Abstract
The phototransformation of two organophosphorus pesticides, parathion and chlorpyrifos, by hydroxyl radicals and carbonate radicals in aqueous solution were studied. The second-order rate constants of parathion and chlorpyrifos with hydroxyl radical were determined to be 9.7 ± 0.5 × 109 and 4.9 ± 0.1 × 109 M−1 s−1, respectively. Addition of hydrogen peroxide increased the UV degradation rates of both pesticides and data were simulated through kinetic modeling. The presence of bicarbonate ions reduced the pesticide degradation rates via scavenging of hydroxyl radical but the formation of carbonate radical during the hydroxyl radical scavenging also contributed to the degradation of the pesticides with second-order reaction rate constants of 2.8 ± 0.2 × 106 and 8.8 ± 0.4 × 106 M−1s−1 for parathion and chlorpyrifos, respectively. The dual roles of bicarbonate ion in UV/H2O2 treatment systems, i.e., scavenging of hydroxyl radicals and formation of carbonate radicals, were examined and discussed using a simulative kinetic model. The transformation of pesticides by carbonate radicals at environmentally relevant bi/carbonate concentrations was shown to be a significant contributor to the environmental fate of the pesticides and it reshaped the general phototransformation kinetics of both pesticides in UV/H2O2 systems.
Keywords: ultraviolet light, photochemistry, advanced oxidation, water treatment
Introduction
Trace amounts of pesticides have been detected in surface waters and groundwater, and have caused increasing public and scientific concerns (Gilliom et al., 2006). The aquatic fate of pesticides is controlled by several degradation pathways, of which the direct and indirect sunlight-induced phototransformation may be major routes. Whereas direct photolysis proceeds by absorbing light, indirect photolysis proceeds through the reaction between contaminants and transient oxidizing radicals such as hydroxyl radical, carbonate radical and singlet oxygen. The direct photolysis rate is related to specific chemical properties, i.e. quantum yield and molar absorption coefficient of pesticides, and may be small compared to other pathways in natural waters due to usually small quantum yields for many pesticides. On the other hand, indirect photolysis, controlled by the reaction rate constants of pesticides with transient oxidizing species and the steady-state concentrations of these species, is typically the more dominant pathway. Particularly, reactions of hydroxyl and carbonate radicals with pesticides are deemed major contributors to indirect phototransformation.
Hydroxyl radical, a powerful oxidant (E0 = 2.3V, pH7) that quickly reacts with most pesticides through electron transfer with second-order rate constants varying between 107–1010 M−1s−1 (Buxton et al., 1988; Augusto et al., 2002), is formed through sunlight photolysis of nitrate and natural organic matter (NOM) in natural waters at concentrations of 10−14–10−18 M (Haag and Hoigne 1985)). Formation of hydroxyl radical in engineered water treatment systems can be achieved using advanced oxidation processes (AOPs) such as ozone, UV/H2O2, ozone/H2O2. The role of hydroxyl radicals in the treatment of pesticides utilizing the AOP processes has been investigated (Acero et al., 2000; Acero et al., 2001; Shemer and Linden 2006).
Unlike the hydroxyl radical, the carbonate radical is a selective transient species toward organic compounds with a high oxidization potential (E0=1.78V, pH7), and is mainly formed through reaction between bi/carbonate ions and hydroxyl radicals or through the quenching of aromatic ketone triplet excited state. It exists in natural waters at much higher concentrations (10−13–10−15 M) (Buxton and Elliot 1986; Huang and Mabury 2000a, Augusto et al., 2002; Lam et al., 2003; Canonica et al., 2005) than the hydroxyl radicals. Carbonate radicals react rapidly with organic pesticides through electron transfer with second-order rate constants varying between 103–109 M−1s−1 (Neta et al., 1988; Canonica et al., 2005). The reaction rate constants are low for simple aliphatic compounds such as methanol and ethanol (103–105 M−1s−1) (Clifton and Huie 1993) but higher (106–108 M−1s−1) for sulfur-containing compounds and aromatic compounds (Mazellier et al., 2002). For electron-rich chemicals such as indole heterocyclic nucleus containing compounds the rate constants could be even higher (Chen and Hoffman 1975). Taking into account its relatively high steady-state concentration in natural waters, the carbonate radical is likely to play as important a role as the hydroxyl radical in the indirect photolysis of pesticides. The reactions between carbonate radical and sulfur-containing chemicals such as thioanisole, dibenzothiophene (DBT) and fenthion (Huang and Mabury 2000b), s-triazine and phenylurea herbicides (Canonica et al., 2005), phenol (Busset et al., 2005) and fenuron (Mazellier et al., 2007) have been studied. Nevertheless, despite the long regarded importance of carbonate radicals in the water environment, studies discussing the reactivity of carbonate radical toward organic contaminants are far fewer than that for hydroxyl radicals, and the roles of bi/carbonate and the carbonate radical in connection with hydroxyl radical in AOP processes are not well reported.
Organophosphorus (OP) are a large group of pesticides that have been widely used since the 1970s replacing organochlorine pesticides for pest control in agriculture and in households owing to their higher acute toxicity, relatively higher decomposition rate, and lower accumulation in the environment. Among them, parathion (O,O-diethyl-O-4-nitro-phenylthiophosphate) and chlorpyrifos (O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate) are two popular pesticides with similar structures containing a pyridinyl or an aromatic ring and a phosphorothionate ethyl ester. The annual usages in the USA are more than one and 11 Million pounds for parathion and chlorpyrifos, respectively. Both pesticides have been detected in surface water and groundwater (Gilliom et al., 2006), and there are growing concerns about their ecological effects because both pesticides are associated with acute toxicity to mammals through inhibiting the enzyme acetylcholinesterase (AChE) in the nervous system with an oral LD50 of 2–30 and 60 mg Kg−1 in rats for parathion and chlorpyrifos respectively. Indeed, parathion has been classified as an acutely toxic pesticide by US EPA (US EPA 1999) and listed as ‘extremely hazardous’ by the World Health Organization (WHO).
A few studies on phototransformation of parathion and chlorpyrifos exist. Most of these studies focused on byproduct formation during the photodegradation of parathion (Grounwell and Erickson 1973; Mansour et al., 1997; Sakellarides et al., 2003; Wu and Linden 2008) and chlorpyrifos (Walia et al., 1988; Barcelo et al., 1993). The degradation kinetics of both pesticides vary among studies as do reported quantum yields at 254nm for parathion [6×10−4 (Mok et al., 1987) and 1.6×10−3 M E−1 (Gal et al., 1992)] and for chlorpyrifos [0.016 (Wan et al., 1994) and 4.8×10−3 M E−1 (Dilling et al., 1984)]. The only study on the reaction mechanism with oxidant radicals reports chlorpyrifos reacts with hydroxyl radical with a second-order rate constant of 4.17 ×109 M−1s−1 (Armbrust 2000).
The objectives of this study were to investigate the phototransformation of parathion and chlorpyrifos in the presence of H2O2 and bi/carbonate under irradiation by low-pressure Hg lamps emitting at 254nm. Based on the measured reaction rate constants of both pesticides with hydroxyl and carbonate radicals obtained through competition experiments and available kinetic parameters in the literature, a kinetic model is proposed that predicts the dynamics of this system and describes the relative importance of and interaction between hydroxyl radical and carbonate radical in indirect photolysis (e.g., AOP) processes.
Materials and Experiments
Materials and reagents
Parathion, chlorpyrifos and aniline were obtained from Chem Service (West Chester, PA, USA) and used as received. Hydrogen peroxide (30% w/w) was obtained from VWR (USA). HPLC grade acetonitrile and water were obtained from Fisher, NJ, USA. All solutions were prepared with 18M Milli-Q water. All other reagents were analytical grade and were used without further treatment. Potassium peroxonitrite (ONOOK) was prepared by photolysis of KNO3 under LP UV lamp for 4 hours (King et al., 1992). Bovine liver catalase was obtained from Sigma-Aldrich and used to destroy any residual hydrogen peroxide in samples prior to HPLC analysis.
UV and UV/H2O2 photolysis experiments
Photodegradation experiments were carried out in a collimated-beam bench reactor described elsewhere (Wu et al., 2007). The UV reactor is configured with four low pressure mercury vapor germicidal lamps (ozone-free, General Electric No. G15T8), which essentially emit monochromatic light at 254nm. Incident irradiance was measured by a calibrated radiometer (IL1700, SED 240/W, International Light, Peabody, MA) and the delivered UV fluence was calculated as the average irradiance multiplied by the exposure time where the average irradiance was determined using an integration of the Beer-Lambert law over the sample depth.
Stock solutions of both pesticides were prepared in methanol and kept at 4°C. To make working solutions, measured amount of stock solutions were added to volumetric flasks and methanol was evaporated with a gentle stream of N2 till dryness. The pesticides were re-dissolved in 20mM phosphate buffer at pH7 through overnight stirring. The initial concentrations were measured just prior to the UV exposure and thus any loss of pesticides due to hydrolysis or volatilization was controlled for in the photodegradation process. In a typical photolysis experiment, 120 mL working solution was placed in a 70×50 mm crystallization dish (surface area of 34.2 cm2 and solution depth of 3.51 cm) and exposed to UV irradiance under completely mixed batch conditions. At specific delivered UV fluence intervals, a 0.6 mL sample was taken by a syringe and transferred to HPLC auto-sampler vials. A maximum of 3 mL solution was removed throughout each exposure experiment, minimizing effects to the solution volume and optic path length. Hydrogen peroxide assisted photodegradation was conducted by adding H2O2 from a 30% stock solution just before UV irradiation. For competition kinetic experiments, aqueous nitrobenzene stock solution was added to the solution just prior to UV irradiation.
Competition experiments measuring carbonate radical reaction rate constants
Carbonate radicals were generated through a reaction between bicarbonate and potassium peroxonitrite (ONOOK) which was generated through the direct photolysis of solid-state potassium nitrate by UV light (254nm) and dissolved in 0.1 N NaOH solution at ratio of 1:4 (m:v) (solution A) (King et al., 1992). Working solutions were prepared such that they consist of 0.01M NaHCO3, 1 µM of aniline (as a probe) and appropriate concentration of pesticides. For a typical experiment, 100 µl of solution A was added into 20 mL of working solution in a Petri-dish under mixing conditions using a magnetic stir bar, allowing 5 minutes for reaction between target chemicals and hydroxyl radicals (generated through homolytic fission of peroxynitrous acid) to be completed (King et al., 1992; Huang and Mabury 2000c). Samples were taken both before and after the reaction for HPLC analysis. Under these conditions, the pH of the final test solution was approximately 9.0, which results in the yield of hydroxyl radical from peroxonitrite to be less than 5% (Crow et al., 1994).
Analytical methods
Concentrations of pesticides and probe chemicals were analyzed using a Varian Pro Star High Performance Liquid Chromatography (HPLC, Varian, Inc., Palo Alto, CA) equipped with a C18 reverse phase column (7.5 × 150 mm) by direct injection (50µL). Isocratic elution was used with a mobile phase of acetonitrile and water (60/40 for parathion, 80/20 for chlorpyrifos and aniline) at a flow rate of 1 mL min−1. A model 330 Polychromatic Diode Array detector was used to detect the concentration of these chemicals. Residual H2O2 was measured following the I3− method (Klassen et al., 1994). The solution pH was determined by using a Thermo Orion 920A pH meter.
Kinetic modeling
Because of the complicated and simultaneous reactions among radicals and water constituents in the UV/H2O2 process, an evaluation of hydroxyl and carbonate radical reactivity was performed using a kinetic model that includes all relevant reactions as summarized in Table 1. Kinetic modeling was performed to calculate reaction rates of the pesticides under different concentrations of H2O2 and bi/carbonate using REACT for Windows software (Bozzelli 2000).
Table 1.
Relevant chemical reactions in the UV/H2O2 AOP system
| Reaction number |
Reactions | Rate constants (M−1s−1, unless otherwise stated) |
Reference | |
|---|---|---|---|---|
| 1 | фH2O2Ia,H2O2 (measured for specific systems, s−1) | Photolysis rate for H2O2, experimental setup specified. | ||
| 2 | HO• + H2O2 → H2O + HO2• | 2.7×107 | Buxton et al., 1998 | |
| 3 | 7.5×109 | Buxton et al., 1998 | ||
| 4 | 8.5×106 | Buxton et al., 1998 | ||
| 5 | 3.9×108 | Buxton et al., 1998 | ||
| 6 | HO• + HO2•→ H2O2 + O2 | 6.6 ×109 | Schested et al., 1968 | |
| 7 | HO2• + HO2•→ H2O + O2 | 8.3 ×105 | Bielski et al., 1985 | |
| 8 | 9.7×107 | Bielski et al., 1985 | ||
| 9 | HO• + HO•→ H2O2 | 5.5×109 | Buxton et al., 1998 | |
| 10 | 1.5×105 | Maruthamuthu et al., 1978 | ||
| 11 | 2.0×104 | Maruthamuthu et al., 1978 | ||
| 12 | 1.4×107 | Neta et al., 1988 | ||
| 13 | 4.3×105 | Draganic et al., 1991 | ||
| 14 | 3.7×107 | Draganic et al., 1991 | ||
| 15 | Forward: 0.45 Back: 1010 |
Stumm and Morgan 1985 | ||
| 16 | Forward:4.5×10−3 | Stumm and Morgan 1985 | ||
| 17 | H2O → OH−+ H+ | Forward: 1×10−4 | ||
| 18 | H2O2 → H+ + HO2− | Forward: 1.6×10−2 | Mazellier et al., 2002 | |
| 19 | HO•+OP → Product | This work | ||
| 20 | CO3•−+OP → Product | This work |
The formation rate of hydroxyl radical (RP,OH) is related to the UV irradiance as shown in Eq. 1 (Glaze et al., 1995; Stefan et al., 1996; Einschlag et al., 2002).
| (1) |
Where is the specific rate of light absorption by hydrogen peroxide (E mol−1 s−1), which integrates the incident UV light irradiation, absorbance of hydrogen peroxide and solution depth in the reactor; is the incident UV irradiance (10−3 E cm−2s−1); α is the solution absorbance (cm−1); Z is the depth of solution ΦH2O2 is the quantum yield of hydrogen peroxide at 254nm (the apparent quantum yield equals 1) (Baxendale and Wilson 1957), and is the fraction of UV light absorbed by hydrogen peroxide; εH2O2 and εOPare molar absorption coefficients of hydrogen peroxide and pesticides, respectively (Sharpless and Linden 2003).
The hydroxyl radical production is countered by reactions between hydroxyl radical and scavenging species in aqueous systems. In the experimental design, major scavenging species include hydrogen peroxide, pesticides and bi/carbonate, and the overall degradation rate of hydroxyl radical is expressed as Eq.2:
| (2) |
Where RS,OH is the scavenging rate of hydroxyl radical;kH2O2, kOP, and ksare the second order reaction rate constants between hydroxyl radicals and H2O2, pesticides, bi/carbonate and other scavenging species, respectively.
By assuming steady state of hydroxyl radical in the system, the expression for hydroxyl radical concentration in the absence of bi/carbonate ions is as Eq. 3:
| (3) |
In the controlled experiment where only Milli-Q ultra pure water was used, the ∑ ks[S] is insignificant. Organic intermediates are generated during the UV/H2O2 process. However, their concentrations are small compared to the parent pesticides, and are much smaller than the concentrations of hydrogen peroxide. These intermediates had no discernable effect on the destruction of parent pesticides as shown by the first order decay in Fig. 1 as well as in literature (Wu and Linden 2008). Therefore, the destruction rate of pesticides is simply:
| (4) |
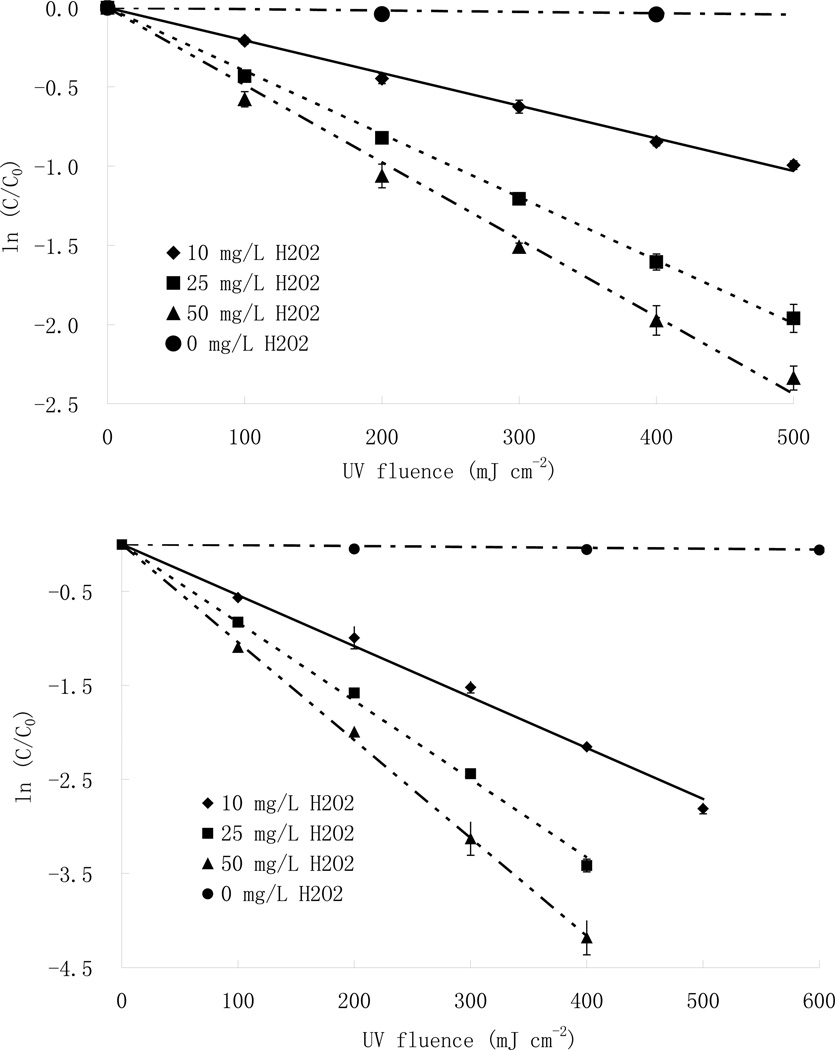
Fig.1.
Photodegradation of parathion and chlorpyrifos with different initial hydrogen peroxide concentrations at pH 7, without carbonate. (a: [parathion]=7.4 µM (top) (Wu and Linden, 2008); b:[chlorpyrifos]=2.13 µM (bottom)). Error bars are one standard deviation.
Results and Discussions
Transformation of Pesticides by UV/H2O2 system without HCO3−
Direct photolysis is insignificant for parathion and chlorpyrifos over the timeframe of the UV exposures, due to their low quantum yields and molar absorption coefficients. However, the addition of hydrogen peroxide significantly increases the destruction rates for both pesticides mainly due to the formation of hydroxyl radicals through the photolysis of H2O2 (Fig. 1) (Wu and Linden 2008).
Aqueous solutions of parathion (7.4 µM) and chlorpyrifos (2.1 µM) with different concentrations (0–50 mg/L) of hydrogen peroxide were examined. Fig. 1 shows that pesticide degradation follows pseudo-first-order kinetic at given pesticide and hydrogen peroxide concentrations. Addition of H2O2 resulted in faster degradation rates for both pesticides, and the reaction rates (represented by the slopes of the straight lines in Fig. 1) increased non-linearly with increasing H2O2 concentration, suggesting their dependence on initial H2O2 concentration (Eq. 4) as well as the H2O2 fH2O2 value (Wu and Linden 2008). In addition, because the scavenging effect of H2O2 dominated the process at higher peroxide concentration, the increase in reaction rate slowed, and the overall rate leveled off, at the higher H2O2 concentrations.
Role of hydroxyl radical in UV/H2O2 pesticide degradation process
To investigate the effect of hydroxyl radical, we applied a competition experiment that utilized nitrobenzene as a probe to determine the second-order reaction rate constants of both pesticides with hydroxyl radical. Nitrobenzene (kNB = 4.0 × 109 M−1 s−1) was chosen as the reference compound because it essentially does not undergo significant direct photolysis, is easy to analyze using HPLC, and has been successfully applied in similar experiments (Wu and Linden 2008). Nitrobenzene was added into working solutions and irradiated by 254nm UV light with appropriate additions of H2O2. Assuming reactions of hydroxyl radical with pesticides and nitrobenzene proceed independently in parallel, the second-order rate constants of pesticides can be expressed by Eq 5:
| (5) |
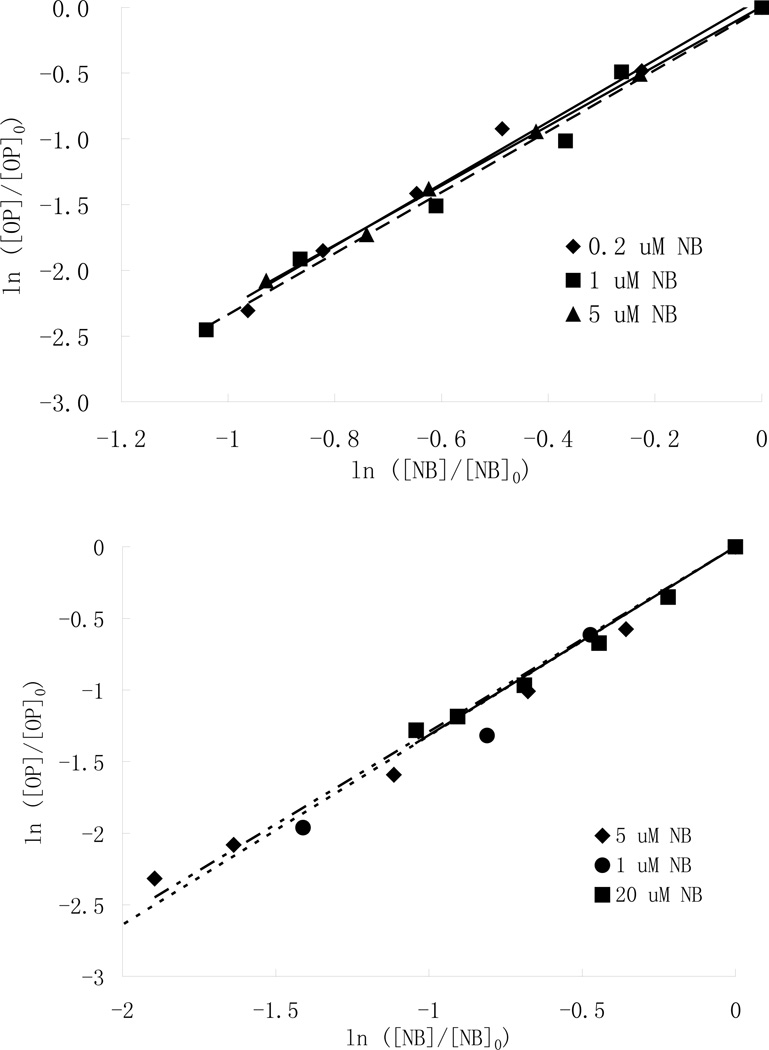
A plot of ln([OP]/[OP]0) versus ln [NB]/NB]0 (Fig. 2) results in a straight line whose slope represents the ratio of rate constants of pesticides and nitrobenzene (Eq. 5). The experiments were conducted with different initial nitrobenzene concentrations and an average value was obtained for calculating the kOP value of each pesticide.
Fig.2.
Relative destruction of parathion and chlorpyrifos in the presence of nitrobenzene (NB) and 25 mg /L H2O2, pH=7 (a: [parathion]0=5µM, b: [chlorpyrifos]=3.04µM)
Both parathion and chlorpyrifos are highly reactive toward hydroxyl radical as reflected by their second-order rate constants: 9.7 ± 0.5×109 and 4.9 ± 0.1 ×109 M−1 s−1 (mean value of experiments), respectively, suggesting hydroxyl radicals could play an important role in limiting their presence in waters (Wu and Linden, 2008). A previous study has reported a similar value of 4.17×109 M−1 s−1 for chlorpyrifos by Armbrust (2000) using acetonphenone as the reference compound.
Photodegradation of pesticides in the presence of bi/carbonate
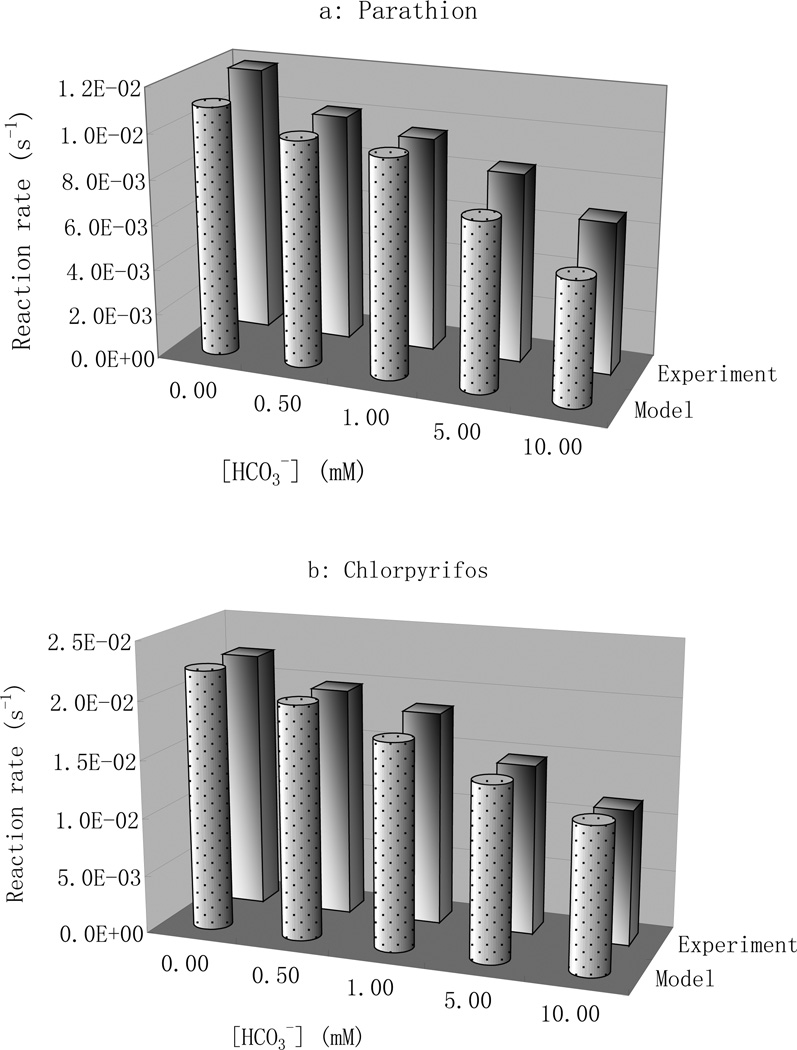
Effects of bi/carbonate on pesticide degradation in UV/H2O2 processes were examined in experiments in which HCO3− was added at concentrations relevant to natural waters. Bi/carbonates acted as a major hydroxyl radical scavenger (Eq. 2) and resulted in a decrease of the overall degradation rate for both pesticides as shown in Fig. 3 (back row). For parathion, the reaction rate decreased 15 percent with addition of a small amount of bicarbonate ions (0.5 mM). The trend continued with increasing bicarbonate concentrations to where a dose of 10 mM of bicarbonate reduced the reaction rate by about 50 percent. A similar trend was also measured for chlorpyrifos where a 10 percent drop in the reaction rate occurred with 0.5 mM bicarbonate added and a 50 percent reduction resulted from 10mM bicarbonate addition (Fig. 3).
Fig.3.
Photodegradation rate constants of parathion and chlorpyrifos in the presence of bi/carbonate, pH=7.0 : (a) Parathion, [para]= 5.13 µM; [H2O2]= 25 mg/L. (b) Chlorpyrifos, [CPF]= 3.5µM, [H2O2]= 50 mg/L
Role of carbonate radical in UV/H2O2 systems
The effects of bi/carbonate on oxidation of pesticides is not limited to a hydroxyl radical scavenging effect, but must also include the formation of the carbonate radical, which typically exists in natural water bodies at 2–3 orders of magnitude higher than the hydroxyl radical and can also react with the target pesticides. To evaluate the contribution of carbonate radicals in the oxidation process, the second-order rate constants between pesticides and carbonate radical needs to be established.
Carbonate radicals may be generated through the photolysis of complex of cobalt (III) (Stumm and Morgan 1996), photosensitizing of aromatic ketones (Canonica et al., 2005), the oxidation of bi/carbonate by sulfate radical anion (Huie and Clifton 1990) and the reaction between peroxynitrite and HCO3− (Huang and Mabury 2000c; Goldstein et al., 2001). A competition method utilizing potassium peroxonitrite (ONOOK) was adopted in this study (Huie and Clifton 1990). This method avoided the complication of direct photolysis of target compounds in the method of photolysis of hydrogen peroxide and minimizes the complication from hydroxyl radical and other secondary radicals.
Upon dissolving ONOOK in waters, hydroxyl radicals are produced as a result of homolytic fission of the peroxonitrous acid (King et al., 1992):
| (6) |
The generated hydroxyl radicals subsequently react with carbonate ions and generate carbonate radicals (Reactions 4 and 5 in Table 1). The NO2 • radical, which is unstable in water, has little effect on the destruction of aniline for three reasons: 1) aniline is an electron-rich chemical that has a high reaction rate constant with carbonate radical; 2) nitrogen dioxide radical proceeds rapidly through the following pathway (Eq. 7) and has a negligible impact on pesticide degradation; and 3) nitrogen dioxide radical can be scavenged by carbonate radical with a high reaction rate of 109 M−1s−1 (King et al., 1992; Huang and Mabury 2000c):
| (7) |
In order to measure the pesticide carbonate radical rate constants, aniline, which has a carbonate radical second-order rate constant of 4.6 × 108 M−1s−1 (Neta et al., 1988), was chosen as probe compound in the competition experiments. Assuming the only reactants in the system were pesticides and probe, the disappearance of probe can be expressed by Eq. 8 (Willson et al., 1971; Huang and Mabury 2000c):
| (8) |
Where R0 is the rate of loss of aniline without pesticide, R is the rate of loss of aniline when pesticide is present in the solution, and kOP,CO3•and kprobe,CO3 are the rate constants at which carbonate radical reacts with pesticides and aniline, respectively.
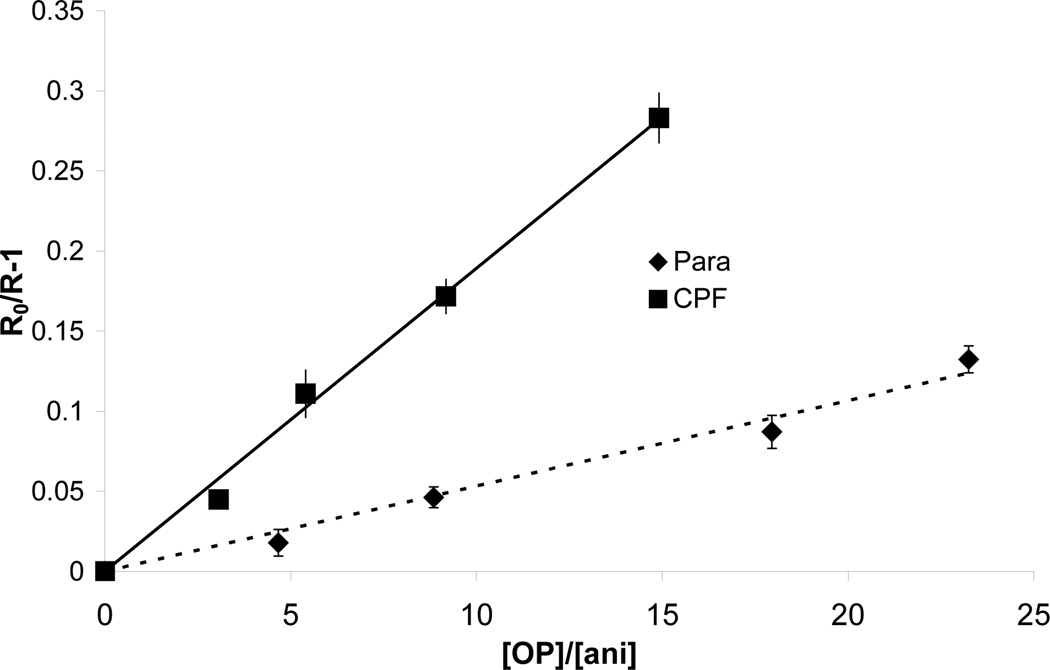
Rate constants between the carbonate radical and pesticides can be determined through the rate change of the probe in different experiments. The plot of [OP]/[probe] versus R0/R-1 yields a straight line with a slope of kOP,CO3• / kprobe,CO3 from which the absolute values of kOP,CO3• of parathion and chlorpyrifos were calculated to be 2.8 ± 0.2×106 and 8.8 ± 0.4×106 M−1s−1 (mean value of experiments), respectively, in this study (Fig. 4). It is noteworthy that the rate constant of chlorpyrifos is about three times higher than parathion, and that, after taking into account the hydroxyl radical rate constants, the overall pesticide degradation kinetics of chlorpyrifos differs substantially from parathion.
Fig.4.
Reactivity toward carbonate radicals for parathion and chlorpyrifos. Error bars are one standard deviation.
The carbonate radical rate constants were integrated into modeling of the reaction rates of parathion and chlorpyrifos at different bi/carbonate concentrations (Fig. 3 front row) The simulation agreed well with experimental results for parathion at all bicarbonate concentration levels and for chlorpyrifos at low bicarbonate levels. The simulation, however, slightly overestimated the rate constant of chlorpyrifos at the highest concentration of bi/carbonate, probably due to the smaller molar ratio of hydrogen peroxide and bicarbonate ions to chlorpyrifos in the experiments. Reactions between hydroxyl radical and phosphorus ions and other secondary radicals such as 2 HO2 • proved insignificant because of their extremely low concentrations and/or low reaction rate constants.
Comparison of the effects of hydroxyl and carbonate radicals
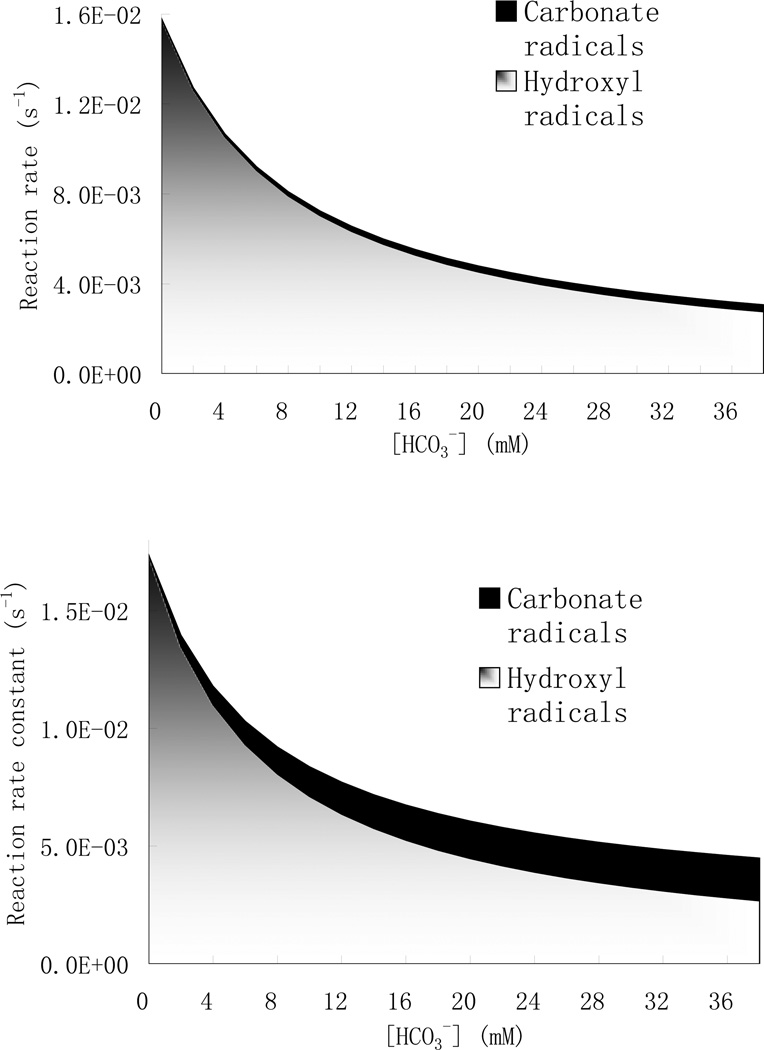
Hydroxyl and carbonate radicals are both important oxidant species in engineered systems and natural waters, and their contributions to the destruction of pesticides is governed by the reaction rate constants and steady-state concentrations. In our UV/H2O2 process, the simulated steady-state carbonate radical concentration was about two orders of magnitude higher than that of hydroxyl radical in the presence of bicarbonate ions at mM level and it increased steadily with increasing bicarbonate concentrations. Contributions of hydroxyl and carbonate radicals to the overall reaction rates of pesticides in the UV/H2O2 process with identical UV intensity and initial concentrations were simulated and compared in Fig. 5. The fact that parathion and chlorpyrifos have quite different reaction rates toward hydroxyl and carbonate radical affects their relative reaction kinetics in the presence of bi/carbonate. As overall reaction rates decrease with the addition of bi/carbonate ions, the contribution of carbonate radical increases sharply while that of hydroxyl radical decreased. Whereas contribution from carbonate radical accounts for about 4 and 10 percent of degradation rate at 10 and 38 mM of bi/carbonate, respectively for parathion, it is more important for chlorpyrifos where the carbonate radical contributes to about 15 and about 45 percent of overall reaction rate at 10 and 38mM of bi/carbonate at the given hydrogen peroxide level. Taking into consideration that bi/carbonate are major components of surface water with concentrations of several mM, the carbonate radical plays an important role in limiting the presence of both pesticides, especially for chemicals with a high kOP,CO3• kOP value like chlorpyrifos. In case of pesticides with even much higher kOP,CO3 values, e.g. fenthion (2 × 107 M−1 s−1) and phorate (1.2 × 107 M−1 s−1) (Huie and Clifton 1990), the contribution of carbonate radical would be even greater.
Fig.5.
Evaluation of the different degradation pathways of parathion and chlorpyrifos as a function of [bi/carbonate]. [H2O2]=40 mg/L, [Para]0=5uM (top), [CPF]0=5uM (Bottom), pH=7
In natural surface waters, the steady-state concentration of carbonate radical varies between 10−13–10−15 M, and is about two-orders of magnitude higher than that of hydroxyl radicals (10−14–10−18 M) (Lam et al., 2003). Even though the rate constants of the carbonate radical are typically two-orders of magnitude lower than that of hydroxyl radicals for most pesticides (105–107 M−1s−1 compared to 107–109 M−1s−1) (Buxton and Greenstock 1988; Lam et al., 2003), the combined effect suggests the contribution of carbonate radical should be significant at least for some pesticides. Suppose that the steady-state concentrations of carbonate and hydroxyl radicals in sunlit natural waters are equal to 5.0 × 10−15 and 1.0 × 10−17 M respectively. The theoretical half-life of chlorpyrifos resulting from hydroxyl radicals alone (without considering direct photolysis, hydrolysis or other oxidizing species such as carbonate radicals) would be 164 d. In contrast, the theoretical half-life would be reduced to 87 d when the contribution of the carbonate radical is taken into account. These results indicate the carbonate radical can not be ignored in the phototransformation of pesticides both in natural and engineered water systems.
Role of bi/carbonate and hydrogen peroxide
Because it acts both as scavenger of hydroxyl radical and source of carbonate radical, bi/carbonate affects the effectiveness of the removal of pesticides in an engineered AOP process. Fig. 3 (back row) shows simulation results for the phototransformation of the pesticides with varying bi/carbonate concentration levels at one hydrogen peroxide concentration. In order to comprehend the effect of bi/carbonate, further simulations were carried out based on rate constants from this study to model the combined effects of H2O2 and bi/carbonate on parathion and chlorpyrifos degradation. For these simulations, the same initial concentrations (5 µM) of both pesticides were chosen and assumption of identical reactor geometry was made while H2O2 and pesticides concentrations varied over a wide range.
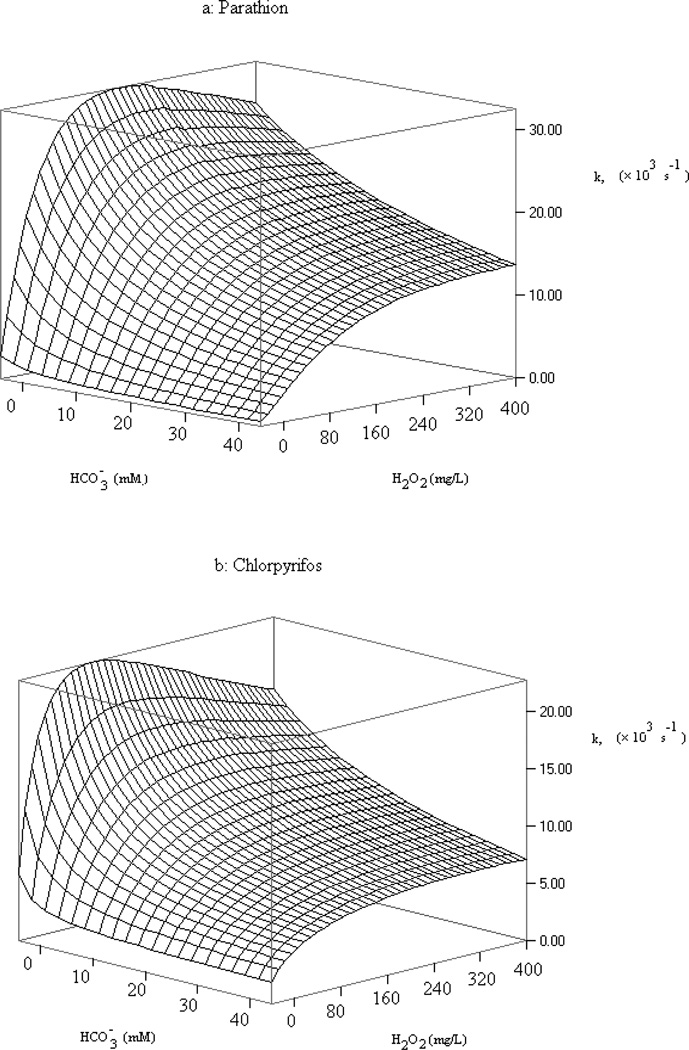
The simulation results are plotted in Fig. 6, which shows the overall reaction rates for parathion and chlorpyrifos at varying hydrogen peroxide and bi/carbonate concentrations. Perhaps the most striking feature of the figure is the similarity of changes of the overall reaction rates for both chemicals over a wide range of H2O2 and bi/carbonate despite their different hydroxyl and carbonate radical rate constants. Reaction rates in both cases initially increased rapidly with H2O2 concentrations but decrease or slow at higher H2O2 concentrations for given bi/carbonate levels. The initial increase was more rapid for parathion as peak reaction rate was reached faster compared to chlorpyrifos, presumably because parathion reacts faster with hydroxyl radical. The reaction rates of both pesticides decreased at very high H2O2 concentrations and this was caused by the scavenging of hydroxyl radical and light attenuation by H2O2 (Eq. 3 and fH2O2). Bi/carbonate caused reduction of the overall reaction rates at all H2O2 levels but such effects were more weighted at low H2O2 concentration levels, i.e. 0–10 mM, for both pesticides. Effects at low concentrations are more pertinent both to natural waters and engineered water systems. At higher bi/carbonate concentrations, the reaction rate constants at higher H2O2 concentration slowed down and the optimal H2O2/pesticide ratio was much higher than without carbonate. The relative contribution of carbonate radical in reducing the pesticide degradation rate constant decreased as the concentration of H2O2 increased.
Fig.6.
Modeled photodegradation rate constants of parathion and chlorpyrifos in aqueous solution as a function of hydrogen peroxide and bicarbonate ion concentrations. (pH=7, [OP]0=5µM)
While H2O2 and bi/carbonate were both proven important for the parathion and chlorpyrifos removal rate in this study, it should be emphasized that these experiments were conducted on the bench-scale with a fixed optical path length and the model here omits consideration of UV reactor hydraulics. To develop this analysis into a predictive model for UV reactors, the fluence rate distribution, hydraulics and optical path length should be taken into account for predicting pesticide removal in different engineered UV reactors.
Conclusions
Hydroxyl radicals are often the primary contributor in the removal rate of many organic contaminants by UV/H2O2 AOP process in engineered water/wastewater treatment systems. It is also critical to understanding the environmental fate of many organic contaminants in natural water systems. Carbonate radical, which exists in natural water systems at higher concentrations (10−13–10−15 M) than hydroxyl radicals, may also play an important role. In UV/H2O2 engineered systems, carbonate radical concentrations can be considerable due to high hydroxyl radical concentration, promoting its formation. Therefore the effect of carbonate radicals should also be considered in order to accurately predict phototransformations of organic contaminants in water systems.
The major source of carbonate radical is through a reaction between bi/carbonate and hydroxyl radical. This reaction has two different effects: the scavenging of hydroxyl radical reducing its availability to target contaminants; and the generation of carbonate radical that subsequently contributes to the destruction of target contaminants. In this study, the reaction rate constants between hydroxyl radicals and two pesticides, i.e. parathion and chlorpyrifos, were determined to be 9.7 ± 0.5×109 and 4.9 ± 0.1 ×109 M−1 s−1, respectively. The reaction rate constants between carbonate radicals and the pesticides were determined to be 2.8 ± 0.2×106 and 8.8 ± 0.4×106 M−1s−1, respectively.
It was experimentally proven in this study that addition of hydrogen peroxide at lower concentrations improves phototransformation rate of these pesticides. However, at higher concentrations, additional hydrogen peroxide may not necessarily increase the overall reaction rates. On the other hand, addition of bi/carbonate always reduces the overall reaction rates due to scavenging of hydroxyl radicals despite the contributions of carbonate radicals to the decay of the target pollutant. In this study, we subsequently used a kinetic model that included all relevant reactions to simulate reaction rates of the two pesticides over a wide range of hydrogen peroxide and bi/carbonate concentrations. These results provide insightful information in guiding UV/H2O2 AOP phototransformation of organic contaminants in engineered systems. They will also be helpful in understanding the environmental fate of organic contaminants subject to these radical species.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acero JL, Stemmler K, von Gunten U. Degradation kinetics of atrazine and its degradation products with ozone and OH radicals: A predictive tool for drinking water treatment. Environ. Sci. Technol. 2000;34(4):591–597. [Google Scholar]
- Acero JL, von Gunten U. Characterization of oxidation processes: ozonation and the AOP O3/H2O2 . J. American Water Works Association. 2001;93(10):90–100. [Google Scholar]
- Armbrust KL. Pesticide hydroxyl radical rate constants: measurements and estimates of their importance in aquatic environments. Environ. Toxicol. Chem. 2000;19(9):2175–2180. [Google Scholar]
- Augusto O, Bonini MG, Amanso AM, Linares E, Santos CCX, De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Racial Biology & Medicine. 2002;32(9):841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- Barcelo D, Durand G, Bertrand ND. Photodegradation of the organophosphorus pesticides chlorpyrifos, fenamiphos and vamidothion in water. Toxicological and Environmental Chemistry. 1993;38:183–199. [Google Scholar]
- Baxendale JH, Wilson JA. Photolysis of hydrogen peroxide at high light intensities. Trans. Farady Sco. 1957;53:344–356. [Google Scholar]
- Bielski BH, Cabelli DE, Arudi RL, Ross AB. Reactivity of HO2/O2- radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1985;14(4):1041–1077. [Google Scholar]
- Bozzelli JW. REACT for Windows: Chemical kinetics emulation and application (by Michael Manka) J. Chem. Edu. 2000;77(2):165–166. [Google Scholar]
- Busset C, Mazellier P, Sarakha M, De Laat J. Photochemical generation of ACCEPTED MANUSCRIPT carbonate radicals and their reactivity with phenol. J. Photochemistry and photobiology A: Chemistry. 2007;185:127–132. [Google Scholar]
- Buxton GV, Elliot AJ. Rate constant for reaction of hydroxyl radicals with bicarbonate ions. Radiat. Phys. Chem. 1985;27:241–243. [Google Scholar]
- Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (.OH/.O−) in aqueous-solution. J. Phys. Chem. Ref. Data. 1988;17:513–886. [Google Scholar]
- Canonica S, Kohn T, Mac M, Real FJ, Wirz J, von Gunten U. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds. Environ. Sci. Technol. 2005;39:9182–9188. doi: 10.1021/es051236b. [DOI] [PubMed] [Google Scholar]
- Chen S, Hoffman MZ. Effect of pH on the reactivity of the carbonate radical in aqueous solution. Radiation Research. 1975;62:18–27. [PubMed] [Google Scholar]
- Clifton CL, Huie RE. Rate constants for some hydrogen abstraction reactions of the carbonate radical. Int. J. Chem. Kinet. 1993;25:199–203. [Google Scholar]
- Crow JP, Spruell C, Chen J, Gunn C, Ischiropoulos H, Tsai M, Smith CD, Radi R, Koppenol WH, Beckman JS. On the pH-dependent yield of hydroxyl radical products from peroxynitrite. Free Radic Biol Med. 1994;16(3):331–338. doi: 10.1016/0891-5849(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Dilling WL, Lickly LC, Lickly TD, Murphy PG, McKellar RL. Organic photochemistry. 19. Quantum yields for O,O-diethyl O-(3,5,6-trichloro-2-pyridinyl) phosphorothioate (chlorpyrifos) and 3,5,6-trichloro-2-pyridinol in dilute aqueous solutions and their environmental phototransformation rates. Environ. Sci. Technol. 1984;18:540–543. [Google Scholar]
- Draganić ZD, Negrón-mendoza A, Sehested K, Vujosevic SI, Navarro-Gonzales R, Albarran-Sánchez MG, Draganić IG. Radiolysis of aqueous solutions of ammonium bicarbonate over a large dose range. Radiat. Phys. Chem. 1991;38(3):317–321. [Google Scholar]
- Einschlag F, Lopez J, Carlos L, Capparelli A. Evaluation of the efficiency of photodegradation of nitroaromatics applying the UV/H2O2 technique. Environ. Sci. Technol. 2002;36:3936–3944. doi: 10.1021/es0103039. [DOI] [PubMed] [Google Scholar]
- Gal E, Aires P, Chamarro E, Esplugas S. Photochemical degradation of parathion in aqueous solutions. Water. Research. 1992;23(7):911–915. [Google Scholar]
- Gilliom R, Barbash JE, Grawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP, Wolock DM. Pesticides in the Nation’s streams and ground water. 2006:1992–2001. USGS open file. [Google Scholar]
- Glaze W, Lay Y, Kang J. Advanced oxidation processes. A kinetic model for the oxidation of 1,2-dibromo-3-chloropropane in water by the combination of hydrogen peroside and UV radiation. Ind. Eng. Chem. Res. 1995;34:2314–2323. [Google Scholar]
- Goldstein S, Czapski G, Lind J, Merenyi G. Carbonate radical ion is the only observable intermediate in the reaction of peroxynitrite with CO2 . Chem. Res. Toxicol. 2001;14:1273–1276. doi: 10.1021/tx0100845. [DOI] [PubMed] [Google Scholar]
- Grounwell JR, Erickson RH. Photolysis of parathion (O,O-Diethyl-O-(4-nitrophenyl)thiophosphate): new products. J. Agric. Food Chem. 1973;21(5):929–931. doi: 10.1021/jf60189a003. [DOI] [PubMed] [Google Scholar]
- Haag WR, Hoigne J. Photo-sensitized oxidation in natural-water via. OH radicals. Chemosphere. 1985;14(11012):1659–1671. [Google Scholar]
- Huang J, Mabury SA. Steady-state concentrations of carbonate radicals in the field waters. Environ Toxicol. Chem. 2000a;19:2181–2188. [Google Scholar]
- Huang J, Mabury SA. The role of carbonate radical in limiting the persistence of sulfur-containing chemicals in sunlit natural waters. Chemosphere. 2000b;41:1775–1782. doi: 10.1016/s0045-6535(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Huang J, Mabury SA. A new method for measuring carbonate radical reactivity toward pesticides. Environ Toxicol. Chem. 2000c;6:1501–1507. [Google Scholar]
- Huie RE, Clifton CL. Temperature dependence of the rate constants for reactions of the sulfate radical, SO4- with anions. J. Phys. Chem. 1990;94:8561–8567. [Google Scholar]
- King PA, Anderson VE, Edwards JO, Gustafson G, Plumb RC, Suggs JW. A stable solid that generates hydroxyl radical upon dissolution in aqueous solutions: Reaction with proteins and nucleic acid. J. Am. Chem. Soc. 1992;114:5430–5432. [Google Scholar]
- Klassen N, Marchington D, McGrowan HCC. H2O2 determination by the I3- method and KMnO4 titration. Anal. Chem. 1994;66:2921–2925. [Google Scholar]
- Lam ML, Tantuco K, Mabury SA. Photofate: A new approach in accounting for the contribution of indirect photolysis of pesticides and pharmaceuticals in surface water. Environ. Sci. Technol. 2003;37:899–907. doi: 10.1021/es025902+. [DOI] [PubMed] [Google Scholar]
- Mansour M, Feicht EA, Behechti A, Scheunert I. Experimental approaches to studying the photostability of selected pesticides in water and soil. Chemosphere. 1997;35:39–50. [Google Scholar]
- Maruthamuthu P, Neta P. Phosphate radicals. Spectra, acid-base equilibria, and reactions with inorganic compounds. J. Phys. Chem. 1978;82:710–713. [Google Scholar]
- Mazellier P, Leroy E, De Laat J, Legube B. Transformation of carbendazim induced by the H2O2/UV system in the presence of hydrogencarbonate ions: involvement of the carbonate radical. New J. Chem. 2002;26:1784–1790. [Google Scholar]
- Mazellier P, Busset C, Delmont A, De Laat J. A comparison of fenuron degradation by hydroxyl and carbonate radicals in aqueous solution. Water Research. 2007;41:4585–4594. doi: 10.1016/j.watres.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Minero C, Pellizzari P, Maurino V, Pelizzetti E, Vione D. Enhancement of dye sonochemical degradation by some inorganic anions present in natural waters. Applied Catalysis B: Environmental. 2008;77:308–316. [Google Scholar]
- Mok CY, Marriott P, Ong L, Yeo GN. Photodegradation of parathion. Bull. Environ. Contam. Toxicol. 1987;38:820–826. doi: 10.1007/BF01616707. [DOI] [PubMed] [Google Scholar]
- Neta P, Huie RE, Ross AB. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17(3):1027–1229. [Google Scholar]
- Sakellarides TM, Siskos MG, Albanis TA. Photodegradation of selected organophosphorus insecticides under sunlight in different natural waters and soils. Inter J. Environ. Anal. Chem. 2003;83(1):33–50. [Google Scholar]
- Schested K, Rasmussen OL, Fricke H. Rate constants of OH with HO2, O2- and H2O2+ from hydrogen peroxide formation in pulse-irradiated oxygenated water. J. Phys. Chem. 1968;72:626–631. [Google Scholar]
- Sharpless CM, Linden KG. Experimental and model comparisons of low- and medium- pressure Hg lamps for the direct and H2O2 assisted UV photodegradation of N-nitrosodimethylamine in simulated drinking water. Environ. Sci. Technol. 2003;37:1933–1940. doi: 10.1021/es025814p. [DOI] [PubMed] [Google Scholar]
- Shemer H, Linden KG. Degradation and byproduct formation of diazinon in water during UV and UV/H2O2 treatment. J. Hazardous Materials. 2006;B136:553–559. doi: 10.1016/j.jhazmat.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Stefan M, Hoy A, Bolton JR. Kinetics and mechanism of the degradation and mineralization of acetone in dilute aqueous solution sensitized by the UV photolysis of hydrogen peroxide. Environ. Sci. Technol. 1996;30:2382–2390. [Google Scholar]
- Stumm W, Morgan JJ. Aquatic Chemistry. 3rd ed. New York: Wiley Interscience; 1996. [Google Scholar]
- Walia S, Dureja P, Mukerjee SK. New photodegradation products of chlorpyrifos and their detection on glass, soil and leaf surfaces. Arch. Environ. Contam. Toxicol. 1988;17:183–188. [Google Scholar]
- Wan HB, Wong MK, Mok CY. Comparative study on the quantum yields of direct photolysis of organophosphorus pesticides in aqueous solution. J. Agric. Food Chem. 1994;42:2625–2630. [Google Scholar]
- Willson R, Greenstock CL, Adams G, Wageman R, Dorfman L. The standardization of hydroxyl radical rate data from radiation chemistry. Int J. Phys. Chem. 1971;3:221–220. [Google Scholar]
- Wu CL, Shemer H, Linden KG. Photodegradation of metolachlor applying UV and UV/H2O2 . J. Agric. Food Chem. 2007;55:4059–4065. doi: 10.1021/jf0635762. [DOI] [PubMed] [Google Scholar]
- Wu CL, Linden KG. Degradation and byproduct formation of parathion in aqueous solutions by UV and UV/H2O2 treatment. Water Research. 2008;42(19):4780–4790. doi: 10.1016/j.watres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]