Here, we are reporting the creation of a high-throughput assay for small-molecule inhibitors of miR-122 by developing a stable Huh7 cell line that constitutively expresses an miR-122 reporter system. Using a stable cell line instead of a transient transfection not only will be more cost efficient and less time-consuming but will also remove variation associated with transient transfection efficiency and additional manipulations. The reported steps to create that cell line can be applied not only to Huh7 cells and miR-122 but also to any other cell line and miRNA combination.
Materials and Methods
Cell Culture
Experiments were performed using the Huh7 human hepatoma cell line (ATCC) cultured in Dulbecco’s Modified Eagle Medium (DMEM; Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 2% penicillin/streptomycin (Mediatech, Manassas, VA) and maintained at 37 °C in a 5% CO2 atmosphere. Huh7-psiCHECK-miR122 cells were cultured in DMEM (Hyclone) supplemented with 10% FBS (Hyclone), 500 μg/ mL of G418 (Sigma Aldrich, St. Louis, MO), and 2% penicillin/streptomycin (Mediatech) and maintained at 37 °C in a 5% CO2 atmosphere.
Reporter Plasmid Construction
The psiCHECK-2 plasmid (1 μg; Promega) was sequentially digested with SgfI (10 U, 50 μL reaction; Promega) followed by PmeI (10 U; New England Biolabs, Ipswich, MA) and gel purified. Insert DNA containing the miR-122 binding site was purchased from IDT DNA (5′ CGCAGTAGAGCTCTAGTACAAACACCATTG TCACACTCCAGTTT 3′ and 5′ AAACTGGAGTGTGAC A AT G G T G T T T G T A C T A G A G C T C T A C T G CGAT 3′), hybridized (90 °C, cooled to 4 °C over 5 min, then 4 °C for 60 min), and ligated with T4 ligase (200 U, 10 μL reaction, 1:10 vector/insert ratio; New England Biolabs) into the digested psiCHECK-2 vector. Positive colonies were identified by PCR colony screens, and the construction of the psiCHECK-miR122 vector was confirmed by sequencing (sequencing primer: 5′ GCTAAGA AGTTCCCT 3′; IDT DNA).14
Determination of G418 Concentration for Selection of Stable Cells
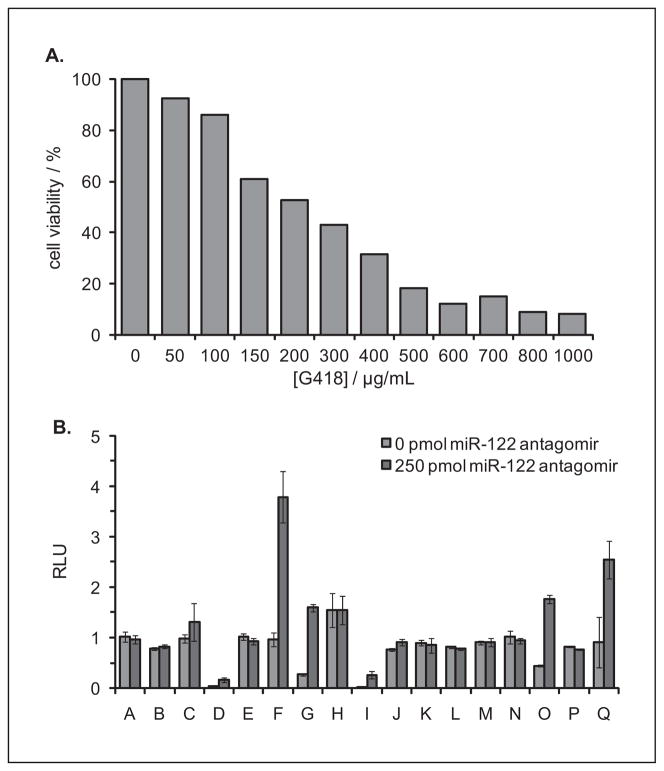
Huh7 cells were passaged into a 12-well plate and were grown to 30% confluency. The cells were treated with DMEM supplemented with increasing concentrations of G418. The cells were incubated at 37 °C for 7 days, and the media was removed and replaced with freshly prepared selective media every 2 to 3 days. The cells were then assayed for viability using a Cell Titer-Glo Luminescent Cell Viability Assay (Promega), and the luminescence was recorded on a microplate reader (Biotek Synergy 4). Selection with 500 μg/mL G418 led to 80% cell death after 48 h and 100% cell death after 7 days and was therefore used in the selection of stable clones.
Generation of the Huh7-psiCHECK- miR122 Reporter Cell Line
Stable transfectants were generated by cotransfection of psiCHECK-miR122 with the pcDNA3 plasmid (Invitrogen, Carlsbad, CA), which encodes a neomycin resistance marker. The psiCHECK-miR122 and pcDNA3 plasmids were linearized with BamHI (20 U, 100 μL reaction; New England Biolabs) and BglII (20 U, 100 μL reaction; New England Biolabs), respectively, and the DNA was ethanol precipitated. Huh7 cells were passaged into a six-well plate and were cotransfected at approximately 90% confluency with the linearized psiCHECK-miR122 (2.0 μg) and linearized pcDNA3 (0.25 μg) using lipofectamine 2000 transfection reagent (3:1 reagent/psiCHECK-miR122 DNA ratio; Invitrogen) in Opti-Mem media (Invitrogen) according to the manufacturer’s protocol. The cells were incubated at 37 °C for 5 h followed by the replacement of transfection media with standard DMEM growth media. After 48 h of incubation, the media was removed and cells were passaged into a 15 cm cell culture plate. Stables clones were selected with DMEM growth media containing 500 μg/mL of G418. This represents the minimum concentration of G418 that kills all of the nonresistant cells within 7 days. G418-resistant clones were assayed for luciferase expression in triplicate using a Dual Luciferase Assay Kit (Promega) according to the manufacturer’s protocol, and the luminescence was recorded on a microplate reader (Biotek Synergy 4).
Selection of the Stable Huh7-psiCHECK-miR122 Reporter Cell Line
Promising clones obtained from the G418 selections were transfected with a miR-122 antagomir antisense agent (250 pmol; 2′-OMe PS modified oligonucleotide 5′ ACAAACACCAUUGUCACACUCCA 3′; IDT DNA) using X-tremeGENE (Roche, Basel, Switzerland) in Opti-Mem media to verify that the Renilla luciferase expression could be restored by miR-122 knockdown. The cells were incubated at 37 °C for 4 h followed by the replacement of transfection media with standard DMEM growth media (supplemented with 10% FBS and 2% penicillin/streptomycin). After 48 h of incubation, the media was removed and the cells were lysed and assayed with a Dual Luciferase Assay Kit. Here, and in all subsequent experiments, the ratio of Renilla to firefly luciferase expression was calculated for each of the triplicates, the data were averaged, and standard deviations were calculated. From these clones, the Huh7-psiCHECK-miR122 cell line was selected based on the level of luciferase expression and the response to miR-122 antagomir transfection.
Effect of DMSO on the Huh7-psiCHECK-miR122 Reporter Cell Line
Huh7-psiCHECK-miR122 cells were seeded at 10 000 cells per well in white, clear-bottom, 96-well plates (BD Falcon, San Jose, CA). After an overnight incubation, the cells were treated with increasing concentrations of DMSO. The cells was incubated at 37 °C for 48 h, and then the media was removed and the cells were lysed and assayed with a Dual Luciferase Reporter Assay Kit (Promega).
Determination of the Z′ Factor
To assess the suitability of the assay for high-throughput screening, the statistical parameter Z′ was determined using both an miR-122 antagomir (positive control) and the previously identified small-molecule miR-122 inhibitor 1. The Huh7-psiCHECK-miR122 cells were seeded at 10 000 cells per well in white, clear-bottom, 96-well plates (BD Falcon). After an overnight incubation, the cells were transfected with the miR-122 antagomir (0 or 250 pmol, negative and positive control, respectively) as previously described. After 48 h of incubation, the media was removed and the cells were lysed and assayed with a Dual Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol. For the small-molecule treatment, Huh7-psiCHECK-miR122 cells were seeded at a density of 5000 cells per well in black, clear-bottom, 384-well plates (BD Falcon). After an overnight incubation, the medium was removed and replaced with 50 μL of DMEM supplemented with 10 μM of 1 or a DMSO control (0.1% DMSO final concentration) in triplicate. The cells were incubated for 48 h followed by analysis with a Dual Luciferase Reporter Assay Kit (Promega). The assays were repeated on multiple days to ensure reproducibility. The Z′ factors were determined using the equation Z′ = 1 – (3 × SDpositive control + 3 × SDnegative control)/(Avgpositive control – Avgnegative control).15
Transfection of the miR-122 Antagomir
The Huh7-psiCHECK-miR122 cells were seeded at 10 000 cells per well in white, clear-bottom, 96-well plates (BD Falcon). After an overnight incubation, the cells were transfected with the miR-122 antagomir at increasing concentrations as previously described. After 48 h incubation, the media was removed and the cells were lysed and assayed with a Dual Luciferase Reporter Assay Kit (Promega).
Treatment with Small-Molecule miR-122 Inhibitors
Huh7-psiCHECK-miR122 cells were seeded at a density of 5000 cells per well in black, clear-bottom, 384-well plates (BD Falcon). After an overnight incubation, the medium was removed and replaced with 50 μL of DMEM supplemented with increasing concentrations of 1-3 or a DMSO control (0.1% DMSO final concentration) in triplicate. The cells were incubated for 48 h followed by analysis with a Dual Luciferase Reporter Assay Kit (Promega).
Results and Discussion
To conduct a high-throughput screen, a stable reporter cell line needed to be constructed to reduce the number of manipulations, to increase the density of wells screened per plate, and to enhance the reproducibility and robustness of the assay. Toward that goal, a psiCHECK-2 vector was constructed by inserting the complementary sequence of miR-122 downstream of a Renilla luciferase gene, enabling the detection of endogenous miR-122 levels and allowing for the screening of small-molecule inhibitors of miR-122 function. The psiCHECK-miR122 reporter did not contain a selectable marker for the generation of a stably transfected mammalian cell line. Therefore, the reporter was cotransfected with the pcDNA3 plasmid (Invitrogen) that encodes a neomycin resistance marker. Stable clones were selected using an optimal G418 concentration of 500 μg/mL, as determined by a cell viability dose-response experiment (Fig. 3A), and resistant clones were assayed for the presence of the luciferase reporter using a Dual Luciferase Assay Kit (Promega). Antagomirs (oligonucleotide antisense agents that are perfectly complementary to the mature miRNA) are known to silence targeted, endogenous miRNAs. Therefore, resistant clones that produced high firefly luciferase readings and low relative luciferase signals were further validated by transfection with an miR-122 antagomir antisense agent to verify that the Renilla luciferase expression responds to miR-122 knockdown (Fig. 3B). From these clones, an optimal Huh7-psiCHECK-miR122 cell line (Clone G) was selected based on the level of luciferase expression and the response to miR-122 antagomir transfection.
Figure 3.
(A) Cell viability assay (Cell Titer-Glo) of Huh7 cells treated with increasing concentrations of G418. Luminescence units were normalized to untreated cells and are represented as percentage cell viability. A total of 500 μg/mL of G418 was used in the selection of stable Huh7-psiCHECK-miR122 clones. (B) Relative luminescence units (RLU) of Huh7-psiCHECK-miR122 clones A-Q transfected with a miR-122 antagomir in a 96-well format. All assays were conducted in triplicate and were normalized to a control containing only transfection reagent. Error bars represent standard deviations from three independent experiments. From these clones, the Huh7-psiCHECK-miR122 cell line G was selected based on the level of luciferase expression and the response to miR-122 antagomir transfection.
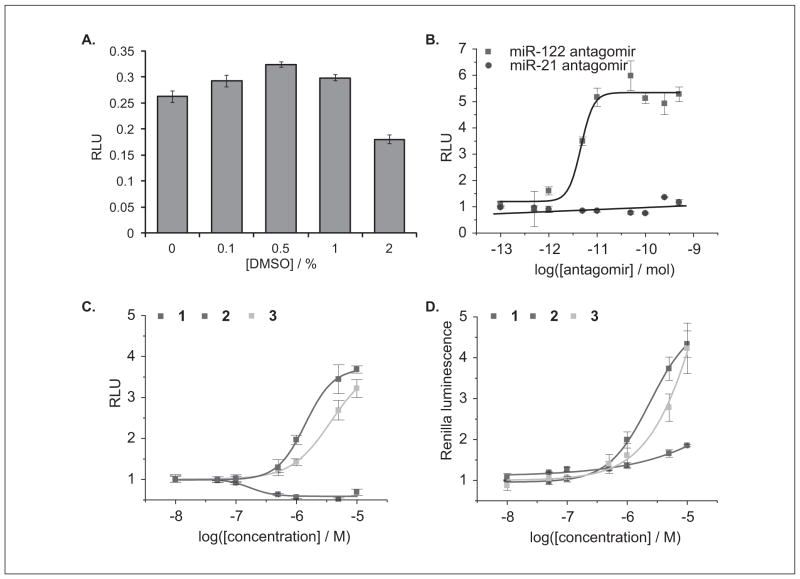
To validate that the Huh7-psiCHECK-miR122 reporter cell line could be used in a high-throughput assay for small-molecule inhibitors of miR-122, the stable cells were treated with increasing concentrations of DMSO because small-molecule libraries are commonly stored in DMSO as a solvent. The DMSO effect on the Huh7-psiCHECK-miR122 assay was tested on multiple days of assay performance. Importantly, DMSO has only a small effect on the relative luciferase signal up to 1%, but this is effect is minimal at the 0.1% DMSO used for the small-molecule experiments (Fig. 4A).
Figure 4.
(A) Relative luminescence units (RLU) of Huh7-psiCHECK-miR122 cells treated with increasing concentrations of DMSO. All assays were conducted in triplicate in a 96-well format. (B) Luciferase assay dose-response curves for Huh7-psiCHECK-miR122 cells transfected with either an miR-122 or miR-21 antagomir in a 96-well format. All assays were conducted in triplicate and were normalized to a control containing only transfection reagent. (C) Dose-dependent response of RLU for Huh7-psiCHECK-miR122 cells treated with increasing concentrations of the miR-122 inhibitors 1-3. All assays were conducted in triplicate in a 384-well format and were normalized to a DMSO control. (D) Dose-dependent response of absolute Renilla luminescence units for Huh7-psiCHECK-miR122 cells treated with increasing concentrations of the miR-122 inhibitors 1-3. All assays were conducted in triplicate in a 384-well format and were normalized to a DMSO control. Error bars represent standard deviations from three independent experiments.
The Huh7-psiCHECK-miR122 assay was further validated by calculating the statistical parameter Z′, which represents a quantitative and well-established measure of the quality of the assay.15 The Z′ factor takes into account the precision of measuring the maximum and minimum control signals in replicate wells. An miR-122 antagomir antisense agent was used as a positive control for miR-122 inhibition. Using this antagomir, the signal-to-background ratio was 6.0 ± 0.4 and the Z′ factor was 0.74, both sufficient for high-throughput screening,15 and these results were reproducible over multiple days of assay performance. The Huh7-psiCHECK-miR122 cell line delivered an improved Z′ factor over the previously reported transient transfection assay. Upon transfection of the Huh7-psiCHECK-miR122 cells with the miR-122 antagomir, a dose-dependent restoration of luciferase signal was observed, whereas transfection with an miR-21 antagomir (negative control) showed no change in luciferase signal, indicating that the increased Renilla luciferase signal was specifically due to the inhibition of miR-122 (Fig. 4B).
Because the goal of developing an Huh7-psiCHECK-miR122 stable cell line was to conduct high-throughput screening for small-molecule inhibitors of miR-122, the previously identified miR-122 inhibitor 1 was used to further validate the assay. To adapt the assay for a high-throughput screening format, it was optimized using the inhibitor 1 in a 384-well format instead of the 96-well format used in the pilot screen.14 In addition, the luciferase assay results were processed using both the absolute Renilla luciferase data (without normalization) and the Renilla luciferase data normalized to the firefly luciferase data to provide relative luciferase units (RLU) to determine if the dual luciferase reporter is necessary when using the stable miR-122 reporter cell line. An advantage of the dual luciferase reporter is that it accounts for variations in transfection efficiency; however, this is accounted for by using the stable miR-122 reporter cell line. A disadvantage of the dual luciferase reporter readout is the substantially higher cost per screened compound. Importantly, exposure of the Huh7-psiCHECK-miR122 cells to increasing concentrations of the miR-122 inhibitor 1 led to a dose-dependent restoration of the luciferase signal (Fig. 4C, D) as demonstrated by both the Renilla luciferase data and the normalized RLU data. In addition, exposure of the Huh7-psiCHECK-miR122 cells to increasing concentrations of the structurally similar analog 2 (Fig. 2) showed no increase in luciferase signal (Fig. 4C, D), indicating that the sulfonamide functionality is crucial for the miR-122 inhibitory activity. Treatment of the Huh7-psiCHECK-miR122 cells with 1 provided Z′ factors of 0.67 (Renilla luciferase data normalized to firefly luciferase data, RLU data) and 0.71 (Renilla luciferase data only, without normalization), demonstrating a robust assay,15 and the obtained results were reproducible over multiple days of assay performance. Based on the Renilla luciferase and RLU results obtained for the treatment of the Huh7-psiCHECKmiR122 cells with 1, the dual luciferase reporter assay is not necessary when using the stable miR-122 reporter cell line, which is an important improvement over the previous transient transfection assay. Thus, an assay for Renilla luciferase only will be sufficient in a high-throughput screening. However, the dual luciferase reporter assay does offer an advantage when assaying possibly toxic compounds. Without normalization to an internal control, small molecules that are active modifiers (inhibitors or possibly activators) of miR-122 but toxic at the concentration used for screening could be disregarded as inactive.
The Huh7-psiCHECK-miR122 stable cell line was then used to further validate potential miR-122 inhibitors from a 1364-compound screen (Diversity Set II from the NCI Developmental Therapeutics Program). Exposure of the Huh7-psiCHECK-miR122 cells to increasing concentrations of the benzothiazole 3 (Fig. 2) led to a dose-dependent restoration of the luciferase signal (Fig. 4C, D) as demonstrated by both the Renilla luciferase data and the normalized RLU data, suggesting that 3 is an miR-122 inhibitor. Importantly, the assay results indicate that 3 has similar activity to the previously identified miR-122 inhibitor 1. An MTT assay of Huh7 cells treated with increasing concentrations of compounds 1-3 demonstrated that these small molecules have no effect on Huh7 cell proliferation (data not shown). Interestingly, both compounds 1 and 3 share similar structural motifs, including a benzamine and a sulfonamide, which was shown to be essential because of the inactivity of compound 2.
In summary, a small-molecule screen for inhibitors of miR-122 was developed using a Huh7 human hepatoma cell line stably transfected with a Renilla luciferase sensor for endogenous miR-122. The assay was optimized and validated through multiple experiments on independent days using an miR-122 antisense agent and a small-molecule miR-122 inhibitor identified from a small pilot screen. This assay has been tested at various DMSO concentrations, and the DMSO has only minimal effect on the assay results up to 1%. A dose-dependent restoration of luciferase signal was observed and Z′ factors sufficient for high-throughput screening were obtained. Moreover, the assay was adapted to a 384-well format and was validated. The stable cell line was used to further investigate the structural requirements of the identified miR-122 inhibitor 1 and to discover a second potential miR-122 inhibitor, the benzothiazole 3. The optimized assay will be used in the screening of large small-molecule libraries of 105 to 106 compounds to identify specific small-molecule inhibitors of miR-122 with enhanced activity, which can be used as unique probes to elucidate the currently unknown biogenesis and regulation of miR-122 in the liver. The discoveries made with these small molecules could provide insight into the role of miR-122 in hepatitis C infection and liver cancer. In addition, the approach for the development of the described reporter assay can be easily extended to other miRNAs and other cell lines.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support was provided by the Teva USA Scholars Grants Program, administered by the American Chemical Society Office of Research Grants, and the NIH (1R21NS073068). C.M.C. acknowledges an NIH/NCSU Molecular Biotechnology Traineeship (2T32GM008776-11).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Carthew R. Gene Regulation by MicroRNAs. Curr Opin Genet Dev. 2006;16(2):203–208. doi: 10.1016/j.gde.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Kozomara A, Griffiths-Jones S. miRBase: Integrating MicroRNA Annotation and Deep-Sequencing Data. Nucleic Acids Res. 2011;39(database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths-Jones S, Grocock RJ, vanDongen S, Bateman A, Enright AJ. miRBase: MicroRNA Sequences, Targets and Gene Nomenclature. Nucleic Acids Res. 2006;34(database issue):D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis BP, Burge CB, Bartel DP. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are MicroRNA Targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Tong AW, Nemunaitis J. Modulation of miRNA Activity in Human Cancer: A New Paradigm for Cancer Gene Therapy? Cancer Gene Ther. 2008;15(6):341–355. doi: 10.1038/cgt.2008.8. [DOI] [PubMed] [Google Scholar]
- 6.Deiters A. Small Molecule Modifiers of the MicroRNA and RNA Interference Pathway. AAPS J. 2010;12(1):51–60. doi: 10.1208/s12248-009-9159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumireddy K, Young D, Xiong X, Hogenesch J, Huang Q, Deiters A. Small-Molecule Inhibitors of MicroRNA miR-21 Function. Angew Chem Int Ed Engl. 2008;47(39):7482–7484. doi: 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esau C, Davis S, Murray S, Yu X, Pandey S, Pear M, Watts L, Booten S, Graham M, McKay R, Subramaniam A, Propp S, Lollo B, Freier S, Bennett C, Bhanot S, Monia B. miR-122 Regulation of Lipid Metabolism Revealed by In Vivo Antisense Targeting. Cell Metab. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu C, Calin G, Giovannini C, Ferrazzi E, Grazi G, Croce C, Bolondi L, Negrini M. Cyclin G1 Is a Target of MiR-122a, a MicroRNA Frequently Down-Regulated in Human Hepatocellular Carcinoma. Cancer Res. 2007;67(13):6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 10.Lin C, Gong H, Tseng H, Wang W, Wu J. miR-122 Targets an Anti-apoptotic Gene, Bcl-w, in Human Hepatocellular Carcinoma Cell Lines. Biochem Biophys Res Commun. 2008;375(3):315–320. doi: 10.1016/j.bbrc.2008.07.154. [DOI] [PubMed] [Google Scholar]
- 11.Jopling C, Yi M, Lancaster A, Lemon S, Sarnow P. Modulation of Hepatitis C Virus RNA Abundance by a Liver-Specific MicroRNA. Science. 2005;309(5740):1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 12.Jopling CL, Schütz S, Sarnow P. Position-Dependent Function for a tandem MicroRNA miR-122-Binding Site Located in the Hepatitis C Virus RNA Genome. Cell Host Microbe. 2008;4(1):77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanford R, Hildebrandt-Eriksen E, Petri A, Persson R, Lindow M, Munk M, Kauppinen S, Ørum H. Therapeutic Silencing of MicroRNA-122 in Primates With Chronic Hepatitis C Virus Infection. Science. 2010;327(5962):198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young D, Connelly C, Grohmann C, Deiters A. Small Molecule Modifiers of MicroRNA miR-122 Function for the Treatment of Hepatitis C Virus Infection and Hepatocellular Carcinoma. J Am Chem Soc. 2010;132(23):7976–7981. doi: 10.1021/ja910275u. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Chung T, Oldenburg K. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]