Summary
Monoclonal antibodies are effective therapies for many disorders, but their nature and use continues to evolve as technologies emerge to improve their drug-like qualities. In this issue Schmitz et al. report on structural and biophysical characterizations of single-domain “nanobodies” from llamas that target the epidermal growth factor receptor (EGFR) by both old and new mechanisms.
Over 100 years ago Paul Ehrlich coined the terms “magic bullet” to describe compounds that specifically bind and deliver a toxin to diseased cells and “antibody” to describe the magic bullets in immune sera. After notable successes treating infectious diseases like diphtheria, antibodies failed to live up to much of their initial therapeutic promise owing to difficulties preparing antibodies of appropriate specificity as well as human immune responses to antisera raised in animals. These problems were largely overcome by the development of monoclonal antibody technology in the mid-1970’s (Kohler and Milstein, 1975) and the engineering of humanized antibodies in the late-1980’s (Jones et al., 1986), which enabled large-scale production of antibodies with defined specificities and minimal immunogenicity. These advances opened the gates to modern antibody therapy, and nearly three-dozen monoclonal antibody therapies have received FDA approval since 1994, mostly to treat cancer and autoimmune disorders.
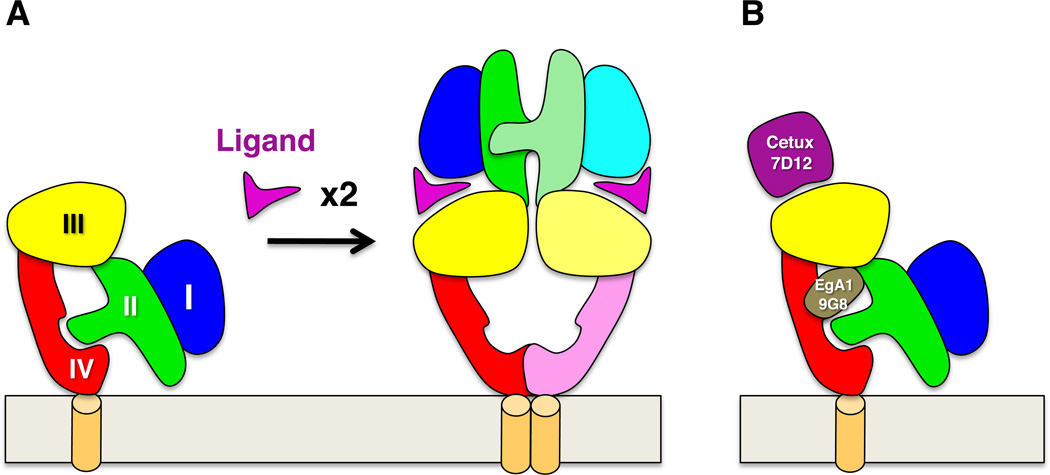
Abnormal activity of the epidermal growth factor receptor (EGFR) is associated with many cancers, and among the FDA-approved antibody therapies are Cetuximab (Erbitux®) and Panitumumab (Vectibix®), which target EGFR and are used to treat colorectal and head-and-neck cancers (Noguchi et al., 2013). EGFR is the archetypal receptor tyrosine kinase and consists of an extracellular ligand-binding region composed of four subdomains, a single membrane-spanning region, a cytoplasmic tyrosine kinase domain, and a C-terminal tail that is phosphorylated during activation and recruits downstream signaling effectors (Burgess et al., 2003). Crystallographic studies have shown that in the absence of ligand the extracellular region adopts a folded-over or “tethered” conformation in which an extended loop from the second domain contacts a membrane-proximal pocket in the fourth domain (Figure 1) (Burgess et al., 2003). Ligand-contacting regions on the first and third domains are held too far apart to bind ligand simultaneously in the tethered conformation, and high-affinity ligand binding requires a large domain rearrangement to an “extended” conformation in which the ligand-binding regions are brought close together. In this extended conformation the previously buried loop on the second domain becomes exposed and mediates reciprocal contacts with the homologous loop on another receptor to form active receptor dimers (Figure 1) (Burgess et al., 2003).
Figure 1.
Activation and inhibition of EGFR extracellular regions. A) The EGFR extracellular region is composed of 4 subdomains: I (blue), II (green), III (yellow), and IV (red). In the absence of ligand a loop from domain II contacts a pocket in domain IV and maintains EGFR in a tethered conformation in which ligand binding surfaces on domains I and III are too far apart to contact ligand simultaneously. Ligand binding stabilizes a domain rearrangement that exposes the domain II loop, which mediates receptor dimerization and conversion to a signaling-competent state. B) Cetuximab and the nanobody 7D12 bind to the EGFR ligand binding site, competing for ligand binding and sterically blocking the extended conformation of the active dimer. The nanobodies EgA1 and 9G8 bind at the hinge region between domains II and III and preclude conversion to the extended conformation without competing for ligand binding.
In previously published work, Ferguson and colleagues showed that Cetuximab binds to the ligand-binding region on the EGFR third domain and blocks both ligand binding and rearrangement of the receptor to the extended conformation (Li et al., 2005). They also showed that another anti-EGFR antibody, Matuzumab, binds to the third domain of EGFR but does not clash with ligand binding and appears to act by sterically preventing EGFR from adopting the extended conformation of active dimers (Schmiedel et al., 2008). In work reported in this issue (Schmitz et al., 2013), these observations are now extended to structural and biophysical characterizations of three EGFR-binding nanobodies, 7D12, EgA1, and 9G8, which are being developed as potential anti-EGFR therapeutics or diagnostics. Nanobodies are antibodies derived from camelids—llamas in this case—that consist of a heavy chain and no light chain (Vincke and Muyldermans, 2012). Nanobodies have generated much interest because of several potential advantages over normal antibodies as therapeutics and diagnostics. Their smaller size may allow greater tumor penetration, they are easier and less costly to produce, and simple modifications have been shown to extend their serum half-life and functionality.
Another potential advantage of nanobodies, that their smaller antigen-binding surface enables them to recognize concave epitopes less accessible to conventional antibodies, is illustrated by the work reported in this issue (Schmitz et al., 2013). Two of the three anti-EGFR nanobodies studied, EgA1 and 9G8, bind to EGFR at a hinge region between the second and third extracellular domains. This hinge is the focal point of domain movement in EGFR when ligand binds, and by contacting the domains flanking this hinge these nanobodies appear to lock EGFR in the inactive, tethered conformation. Like Matuzumab, these nanobodies do not block ligand binding to EGFR and may have an advantage as therapeutics in that their effects cannot be overcome by high ligand concentrations. Unlike the conventional antibody Matuzumab, EgA1 and 9G8 are able to bind EGFR in a small concave pocket owing to their smaller antigen-contacting surface, and it will be interesting to see whether this “lock-down” of the inactive EGFR conformation will translate to better inhibition of EGFR activity.
The third anti-EGFR nanobody studied, 7D12, binds EGFR at a site that overlaps its ligand binding site and blocks ligand binding like Cetuximab showing, perhaps not surprisingly, that nanobodies can functionally mimic conventional antibodies. Moving forward, it will be interesting to parse the contributions of various mechanisms to anti-EGFR therapeutic activity—antibody-dependent cellular cytoxicity (ADCC) vs. blocking ligand vs. blocking an active conformation (Noguchi et al., 2013). These mechanisms may then be engineered into optimal therapeutics or perhaps targeted with combined therapies. The history of antiHER2 antibody therapy illustrates the value of this approach. The EGFR homolog HER2 (Homolog of EGFR 2) is overexpressed and abnormally active in 20–25% of breast and gastric cancers (Slamon et al., 1987), and the anti-HER2 antibody Trastuzumab (Herceptin®) is used to treat these cancers. First approved in 1998, Trastuzumab does not block HER2 dimerization or signaling but rather appears to work through ADCC and other mechanisms (Arteaga et al., 2012). Another anti-HER2 antibody, Pertuzumab (Perjeta®), does block HER2 dimerization and signaling (Arteaga et al., 2012), and the increased the effectiveness of Trastuzumab when given with Pertuzumab led to FDA approval of this combination therapy last year. Not to stop there, and perhaps realizing Ehrlich’s original dream of magic bullets, a toxin-conjugated form of Trastuzumab, Trastuzumab-emtansine (T-DM1 or Kadcyla®) (Arteaga et al., 2012), appears to have even greater anti-HER2 efficacy and was approved earlier this year for treatment of late stage breast cancer. Trials of T-DM1 in earlier stage cancer are ongoing.
The evolution of anti-HER2 therapies highlights the value of determining the mechanism of antibody therapeutics to enable continued optimization of therapies. By uncovering novel inhibitory mechanisms with novel agents, the molecular characterization of anti-EGFR nanobodies described in this issue charts an important new path towards improved cancer drugs and diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Ritter G, Nishikawa H. Antibody-based therapy in colorectal cancer. Immunotherapy. 2013;5:533–545. doi: 10.2217/imt.13.35. [DOI] [PubMed] [Google Scholar]
- Schmiedel J, Blaukat A, Li S, Knochel T, Ferguson KM. Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Can. Cell. 2008;13:365–373. doi: 10.1016/j.ccr.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz KR, Bagchi A, Roovers RC, van Bergen en Henegouwen PMP, Ferguson KM. Structural evaluation of EGFR inhibition mechanisms for nanobodies/VHH domain. Structure. 2013 doi: 10.1016/j.str.2013.05.008. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Vincke C, Muyldermans S. Introduction to heavy chain antibodies and derived Nanobodies. Methods Mol Biol. 2012;911:15–26. doi: 10.1007/978-1-61779-968-6_2. [DOI] [PubMed] [Google Scholar]