Abstract
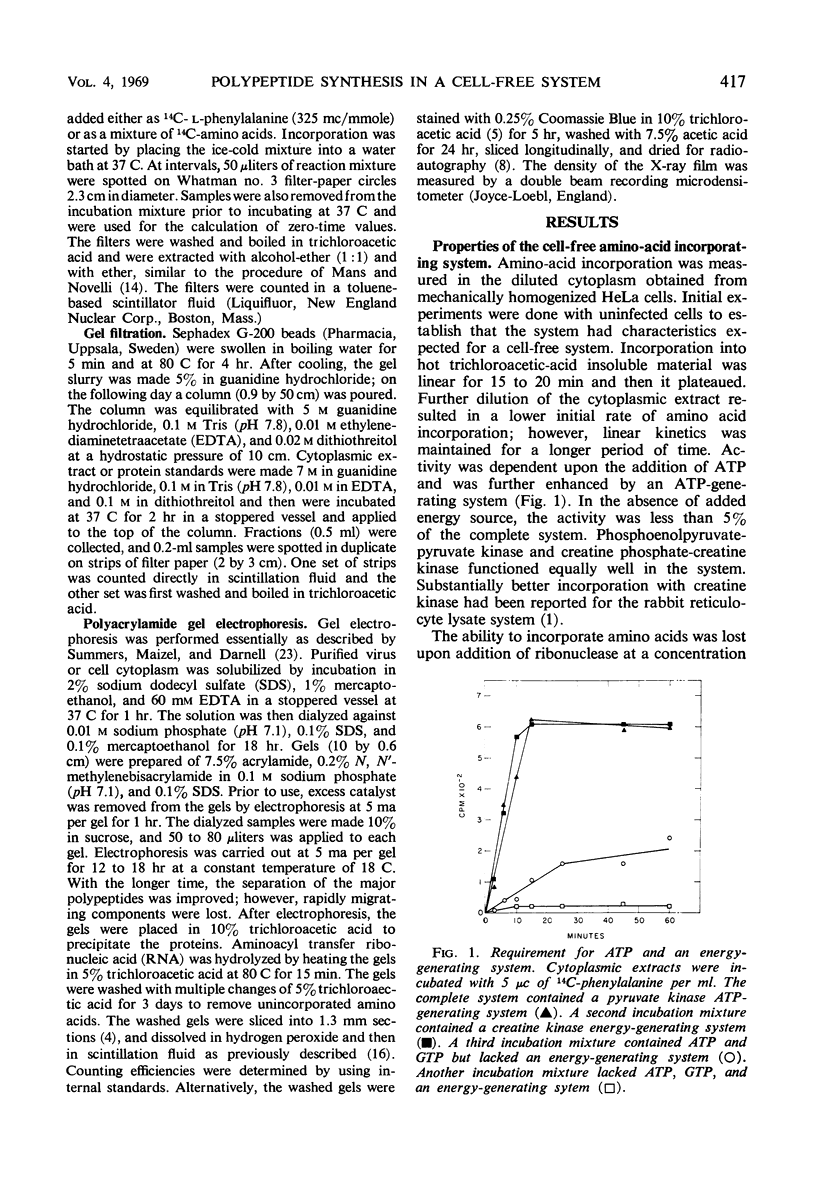
Polypeptide synthesis has been studied in cell-free systems prepared from vaccinia virus-infected and uninfected HeLa cells. Cytoplasmic extracts containing endogenous messenger ribonucleic acid were used. Amino acid incorporation into hot trichloroacetic acid-precipitable material was linear for 15 to 20 min at 37 C. The initial rate of protein synthesis was approximately 15% of the rate in intact cells. Optimal conditions for polypeptide synthesis were similar in cell-free systems prepared from infected or uninfected cells. Requirements for an energy source and Mg++ were demonstrated. The optimal Mg++ concentration was 4 to 5 mm. Ribonuclease, puromycin, and cycloheximide were inhibitory. The molecular weights of the polypeptides labeled in the cell-free systems, as determined by gel filtration in 5 m guanidine hydrochloride, ranged from 16,000 to above 68,000. Polyacrylamide gel electrophoresis indicated that the polypeptides labeled in cell-free extracts of uninfected and infected cells were different. The latter closely corresponded in electrophoretic mobility with the viral polypeptides made in intact, infected cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTARDI G., SMITH J. Virus specific protein and a ribo-nucleic acid associated with ribosomes in poliovirus infected HeLa cells. Cold Spring Harb Symp Quant Biol. 1962;27:271–292. doi: 10.1101/sqb.1962.027.001.026. [DOI] [PubMed] [Google Scholar]
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Burka E. R. Hemin: an inhibitor of erythroid cell ribonuclease. Science. 1968 Dec 13;162(3859):1287–1287. doi: 10.1126/science.162.3859.1287. [DOI] [PubMed] [Google Scholar]
- Chrambach A. Device for sectioning of polyacrylamide gel cylinders and its use in determining biological activity in the sections. Anal Biochem. 1966 Jun;15(3):544–548. doi: 10.1016/0003-2697(66)90121-7. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Farkas W., Marks P. A. Partial purification and properties of a ribonuclease from rabbit reticulocytes. J Biol Chem. 1968 Dec 25;243(24):6464–6473. [PubMed] [Google Scholar]
- LAMFROM H., KNOPF P. M. INITIATION OF HAEMOGLOBIN SYNTHESIS IN CELL-FREE SYSTEMS. J Mol Biol. 1964 Aug;9:558–575. doi: 10.1016/s0022-2836(64)80227-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Salzman N. P. Sequential protein synthesis following vaccinia virus infection. J Virol. 1968 Oct;2(10):1016–1027. doi: 10.1128/jvi.2.10.1016-1027.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHMI M., KELLER R. Titration of vaccinia virus and its neutralizing antibody by the palque technique. Nature. 1962 Jan 13;193:150–151. doi: 10.1038/193150a0. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., EAGLE H. The free amino acid pool of cultured human cells. J Biol Chem. 1958 Mar;231(1):533–545. [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Salzman N. P. Metabolic properties of early and late vaccinia virus messenger ribonucleic acid. J Virol. 1967 Jun;1(3):550–558. doi: 10.1128/jvi.1.3.550-558.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Levintow L. Constitution and function of polyribosomes of poliovirus-infected HeLa cells. Virology. 1965 Sep;27(1):44–53. doi: 10.1016/0042-6822(65)90142-x. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., NOLL H., PENMAN S. EFFECT OF CYCLOHEXIMIDE ON RIBOSOMAL AGGREGATES ENGAGED IN PROTEIN SYNTHESIS IN VITRO. Biochim Biophys Acta. 1964 Jul 22;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]



