Abstract
We reported earlier that exposure of rats to 3-methylcholanthrene [MC] causes sustained induction of hepatic cytochrome P450 (CYP)1A expression for up to 45 days by mechanisms other than persistence of the parent MC (Moorthy, J. Pharmacology. Exp. There., 294, 313–322, 2000). The CYP1A genes are members of the Ah gene battery that also encode CYP1B1 and phase II enzymes such as glutathione S-transferase (GST-α), UDP glucuronyl tranferase (UGT)1A, NAD[P]H: quinone oxidoreductase I [NQO1], aldehyde dehydrogenase (ALDH), etc. Therefore, in this investigation, we tested the hypothesis that MC elicits persistent induction of CYP1B1 and phase II genes, which are in part regulated by the Ah receptor [AHR]. Female Sprague-Dawley rats were treated with MC [100 μmol/kg], i.p., once daily for 4 days, and expression of CYP1B1 and several phase II [e.g., glutathione-S-transferase [GST]-α, NAD[P]H: quinone oxidoreductase I [NQO1] genes and their corresponding proteins were determined in lung and liver. The major finding was that MC persistently induced [3–10 fold] the expression of several phase II enzymes, including GST-α, NQO1, UGT1A1, ALDH, and epoxide hydrolase (EPHX) in both tissues for up to 28 days. However, MC did not elicit sustained induction of CYP1B1. Our results thus support the hypothesis that MC elicits coordinated and sustained induction of phase II genes presumably via persistent activation of the AHR, a phenomenon that may have implications for chemical-induced carcinogenesis and chemopreventive strategies in humans.
Keywords: 3-methylcholanthrene, phase II enzymes, CYP1A1, gene expression, carcinogenesis, CYP1B1, Ah receptor, NAD[P]H: quinone oxidoreductase, rat, in vivo
3-Methylcholanthrene (MC) is one of the most potent polycyclic aromatic hydrocarbon (PAH) carcinogens (Harvey, 1982). Metabolism of MC by cytochrome P450 (P450) enzymes and epoxide hydrolase (EPHX) leads to the formation of chemically reactive intermediates that can bind covalently to DNA, a critical step in the initiation of carcinogenesis (Guengerich, 1990). The CYP enzymes are a very large multi-gene family of constitutive and inducible enzymes that play a pivotal role in the oxidative activation and/or inactivation of a wide range of xenobiotics, including environmental carcinogens and pollutants (Guengerich, 1990). The CYP1A family comprises two proteins CYP1A1 and 1A2, which play important roles in carcinogen bioactivation (Guengerich, 1990). Recent studies have shown that CYP1B1 is also active in PAH-mediated carcinogenesis (Shimada et al., 1996). The hepatic CYP1A1/1A2/1B1 enzymes are inducible by a number of chemicals, including the hepatocarcinogen 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Whitlock, 1999), benzo[a]pyrene (BP) Conney 1986), and cigarette smoke (Kawajiri et al., 1990). Moreover, MC also induces CYP1A1/1B1 in extrahepatic tissues such as lung (Kondraganti et al., 2002) and mammary glands (Christou et al., 1991), which are target organs for carcinogenesis. In terms of specificity of human CYPs toward PAH metabolism, while CYP1A1 is more active in the activation for chrysene-1,2-diols and benz[a]anthracene trans dihdrodiols, CYP1A1 and 1B1 have similar catalytic activities towards towards other procarcinogens, such as benzo[a]pyrene-7,8-diol, 7,12-dimethylbenz[a]anthracene-3,4-diol, etc. (Shimada et al., 2001). These investigators also looked at the catalytic activities of 14 humans CYPs towards the catalytic activities of several PAHs, and found that CYP1A2, 2C9, 3A4, and 2C19 metabolized PAHs at much slower rate than those catalyzed by CYP1A1, and that other enzymes, including CYP2A6, 2B6, 2C8, 2D6, 2E1, 3A5, 3A7, and 4A11 were almost inactive in the bioactivation of PAHs (Shimada et al., 2001).
The mechanisms of induction of CYP1A1/1A2 by MC or TCDD have been investigated extensively (Whitlock et al., 1999), and the Ah receptor (AHR) and AHR nuclear translocator (ARNT) play important roles in CYP1A1/1A2 gene expression. The mechanisms entail binding of the inducer to the Ah receptor [AHR], a cytosolic protein. The inducer-receptor complex is translocated into the nucleus, where it binds to the Ah receptor nuclear translocator (ARNT), and this ligand-activated transcription factor interacts with Ah responsive elements (AHREs), which are located in multiple copies in the upstream portion of the CYP1A1 promoter, resulting in transcriptional activation of many genes on the Ah locus, including CYP1A1, CYP1A2, CYP1B1, and phase II enzymes such as glutathione S-transferases (GST), NAD[P]H: quinone oxidoreductase (NQO1), UDP glucuronyl transferase (UGT), aldehyde dehydrogenase (ADH) (Whitlock, 1999; Kohle and Bock, 2007).
The major function of phase II enzymes is to participate in the further detoxication of drugs by catalyzing the conjugation, hydration, sulfation, and oxidation, resulting in the formation of highly polar compounds that can be excreted from the body through various xenobiotic transporters (Talalay, 1989). The phase II enzymes are often synonymous with antioxidant enzymes since they play a major role in attenuating oxidative stress induced by reactive oxygen species (ROS) and electrophiles that are generated in the cell by xenobiotics, drugs, heavy metals, ionizing radiation, hyperoxia, etc (Talalay, 1989; Dakshinamoorthy et al., 2000). ROS are also generated endogenously during the normal cellular metabolism (Dakshinamoorthy et al., 2000). The genes encoding phase II enzymes are also regulated in a concerted manner at the transcriptional level through the antioxidant response element (ARE) or electrophilic response element (EpRE) Rushmore et al., 1991). The nuclear factor-erythroid 2 factor (Nrf2) activates transcription of phase II genes through the ARE/EpRE (Jaiswal, 2004). The main antioxidant genes that are regulated by the Nrf2 are NQO1, GST-Ya, heme oxygenase-1 (HO-1), epoxide hydrolase (EPHX), ADH, UGT1A1, γ-glutamyl transpeptidase (γ-GCS), etc (Jaiswal, 2004). In fact, it has been shown that PAHs induce GST Ya and other phase II enzyme via AREs, but this requires CYP1A1-dependent metabolism to intermediates that can interact with ARE. Recent studies suggest that Nrf2, a CNC (cap’n collar) basic leucine zipper transcription factor is an AHR target gene (Ma et al., 2004), suggesting that the distinct but overlapping AHR and Nrf2 gene batteries may be coordinately and regulated, thereby offering the possibility that the phase II enzymes can be synergistically activated by a number of phytochemicals that act as cancer chemopreventive agents (Ramos-Gomez et al., 2001). In fact, recently evidence has emerged for a direct coupling of the AHR and Nrf2, which appears to be very effective in protecting against oxidative/electrophlic stress (Miao et al., 2005).
We earlier reported that MC elicits a long-term induction of CYP1A enzymes for up to 45 days (Moorthy et al., 1993). Furthermore, we demonstrated that the sustained induction of CYP1A enzymes is mediated by mechanisms other than persistence of the parent compound (Moorthy, 2000). Using a CYP1A2-null mouse model we provided evidence that CYP1A2 plays a role in the sustained CYP1A1 induction, presumably by catalyzing the formation of a MC metabolite that may be responsible for sustained CYP1A1 induction in liver (Kondraganti et al., 2002).
There is a paucity of data with regard to persistence of induction of CYP1B1, as well as phase II enzymes. TCDD causes persistent induction of CYP, as well as phase II enzymes, such as NQO1 (Gasiewicz et al., 1986), due to its long-term retention in the body. However, it is not known whether MC also elicits persistent induction of phase II enzymes under experimental conditions that result in sustained CYP1A1 induction (Moorthy, 2000). Thus, the goal of this study was to determine whether other enzymes encoded by the Ah locus are also persistently induced. Specifically, we tested the hypothesis that treatment of rats with MC would also lead to persistent induction (for up to 28 days after MC withdrawal) of CYP1B1 and phase II genes, a phenomenon that might contribute to a better understanding of the mechanisms of toxicity and carcinogenesis mediated by MC and other PAHs.
MATERIALS AND METHODS
Chemicals
MC, Tris, sucrose, NADPH, bovine serum albumin, ethoxyresorufin, glutathione reductase, glucose 6-phosphate, and glucose 6-phosphate dehydrogenase were purchased from Sigma Chemical Co. (St. Louis, MO). All reagents for real time RT-PCR were from Applied Biosystems (Foster City, CA). Buffer components for electrophoresis and western blotting, and goat anti-mouse IgG conjugated with horseradish peroxidase were obtained from Bio-Rad laboratories (Hercules, CA). Polyclonal antibody to CYP1B1 was purchased from BD Bioscience (Franklin Lakes, NJ). Polyclonal NQO1 antibody used in the present study was raised in rabbits against purified rat cytosolic NQO1 protein, and these antibodies cross-react with rat, mice, and human NQO1 (Dakshinamoorthy et al., 2005). cDNAs encoding human NQO1 (Robertson et al., 1986) were used to determine NQO1 gene expression in rats.
Animals
Two month-old male Sprague-Dawley rats were from Harlan Sprague-Dawley, Inc. The animals were acclimatized for at least 1 week before beginning of the experiments.
Animal Treatment
Rats were treated with MC (100 μmol/kg) in corn oil (CO) [2 ml/kg], or were given equal volumes of CO, i.p., once daily for 4 days, as reported earlier (Moorthy et al, 1993; Moorthy, 2000; Kondraganti et al., 2005). At the 1, 15, and 28 day time points after the last treatment, the animals (4–5 per group) were sacrificed, and livers and lungs from individual animals were used for isolation of subcellular fractions or stored at −800 C for later isolation of total RNA.
Preparation of microsomes, cytosols, and enzyme Assays
Lungs and livers were perfused with ice-cold phosphate-buffered saline, pH 7.4. Liver microsomes were isolated by the calcium chloride precipitation method (Moorthy et al., 1993; Moorthy, 2000; Kondraganti et al., 2005), whereas lung microsomes were prepared by differential centrifugation, as reported previously (Moorthy, 2000; Kondraganti et al., 2002). The supernatants remaining following sedimentation of microsomes were considered as cytosolic fraction for enzyme and protein analyses. Protein concentrations were estimated by the Bradford dye-binding method (Bradford, 1976).
Enzyme Assays
GST-α̤ NQO1, and ADH activities were assayed in the cytosols according to published protocols (Reddy et al., 1983; Jaiswal et al., 1988; Quistad et al., 1994). UDPGT and EPHX activities were determined in the microsomal fractions of liver and lung tissues, as reported earlier (Bock et al., 1983; Depierre et al., 1984).
Western blotting
Liver or lung microsomes or cytosols (20 μg of protein) prepared from individual animals were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 7.5% acrylamide gels. The separated proteins on the gels were transferred to polyvinylidene difluoride membranes, followed by Western blotting (Moorthy, 2000) using specific CYP1B1 or NQO1 antibodies.
Northern hybridization
Total liver RNA was isolated as reported earlier (Moorthy, 2000), and the purity of RNA was assessed by measuring 260/280 ratios and by agarose gel electrophoresis. The RNA [20 μg/per sample] was loaded on to 1% agarose/formaldehyde denaturing gel, separated by electrophoresis, and transferred to nitrocellulose filters. The filters containing separated RNA were prehybridized by exposure to 20 mM Na2HPO4, pH 7.6, 10X Denhardt’s reagent, 100 μg/ml of heatdenatured salmon sperm DNA, and 7% SDS for approximately 3 h at 55° C. After prehybridization, the solutions were discarded and nitrocellulose filters were hybridized at 55° C for 16 h by using the same solution used for prehybridization and adding 10% dextran and the 32P-labeled NQO1 cDNA probe [20 × 106 cpm], which was prepared by the random prime technique, as reported earlier (Moorthy, 2000; Kondraganti et al., 2002). The hybridized filters were washed 3 times for 20 minutes with 3X SSC (1X SSC, 0.15 M sodium chloride, 0.015 M sodium citrate), and 1% SDS at 68° C to decrease nonspecific binding of radioactive transcripts to the filter. Following these washes, the membranes were exposed to autoradiography. Relative levels of NQO1 mRNAs were quantitated by phosphor-imager analysis. GAPDH cDNA probe was used as a RNA transfer and loading control. For this, the nitrocellulose membranes were stripped, and hybridized with GAPDH cDNA probe, followed by exposure to autoradiography.
Real Time reverse transcriptase-polymerase chain reaction [RT-PCR] Assays
Total RNA [50 ng] from livers of CO- and MC-treated animals was subjected to one step real time quantitative TaqMan RT-PCR. ABI PRISM 7700 Sequence Detection System was used for the RT-PCR reactions. Gene-specific primers in the presence of TaqMan Reverse Transcription Reagents and RT Reaction Mix [Applied Biosystems] were used to reverse transcribe RNA, and TaqMan Universal PCR Master Mix and Assays-on-Demand Gene Expression probes [Applied Biosystems] were used for PCR amplification. Following a RT hold for 30 min at 48°C, the samples were denatured at 95°C for ten minutes. The thermal cycling step was for 40 cycles at 95°C for 15 s, and 40 cycles at 60°C for 1 min. Serial dilutions of RNA were used to optimize and validate RT-PCR conditions UGT1A1, 1A6, 1A7, and 18S genes. Each data point was repeated 3 times. Quantitative values were obtained from the threshold PCR cycle number [Ct] at which the increase in signal was associated with an exponential growth of PCR product after it starts to be detected. The relative mRNA levels for UGT genes were normalized to their 18S content. The relative expression levels of the target gene were calculated according to the equation, 2−ΔcT, where ΔcT = Ct target gene − Ct 18S gene (Jiang et al., 2004).
Statistical Analysis
All data are expressed as means ± S.E.M of values from at least 4 individual animals. Statistical significance between control and treated groups for each time point was assessed by one-way ANOVA, followed by Tukey’s post hoc tests. P values that were < 0.05 were considered significant.
RESULTS
The major goal of this study was to determine the temporal effects of MC on phase II gene and protein expression profiles in rat liver and lung, and their possible relationship to carcinogenesis. We report here that exposure of rats to MC leads to persistent induction in the expression of phase II genes and their corresponding protein contents and enzyme activities for several weeks after MC withdrawal. In this investigation we tested the hypothesis that persistent induction of CYP1A1/1A2 by MC, as described in a previous study (Kondraganti et al., 2002), is accompanied by sustained modulation of other gene members of the Ah battery, a phenomenon that might contribute to a better understanding of the mechanisms of toxicity mediated by MC and other PAHs.
Effect of MC on phase II enzyme activities
As shown in Table 1, MC elicited induction (2-15-fold) of the catalytic activities of each of the hepatic phase II enzyme, i.e. NQO1, ALDH, GST-α, UGT, and EPHX at the day 1, compared to the corresponding controls. The induction of each of these activities was sustained for 15–28 days, with induction declining by day 28. As can be seen in the table, GST-α activities were doubled at day 1 in the MC-treated rats, and declined to control by day 28. NQO1 activities were induced about 11.2-fold by MC at day 1. Induction of NQO1 persisted at 15 days [3.8-fold] and by day 28 the activities were slightly higher than control (Table 1). ADH activities were induced 15- and 6-fold, respectively, by MC at 1 and 15 days. The induction was significant even on day 28 (Table 1). UGT activities were induced about 4.5 fold by MC at 1 day and significant induction was noticed even on day 28 (Table 1). A 2.5 fold induction of EPHX activities was observed on days 1 and 15, but the activities were similar to control on day 28 (Table 1). In lung, MC-mediated induction of ADH, NQO1, and UDPGT, but not GST-α was sustained for 28 days (Table 2). EPHX activities also declined by day 28 (Table 2).
TABLE 1.
EFFECT OF 3-METHYLCHOLANTHRENE ON HEPATIC PHASE II ENZYME EXPRESSION
| TIME | |||||
|---|---|---|---|---|---|
| NQO1 | ALDH | GST-a | UGT | EPHX | |
| [Days] | [nmol/min/mg protein] | [nmol/min/mg protein] | [nmol/min/mg protein] | [nmol/min/mg protein] | [nmol/min/mg protein] |
| 0 | 10.45 ± 1.5 | 5.35 ± 1.7 | 6.5 ± 0.8 | 102.1 ± 5.1 | 1.8 ± 0.141 |
| 1 | 122.6* ± 15.9 | 83.5*± 3.5 | 11.5* ± 1.7 | 445.3* ± 26.16 | 4.65* ± 0.71 |
| 15 | 42.3* ± 3.2 | 33.4* ± 2.5 | 11.2* ± 1.4 | 210.4* ± 12.32 | 4.45* ± 0.80 |
| 28 | 14.4* ± 1.8 | 11.2* ± 1.8 | 7.2 ± 1.2 | 180.3* ± 10.4 | 2.1 ± 0.30 |
Rats were treated with vehicle corn oil, or MC, as described under Materials and Methods, and phase II enzyme activities were determined in liver microsomes or cytosols at 1, 15, or 28 days after MC withdrawal. Values represent mean ± SD of liver samples from 4 individual animals.
Statistically significant differences between vehicle-treated and MC-treated samples at P < 0.05.
TABLE 2.
EFFECT OF 3-METHYLCHOLANTHRENE ON PULMONARY PHASE II ENZYME EXPRESSION
| TIME | |||||
|---|---|---|---|---|---|
| NQO1 | ALDH | GST-a | UGT | EPHX | |
| [Days] | [nmol/min/mg protein] | [nmol/min/mg protein] | [nmol/min/mg protein] | [nmol/min/mg protein] | [nmol/min/mg protein] |
| 0 | 2.45 ± 0.5 | 6.35 ± 0.49 | 2.46 ± 0.23 | 121.5 ± 20.2 | 1.8 ± 0.141 |
| 1 | 22.62* ± 5.91 | 21.5*± 3.5 | 3.48* ± 0.04 | 215.5* ± 26.16 | 2.35* ± .071 |
| 15 | 18.95* ± 0.97 | 22.65* ± 6.15 | 2.39 ± 0.23 | 205* ± 22.32 | 2.45* ± .636 |
| 28 | 14.5* ± 1.2 | 11.2* ± 0.90 | 2.10 ± 0.20 | 185.5* ± 20.4 | 1.9 ± 0.23 |
Rats were treated with vehicle corn oil, or MC, as described under Materials and Methods, and phase II enzyme activities were determined in lung microsomes or cytosols at 1, 15, or 28 days after MC withdrawal. Values represent mean ± SD of lung samples from 4 individual animals.
Statistically significant differences between vehicle-treated and MC-treated samples at P < 0.05.
Effect of MC on CYP1B1 and NQO1 protein expression
MC did not elicit persistent induction in liver or lung of CYP1B1, which also plays an important role in PAH metabolism (Figure 1). The modulation of NQO1 activities was paralleled by similar changes in protein contents, as determined by Western blotting (Figure 2).
Figure 1.
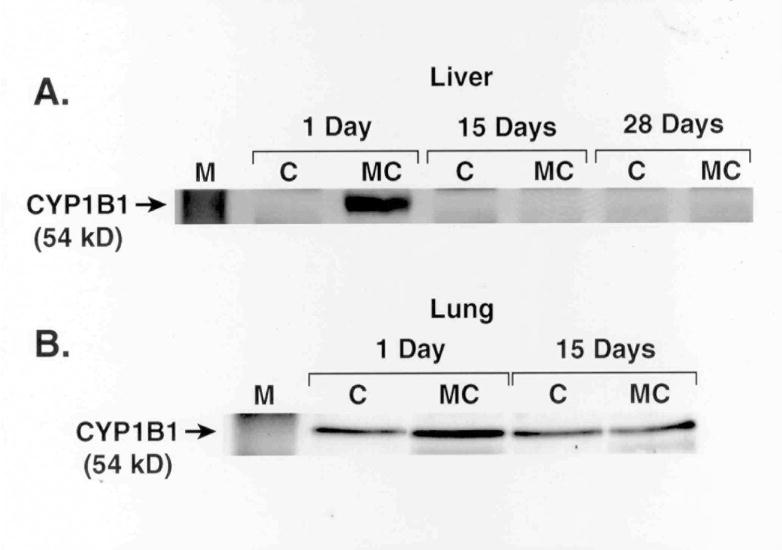
Effect of MC on CYP1B1 apoprotein. Female rats were treated with MC as described under Materials and Methods, and CYP1B1 apoprotein expression in liver (A) and lung (B) microsomes was determined at the indicated time points by Western blotting. Microsomes from 4 individual animals from each group were analyzed, and similar changes were noted. The figure is a representative blot showing the changes elicited by MC at each time point.
Figure 2.
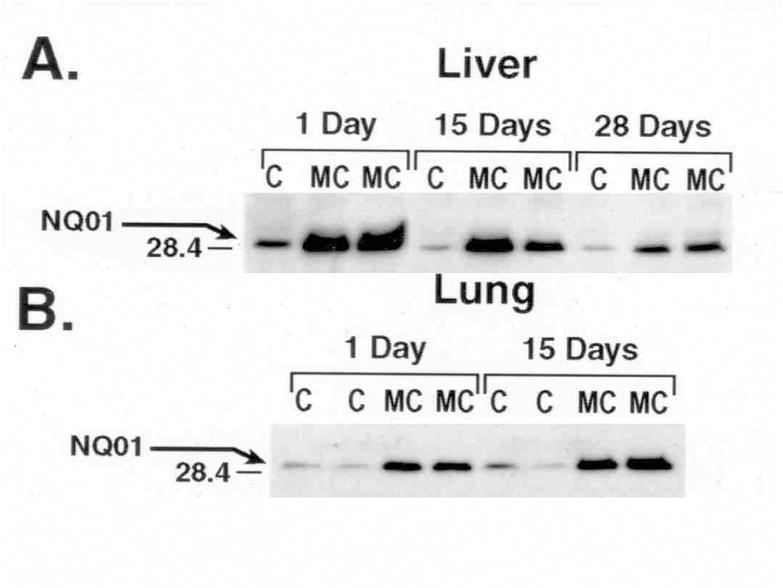
Effect of MC on hepatic and pulmonary NQO1 protein expression. Rats were treated with MC or CO vehicle alone as control as described in Materials and Methods, and NQO1 apoprotein expression in liver (A) and lung (B) cytosols was determined at the indicated time points by Western blotting. Cytosolic fractions from at least 4 individual animals from each group were analyzed, and similar changes were noted. The figure is a representative blot showing the changes elicited by MC in two individual animals at each time point.
Effect of MC on phase II gene expression
Northern blotting experiments revealed significant induction of NQO1 expression in liver (Figure 3) and lungs (data not shown) of rats exposed to MC at 1 day. However, induction declined by 15 days. Quantitative phosphor-imaging analyses, after normalizing the data using GAPDH mRNA, revealed that MC induced NQO1 expression by 20-, 3-, and 1.2-fold compared to the corresponding corn oil controls at the 1, 15, and 28 days, respectively (not shown). MC caused significant induction of UGT1A1 mRNA by 180-, 35-, and 8-fold at the 1, 15, and 28 days, respectively, compared to the corresponding controls (Figure 4). Similar induction was also noticed for the UGT1A2 and 1A7 gene [not shown]. Taken together, our findings indicate that MC differentially and persistently regulates the expression of hepatic and pulmonary phase II enzymes by mechanisms other than persistence of the parent MC (Moorthy, 2000; Kondraganti et al., 2002).
Figure 3.
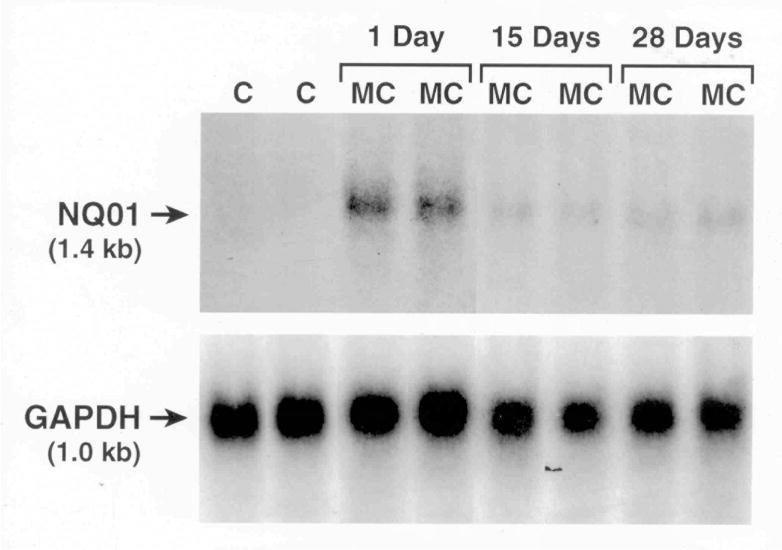
Effect of MC on NQO1 mRNA expression. Total RNA was isolated from livers of control and MC-treated animals at the 1, 15, or 28 day time points, and NQO1 mRNA levels were determined by Northern hybridization. mRNA signals were detected by exposing the membranes to autoradiography for 24 h at −800 C. The membranes were washed and re-probed with GAPDH cDNA probe to assess RNA transfer, loading, and hybridization.
Figure 4.
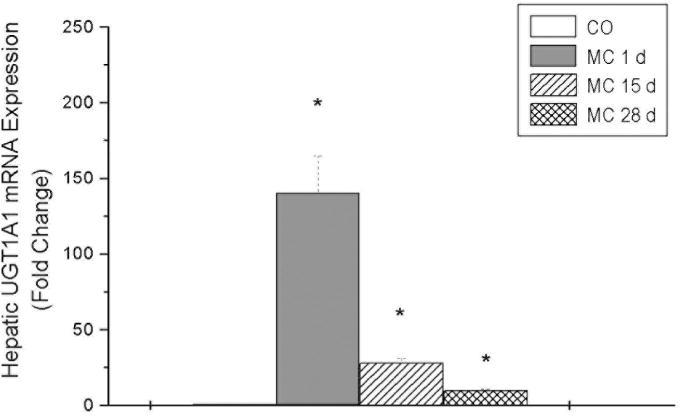
Effect of MC on hepatic UGT1A1 mRNA expression. Rats were treated with MC or CO vehicle alone as control as described in Materials and Methods, and total RNA was isolated from livers of rats 1, 15, or 28 days after the last treatment. The RNA was subjected to real-time RT-PCR using UGT1A1-specific primers, as described in Materials and Methods.
DISCUSSION
In this investigation we conducted experiments to test the hypothesis that persistent induction of CYP1A enzymes by MC, which we reported earlier (Moorthy et al., 1993; Moorthy, 2000) is accompanied by sustained induction of CYP1B1 and phase II enzymes whose genes are also regulated by the AHR. Since phase I as well as phase II enzymes play a pivotal role in the activation and detoxication of PAH carcinogens, the studies reported in this manuscript may have important implications for human carcinogenesis and chemopreventive strategies.
The persistent induction of hepatic and pulmonary phase II enzyme activities [Tables 1 and 2] in rats suggested that AHR might play a role in the sustained induction, since the genes encoding these proteins belong to the Ah gene battery and are coordinately regulated (Whitlock, 1999; Kohle and Bock, 2007; Talalay, 1989; Dakshinamoorthy et al., 2000). That CYP1B1 was not persistently induced may have been due to regulatory mechanisms that involved AHR-independent mechanisms (Kondraganti et al., 2002; Cho et al., 2005).
Our observation of long-term induction of phase II enzymes is in agreement with our previous findings showing sustained induction of CYP1A enzymes by MC in the rat model (Moorthy et al., 1993; Moorthy, 2000). In rats, we recently demonstrated that MC causes a prolonged induction of CYP1A1 by mechanisms other than persistence of the inducer (Moorthy, 2000). In fact, greater than 99% of the parent MC is metabolized and cleared from the body by day 15, and we also found that the intrahepatic concentration of 270 pmol/g of the parent compound at day 15 is insufficient to induce CYP1A1 (Moorthy, 2000). The lack of a persistent effect of MC on hepatic CYP1A1 (EROD) activities in the CYP1A2-null mice (Kondraganti et al., 2002) provides strong evidence for an important role for CYP1A2 in the maintenance of CYP1A1 induction by MC. It is not known if MC would fail to persistently induce phase II enzymes in the CYP1A2-null mice.
Our Northern hybridization experiments (Figure 3) showing sustained induction of NQO1 in the liver indicate that the persistent induction of NQO1 protein expression was preceded by prolonged upregulation of the corresponding gene. The possible contribution of message stabilization to the persistent augmentation of NQO1 has not been excluded, and it is possible that post-transcriptional mechanisms may have also contributed to the sustained induction. Similarly, our real-time RT-PCR experiments showing sustained induction of UGT1A1, 1A2, and 1A7 mRNA levels support the hypothesis that MC persistently induces phase II enzymes in a concerted fashion. The UGT enzymes are expressed in multiple forms, and UGT1A2, UGT1A6 and UGT1A7 are markedly induced in rat liver upon exposure to PAHs (Kondraganti et al., 2005; Cho et al., 2005; Auyeung et al., 2003). Interestingly, the rat UGT1A6 isoform is controlled by two tissue-specific promoters (Auyeung et al., 2003), and the different UGT isoforms are generated by alternate splicing. The induction of UGT1A isoforms by MC is mediated by the AHR, and the presence of multiple AHREs in the promoters of the UGT1A gene may explain the inducibility of multiple UGT1A isoforms by MC (Wells et al., 2004).
The observation that MC elicited sustained induction of phase II enzymes in lung suggested that this phenomenon is of relevance to carcinogenesis as lung is a target organ for carcinogenesis mediated by MC and other PAHs (Li et al., 1992). The findings of Beebe et al. (1992) demonstrating a correlation between persistent induction of CYP1A1 enzyme in lungs of mice exposed to the tumor promoter Aroclor 1254 indicates the significance of sustained CYP1A1 induction to tumorigenesis. We had earlier reported that MC did not elicit sustained induction in kidney, a non-target organ for PAH-mediated carcinogenesis (Li et al., 1992), suggesting that the prolonged induction in the lung may have important implications for carcinogenesis, because if PAHs can elicit sustained induction of CYP1A1 in lung tissues of humans, it is possible that this phenomenon may contribute to activation of PAHs to carcinogenic metabolites.
The molecular mechanisms involved in the persistent induction of phase II genes is not clearly understood. Our recent observation that MC did not cause a persistent induction of the AHR gene by MC (Kondraganti et al, 2005) suggests that the persistent induction of CYP1A1 and phase II enzymes was probably not due to persistent upregulation of AHR gene or protein expression, but may have involved mechanisms involving sustained transcriptional activation of the Ah gene battery through the AHREs, which are present in the promoter regions of each of the members of the Ah gene battery.
We recently provided experimental evidence that MC, upon metabolic activation in vitro, yields metabolite(s) that covalently bind to plasmids containing CYP1A1 promoter, suggesting that some of the MC-DNA adducts are sequence-specific, being targeted towards the CYP1A1 promoter (Moorthy, 2002; Moorthy et al., 2007). Furthermore, we demonstrated that PCR amplification of sequences containing AHREs was inhibited in MC-treated, but not vehicle-treated DNA, supporting the hypothesis that MC-DNA adducts were present on AHREs, resulting in inhibition of taq polymerase activity (Moorthy, 2002; Moorthy et al., 2007). MC treatment did not inhibit PCR amplification of sequences that lacked the AHREs lending further credence to the hypothesis that MC-DNA adducts were targeted to the AHREs of the CYP1A1 as well as phase II genes such as NQO1, UGT1A1, GST-Ya, ADH, etc. (Moorthy et al., 2007).
We provided an explanation for the possible mechanisms by which MC would elicit persistent induction of CYP1A1 in the absence of the parent compound (Moorthy, 2000;2002; Moorthy et al., 2007). In regard to the sustained induction of phase II enzymes, we postulate a similar mechanism (Figure 5). CYP1A2 might possibly catalyze the formation of a MC metabolite, which may complex with the AHR and get targeted to the nucleus, wherein it could covalently bind to the AHREs on the CYP1A1 and phase II gene promoters and maintain CYP1A1 and phase II gene expression. The most likely metabolites produced by CYP1A1 metabolism of MC are 2-hydroxy MC and 1-hydroxy MC, based on preliminary experiments that showed 103- and 45-fold induction of CYP1A1 enzyme activity, respectively, in hepa-1 cells exposed to these compounds (not shown).
Figure 5.
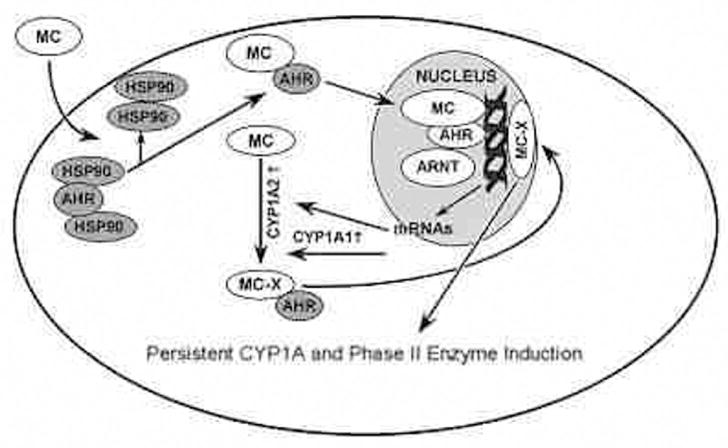
Mechanistic hypothesis for involvement of MC-DNA adducts in persistent CYP1A1 and phase II enzyme induction. We postulate that 3-methylcholanthrene (MC) is converted by CYP1A1 to a reactive metabolite MC-X. MC-X then enters the nucleus and specifically interacts covalently with AhREs or other regulatory elements on the CYP1A1 promoter. The resulting MC-X-DNA adduct mimics the role of MC-AHR-ARNT complex in enhancing transcription of CYP1A1 and phase II genes. Because MC-DNA adducts in liver in vivo are persistent for several weeks after MC withdrawal, we hypothesize that a persistent adduct such as MC-X is responsible for persistent CYP1A1 and phase II enzyme induction by MC.
Because induction of phase II enzymes in response to oxidative stress entails a pivotal role for the Nrf2 signaling pathway (Kohle and Bock, 2007; Talalay, 1989; Dakshinmoorthy et al., 2000; Rushmore et al., 1991; Jaiswal 2004; Ma et al., 2004; Ramos-Gomez et al., 2001), it is possible that the Nrf2-mediated mechanisms might also contribute to the sustained phase II enzyme induction in response to MC, which is known to induce oxidative stress in vivo. Our observation that MC causes persistent upregulation of EPHX, whose gene promoter contains AREs (Chen et al., 2003), is consistent with the hypothesis that sustained Nrf2-mediated gene activation, in part, contributes to the persistent induction of phase II enzymes. Because the Nrf2 accumulates in the nucleus within 15 minutes of antioxidant treatment, and is exported out of the nucleus in 8 hr (Jain et al., 2005), it is unlikely that Nrf2 plays a direct role in the persistent induction of phase II genes by MC. We reported earlier that MC treatment of rats leads to formation of exogenous and endogenous DNA adducts that are persistent for up to 45 days after MC withdrawal (Moorthy et al., 1993). Since endogenous adducts may be formed due to oxidative DNA damage, it is tempting to speculate that some of these adducts may be sequence-specific, and such adducts if formed in the ARE regions may lead to persistent transcriptional activation of phase II genes in a concerted fashion Although we do not have direct evidence to support this hypothesis, in the future, we plan to conduct ligation-mediated PCR (LM-PCR) experiments to test the sequence-specificity of adduct formation in the promoter regions of these genes. Should adducts be formed on the promoters of phase II genes, we will construct plasmids containing these adducts, and determine if transfection of these adducted plasmids into hepa-1 cells would lead to modulation of transcriptional activation of phase II gene expression, similar to what we found with respect to CYP1A1 gene modulation by MC (Moorthy et al., 2007).
Regardless of the mechanism of persistent induction of phase II enzymes by MC, this phenomenon may have significant implications for cancer chemoprevention (Conney, 2003). Since phase II enzymes play important roles in the detoxification of reactive electrophiles to non-carcinogenic metabolites, and, therefore, sustained induction of these enzymes in humans could alleviate cancer risk in individuals exposed to environmental chemicals. Because a number of chemopreventive agents such as sulforaphane, oltipraz, etc. are phase II inducers, future studies on the detailed molecular mechanisms of phase II enzymes by PAHs could lead to the development of novel drugs that might persistently induce phase II enzyme, with little toxic effects. These studies would have immense potential in the development of new strategies in chemoprevention of chemical-induced cancers in humans.
Acknowledgments
We thank Mr. Kathirvel Muthiah for helping us with the real time RT-PCR analyses reported in this paper.
FUNDING
National Institutes of Health (RO1ES009132, RO1HL070921 and R01HL087174 to BM; RO1 ES07943 to AK; Department of Defense (DAMD 17-03-1-0296) to SK; American Heart Association (Texas Affiliate) (0655122Y) to BM.
References
- Auyeung DJ, Kessler FK, Ritter JK. Mechanism of rat UDP-glucuronosyltransferase 1A6 induction by oltipraz: evidence for a contribution of the arylhydrocarbon receptor pathway. Mol Pharmacol. 2003;63:119–127. doi: 10.1124/mol.63.1.119. [DOI] [PubMed] [Google Scholar]
- Beebe L, Fox SD, Riggs CW, Park SS, Gelboin HV, Issaq HJ, Anderson LM. Persistent effects of a single dose of Aroclor 1254 on cytochromes P450IA1 and IIB1 in mouse lung. Toxicol Appl Pharmacol. 1992;114:16–24. doi: 10.1016/0041-008x(92)90091-6. [DOI] [PubMed] [Google Scholar]
- Bock KW, Burchell B, Dutton GJ, Hanninen O, Mulder GJ, Owens IS, Siest G, Tephly TR. UDP-glucuronyl transferase activities: guidelines for consistent interim terminology and assay conditions. Biochem Pharmacol. 1983;32:953–955. doi: 10.1016/0006-2952(83)90610-x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Meford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel antiinflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- Cho YC, Zheng W, Yamamoto M, Liu X, Hanlon PR, Jefcoate CR. Differentiation of pluripotent C3H10T1/2 cells rapidly elevates CYP1B1 through a novel process that overcomes a loss of Ah receptor. Arch Biochem Biophys. 2005;439:139–153. doi: 10.1016/j.abb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Christou M, Savas U, Schroeder S, Shen X, Thompson T, Gould MN, Jefcoate CR. Cytochromes P4501A1 and P4501B1 in mammary gland: cell-specific expression and regulation by polycyclic aromatic hydrocarbons and hormones. Mol Cell Endocrinol. 1991;115:41–50. doi: 10.1016/0303-7207(95)03668-w. [DOI] [PubMed] [Google Scholar]
- Conney AH. Induction of microsomal cytochrome P450 enzymes: the first Bernard Brodie lecture at Pennsylvania State University. Life Sci. 1986;39:2493–2518. doi: 10.1016/0024-3205(86)90103-7. [DOI] [PubMed] [Google Scholar]
- Conney AH. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention. Cancer Res. 2003;63:7005–7031. [PubMed] [Google Scholar]
- Dakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach 1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H: quinone oxidoreductase I gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- Dakshinamoorthy S, Long DJ, Jaiswal AK. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul. 2000;36:201–206. doi: 10.1016/s0070-2137(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Depierre JW, Seidegard J, Morgenstern R, Balk L, Meijer J, Astrom A, Norelius I, Ernster L. Induction of cytosolic glutathione transferase and microsomal epoxide hydrolase activities in extrahepatic organs of the rat by phenobarbital, 3-methylcholanthrene and transstilbene oxide. Xenobiotica. 1984;14:295–301. doi: 10.3109/00498258409151415. [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Rucci G, Henry EC, Baggs RB. Changes in hamster hepatic cytochrome P-450, ethoxycoumarin O-deethylase, and reduced NAD[P]: menadione oxidoreductase following treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Partial dissociation of temporal and dose-response relationships from elicited toxicity. Biochem Pharmacol. 1986;35:2737–2742. doi: 10.1016/0006-2952(86)90183-8. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Enzymatic oxidation of xenobiotic chemicals. Crit Rev Biochem Mol Biol. 1990;25:97–153. doi: 10.3109/10409239009090607. [DOI] [PubMed] [Google Scholar]
- Harvey RJ. Polycyclic hydrocarbons and cancer. Am Scient. 1982;70:386–393. [PubMed] [Google Scholar]
- Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK, McBride OW, Adesnik M, Nebert DW. Human dioxin-inducible cytosolic NAD(P)H:menadione oxidoreductase. cDNA sequence and localization of gene to chromosome 16. J Biol Chem. 1988;263:13572–13578. [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochrome P4501A expression, and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- Kawajiri K, Nakachi K, Imai K, Hayashi S, Watanabe J. Individual differences in lung cancer susceptibility in relation to polymorphisms of P-4501A1 gene and cigarette dose. In: Ernstner L, Gelboin HV, Sugimura T, editors. Xenobiotics and Cancer. Japan Society, Tokyo and Taylor and Francis; London: 1990. pp. 55–61. [PubMed] [Google Scholar]
- Kohle C, Bock KW. Coordinate regulation of phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kondraganti SR, Jiang W, Moorthy B. Differential regulation of expression of hepatic and pulmonary cytochrome P4501A enzymes by 3-methylcholanthrene in mice lacking the CYP1A2 gene. J Pharmacol Exp Ther. 2002;303:945–951. doi: 10.1124/jpet.102.039982. [DOI] [PubMed] [Google Scholar]
- Kondraganti SR, Muthiah K, Jiang W, Barrios R, Moorthy B. Effects of 3-methylcholanthrene on gene expression profiling in the rat using cDNA microarray analyses. Chem Res Toxicol. 2005;18:1634–1641. doi: 10.1021/tx050085n. [DOI] [PubMed] [Google Scholar]
- Li D, Moorthy B, Chen S, Randerath K. Effects of cytochrome P450 inducers on I-compounds in rat liver and kidney DNA. Carcinogenesis. 1992;13:1191–1198. doi: 10.1093/carcin/13.7.1191. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kinner K, Bi Y, Chan JY, Kan YW. Induction of murine NAD(P)H: quinone oxidoreductase by 2,3,7,8-tetrachloro-p-dioxin requires CNC (cap `n’ collar) basic leucine zipper factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem J. 2004;377:205–213. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W, Hu L, Scrivens J, Batist G. Transcriptional regulation of NF-E2 p45-related factor (Nrf2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway. J Biol Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- Moorthy B. Persistent expression of 3-methylcholanthrene-inducible cytochromes P4501A in rat hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 2000;294:313–322. [PubMed] [Google Scholar]
- Moorthy B. 3-Methylcholanthrene inducible hepatic DNA adducts: A mechanistic hypothesis linking sequence-specific DNA adducts to sustained cytochrome P4501A1 induction by 3-methylcholanthrene. Redox Report. 2002;7:9–13. doi: 10.1179/135100002125000127. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Chen S, Li D, Randerath K. 3-Methylcholanthrene-inducible liver cytochrome[s] P450 in female Sprague-Dawley rats: possible link between P450 turnover and formation of DNA adducts and I-compounds. Carcinogenesis. 1993;14:879–886. doi: 10.1093/carcin/14.5.879. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Muthiah K, Fazili IS, Kondraganti SR, Wang L, Couroucli XI, Jiang W. 3-Methylcholanthrene elicits DNA adduct formation in the CYP1A1 promoter region and attenuates reporter gene expression in rat H4IIE cells. Biochem Biophys Res Commun. 2007;354:1071–1077. doi: 10.1016/j.bbrc.2007.01.103. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Aldehyde dehydrogenase of mice inhibited by thiocarbamate herbicides. Life Sci. 1994;55:1537–1544. doi: 10.1016/0024-3205(94)00314-9. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in Nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy CC, Burgess JR, Gong ZZ, Massaro J, Tu CP. Purification and characterization of the individual glutathione S-transferases from sheep liver. Arch Biochem Biophys. 1983;224:87–101. doi: 10.1016/0003-9861(83)90192-3. [DOI] [PubMed] [Google Scholar]
- Robertson JA, Chen HC, Nebert DW. NAD(P)H:menadione oxidoreductase. Novel purification of enzyme cDNA and complete amino acid sequence, and gene regulation. J Biol Chem. 1986;261:15794–15799. [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Picket CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Shimada T, Oda Y, Gillam EM, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos. 2001;29:1176–1182. [PubMed] [Google Scholar]
- Talalay P. Mechanisms of induction of enzymes that protect against chemical carcinogenesis. Adv Enzyme Regul. 1989;28:237–250. doi: 10.1016/0065-2571(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Wells PG, Mackenzie PI, Chowdhury JR, Guillemette C, Gregory PA, Ishii Y, Hansen AJ, Kessler FK, Kim PM, Chowdury NR, Ritter JK. Glucuronidation and the UDP-glucuronyltransferases in health and disease. Drug Metab Dispos. 2004;32:281–290. doi: 10.1124/dmd.32.3.281. [DOI] [PubMed] [Google Scholar]
- Whitlock JP. Induction of cytochrome P4501A1. Ann Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]


