Abstract
Aging-related oxidative stress has been linked to degenerative modifications in different organs and tissues. Using redox proteomic analysis and illustrative MS/MS mapping we demonstrate oxidative post-translational modifications in structural proteins of intervertebral discs (IVD) isolated from aging mice. Increased protein carbonylation was associated to protein fragmentation and aggregation. Complementing these findings, a significant loss of elasticity and increased stiffness was measured in fibrocartilage from aging mice. Studies employing circular dichroism and intrinsic tryptophan fluorescence revealed a significant loss of secondary and tertiary structures of purified collagens following oxidation. Collagen unfolding and oxidation promoted both non-enzymatic and enzymatic degradation. Importantly, induction of oxidative modification in healthy fibrocartilage recapitulated the biochemical and biophysical modifications observed in the aging IVD. Together, these results suggest that protein carbonylation, glycation, and lipoxidation could be an early event in promoting IVD degenerative changes.
Introduction
Intervertebral discs (IVDs) function as shock-absorbers that resist compressive forces exerted on the spine, serve to evenly distribute load across vertebral bodies, and provide for the complex motion of the axial skeleton. The IVD is a complex structure composed of the fibrotic annulus fibrosus (AF) which is integrated with the cartilaginous vertebral endplates and encases the gelatinous nucleus pulposus(NP).
During aging, IVDs undergo degenerative changes that include significant histological, biochemical, and metabolic modifications. Factors known to contribute to IVD failure include changes within the NP involving an increase in cell density, cell senescence, and cell death associated with impaired synthetic activity and increased matrix metalloprotease (MMP) activity. These changes are associated with reduced levels of proteoglycans, and a shift in levels of collagen type II in favor of collagen type I which imparts a fibrous nature to the NP. The cartilaginous endplates tend to calcify and become thinner, thus altering both nutrition and redox potential within the NP. The AF loses its highly organized lamellar structure and becomes fissured (Adams and Roughley, 2006; Antoniou et al., 1996; Johnstone and Bayliss, 1995; Kepler et al., 2013; Maeda and Kokubun, 2000; Urban et al., 2001). From a biochemical perspective the reduction of proteoglycan molecules which attract and bind water via their polar groups results in a loss of hydration and nucleus pressure (Adams and Roughley, 2006; Buckwalter, 1995; Roughley, 2004).
A substantial portion of these biochemical changes are thought to result from post-translational oxidative changes in structural elements of the IVDs tissues. In aging an imbalance between the generation of free radicals and the cellular scavenging mechanisms has been observed, resulting in tissue oxidative stress (Alexeyev, 2009; Haigis and Yankner; Roberts and Sindhu, 2009). Aging-related oxidative stress is associated with: (i) mitochondrial senescence and aging of the mitochondrial redox enzymatic chain and a decrease in the synthesis of free radical scavenging enzymes(Berenbaum; Broadley and Hartl, 2008; Cannizzo et al.; Dufour et al., 2000; Haigis and Yankner); (ii) excessive calorie intake with increased production of free radicals (Roberts and Sindhu, 2009; Terman, 2006; Wellen and Thompson), (iii) increased incidence of pathologies that present with chronic inflammation that activate the oxidative burst (Cathcart, 2004; de la Fuente et al., 2004; Preynat-Seauve et al., 2003), and (iv) accumulation over time of free radicals produced by different environmental sources.
Oxidative stress is clearly an important factor in IVD failure. Indeed, patients with osteoarthritis exhibit elevated synovial fluid and serum levels of F2-isoprostanes, 4-hydroxynonenal, and malondialdehyde which are the major aldehydic products of lipid peroxidation (Basu et al., 2001; Henrotin et al., 2003; Tiku et al., 1999) and dietary intake of antioxidants has been shown to ameliorate the symptoms of osteoarthritis (McAlindon et al., 1996). Increased levels of advanced glycation end products (AGEs) have also been reported in aging IVDs. Among AGEs pentosidine, a product of the oxidative stress-induced Maillard reaction, induces a non-enzymatical crosslink of collagen fibers in the IVD (Pokharna et al., 1995; Sell and Monnier, 1989; Takahashi et al., 1994) (Pokharna and Phillips, 1998). The pentosodine cross-links alter both the anatomical integrity and biological properties of the collagen network through inhibition of matrix turnover resulting in increased cartilage stiffness and fragility (Monnier et al., 1984; Pokharna and Phillips, 1998).
Published studies have also established a firm link between oxidative stress and chondrocytes senescence and apoptosis (Adams and Horton, 1998; Chen et al., 1995; Fay et al., 2006; Hashimoto et al., 1998; Homma et al., 1994; Pelletier et al., 2000; Wruck et al., 2011).
However all the published data so far have been conducted in tissues with well established degenerative and inflammatory damage, thus making it difficult to clearly delineate whether cartilage damage by oxidative stress precedes or follows degenerative changes.
In the present study a different approach is used to understand the early effects of oxidative damage to the IVD. In particular we have investigated whether oxidation of collagen represents an early or even initiating event in cartilage degeneration. To this goal we first mapped all the biochemical changes of structural proteins in aging cartilage, related to oxidative stress. We then determined how such modifications applied to a healthy IVD interfere with protein folding, susceptibility to MMPs degradation, and mechanical properties. The results provide insights into the mechanisms whereby oxidative damage contribute to IVD failure.
Results
Histological and biomechanical alterations exhibited in aging IVD
IVD were isolated from 3, 12, and 22 month old mice. Histological examination of this material from the 3 and 12 month old mice did not show significant differences in the morphology and staining of the nucleus pulposus, annulus fibrosus, and end plate cartilage (Figure 1a and data not shown). The intervertebral discs of 22 month old mice revealed segmental, severe degeneration of the discs with loss of gelatinous matrix (GM) and central notochordal cell (CNC) layer along with proliferation of chondrocyte-like cells (arrowheads) merging with the adjacent annulus fibrosus and end plate cartilage (Figure 1a). A marked decrease in Alcian blue and Safranin O-Fast Green staining was observed in the IVD of aged mice. Complementing these findings, Western blot analysis detected an increase of collagen I and a loss of collagen II in IVD isolated from 22 month old mice, as compared to IVD from younger mice (Figure 1b and 1c).
Figure 1. Structural and biomechanical changes in aging IVD.
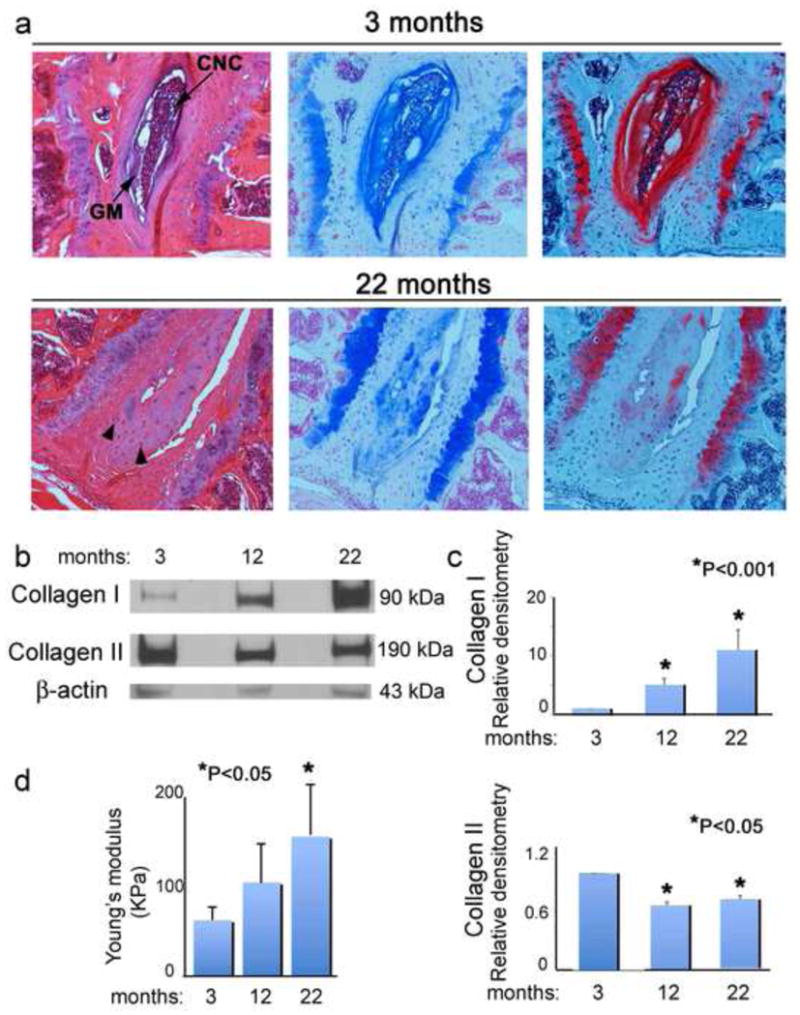
a) Light micrographs of the intervertebral disc (IVD) from 3 and 22 month old mice, H-E, Alcian blue pH 2.5, Safranin O-Fast Green staining respectively. Loss of the central notochordal cell (CNC) layer and total proteoglycans in the gelatinous matrix (GM) in aging mice. Proliferation of chondrocyte-like cells (arrowheads) was observed in the nucleus pulposus (NP) in 22 month old mice. b)Western blotting for collagen I, collagen II, and β-actin (loading control) from 3, 12, and 22 month old mice. c) Bar graphs represent average and s.e.m. of three independent western blots, P<0.001 and P<0.05 values respectively, ANOVA, Tukey. d) Intrinsic biomechanical properties of IVD from 3, 12, and 22 month old mice.
To determine whether the loss of collagen fibril architecture observed in aging IVD would affect biological performance of the fibrocartilage, freshly isolated IVD from 3, 12, and 22 month old mice were subjected to compressive forces using a custom-made mechanical testing device(Soltz and Ateshian, 1998). Young’s modulus (EY), which measures the stiffness of an elastic material, was determined. A significant loss of elasticity and increased stiffness was observed in fibrocartilage from older mice (Figure 1d).
Increased aggregation and oxidative modifications of proteins in aging IVD
Oxidative stress has been linked to tissue degenerative modifications in several diseases. To investigate whether there was an increase in IVD oxidative stress with age, mice were injected intravenously with the CellROX® probe that fluoresces upon binding to reactive oxygen species. An age-dependent increase in IVD fluorescence was observed (Figure 2a, 2b). To determine whether the increase in tissue ROS would elevate the level of oxidatively modified proteins in IVD of aging mice, IVD proteins were extracted from 3, 12, and 22 month old mice and incubated with 2,4-Dinitrophenylhydrazine (DNPH) which specifically binds to carbonyl groups introduced into amino acid side chains as an irreversible oxidative modification. The DNPH derivatized samples were separated on a gradient SDS-PAGE which was followed by western blotting using a primary antibody specific to the DNP moiety of the proteins. Western blot analysis detected the presence of carbonylated proteins in IVD isolated from mice of all ages (Figure 2c). However, the levels of carbonylated proteins detected were significantly higher in IVD isolated from aging mice (Figure 2c).
Figure 2. Detection of oxidatively modified proteins in aging IVD.
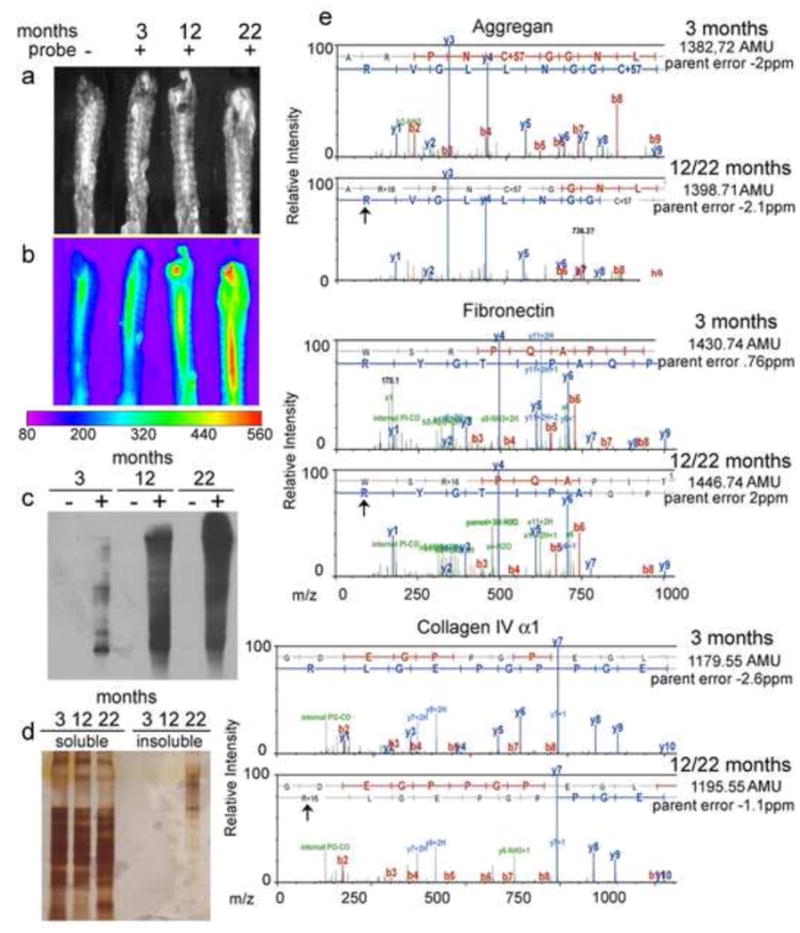
a,b)Phase contrast and oxidative stress measurements in IVD isolated from 3, 12, and 22 month old mice using a fluorogenic probe, CellROX® Deep Red. c) Western blot analysis of carbonylated proteins detected in IVD isolated from 3, 12, and 22 month old mice. Lanes marked as “−” indicate non-derivatized proteins (derivatization control) and “+”indicate derivatized proteins. d) Silver stained SDS-PAGE of sequential protein extractions from IVD using sodium chloride followed by guanidine hydrochloride. Guanidine hydrochloride extractions demonstrate microaggregates in IVD from aged mice. e) Illustrative MS/MS mapping of oxidative modifications of amino acid side chains across the same peptide sequences in IVD proteomes isolated from 3, 12, and 22 month old mice showed the selective Arg oxidation in aggrecan, fibronectin and alpha-1 collagen VI.
To determine whether protein carbonylation increased the level of protein aggregation, IVDs were extracted using NaCl (soluble fraction) followed by guanidine hydrochloride (insoluble fraction) and then examined by SDS-PAGE and silver stain analysis (Figure 2d). Insoluble aggregates were only observed in the guanidine hydrochloride extraction of IVD isolated form aged mice but not from younger mice (Figure 2d).
Redox proteomic analysis of the IVD cartilage of 3, 12, and 22 month old mice was performed to investigate the age-dependent development of neo-epitopes derived from the major proteins comprising the IVD. Sequential NaCl and guanidine hydrochloride extractions of the total proteomes from IVD cartilage isolated from 3, 12, and 22 months aged mice followed by 1D electrophoresis, in gel trypsin digestion, and two-dimensional LC separations coupled to tandem mass spectrometry on a nanoLC/ Orbitrap system demonstrated that cartilage from aging mice contain an increasing amount of chemically oxidized amino acids in the main proteins constituents of the IVD(Figure 2e). These studies identified mapped oxidative modifications of collagen VI (alpha-1, alpha-2 and alpha-3 chains), fibronectin, aggrecan, decorin, versican, and matrix Gla protein (Table 1). Oxidations included: monooxidations on Arg, Cys (sulfenic acid), Phe, Lys, Met (Met-sulfoxide), Trp, Tyr; diooxidations on Arg, Cys (sulfinic acid), Met (Met-sulfone), Lys, Phe, Pro, Trp (N-formyl-kynurenine/dihydroxy Trp/dioxindolylalnine), Tyr; trioxidations on Cys (cysteic acid); Arg-GluSa (glutamic semialdehyde conversion of Arg); Pyrrolidinone (Pro) (Table 1).
Table 1. MS/MS mapping reveals increased oxidative post-translational modifications in aged mice.
MS/MS mapping of oxidative modifications of amino acid side chains across the same peptide sequences in IVD isolated from 3, 12, and 22 month old mice.


|
Oxidative structural modifications of purified collagens I and II
Next, we investigated a possible relationship between oxidation, induced by Fenton reaction, and modification in the proteins secondary and tertiary structures, as a potential causative factor for the degeneration observed in IVD during aging. Collagen is an optically active protein that adopts a polyproline II-like helical conformation. The unique circular dichroism (CD) spectrum of collagen is characterized by a small positive band at 221 nm and a large negative band at 197–198 nm(Shahab et al.). The maxima of the two bands are a measure of the triple helical content of the given sample(Shahab et al.) (Figure 3a, 3b).
Figure 3. Oxidative stress induces secondary and tertiary structural alterations in purified collagen I and II.
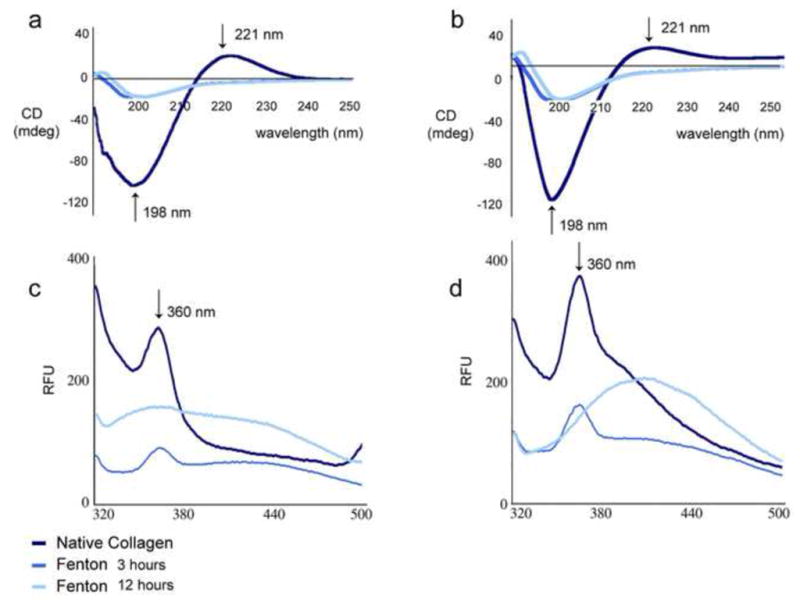
a,b) Circular dichroism analysis of secondary structure changes in collagens I and II following oxidation. The loss of ellipticity (in mdeg) at 198 nm and 221 are observed in each collagen following oxidation for both time points. c,d) Intrinsic Tryptophan fluorescence analysis of tertiary structural changes of collagens I and II upon oxidation with ROS generated by Fenton reaction. At 3 hours, unfolding is evident for both collagens (lower signal in the Trp maximum fluorescence emission signal at 360 nm). Following 12 hours, the unfolding is correlated with partial aggregation for collagen I and II as shown by the red-shift of the fluorescence signal to 420–430 nm.
Structural changes of native and chemically oxidized Collagen I and Collagen II were analyzed by far-UV CD spectroscopy (190–300 nm). Far UV CD spectrum of native collagens exhibited a maximum at 221 nm and a minimum at 198 nm, which are characteristic features of the collagen triple helix (Shahab et al.). However, upon oxidation, there was a distinctive loss of the positive CD signal at 221 nm for both collagen I and II proteins (Figure 3a and 3b). Moreover, the negative signal at 198 nm was sharply decreased from [−138.5] to about [−25.0] mdeg after 3h and 12h incubation in the Fenton reaction. These data reveal that the triple-helical content and packing of the helices in both collagens I and II were disrupted due to changes in the conformation of the oxidized proteins. The CD spectra of the native protein controls in the presence of just one of the reagents of the Fenton reaction (either in presence of [EDTA+FeCl2] or in presence of just H2O2) didn’t show significant changes in the secondary structure (data not shown).
In a hydrophobic environment (such as that within the core of a protein), tyrosine (Tyr) and tryptophan (Trp) have a high quantum yield and therefore a high fluorescence intensity (Pfefferkorn et al.). In contrast, in a hydrophilic environment (such as that at a protein surface in an aqueous solution) their quantum yield decreases, resulting in lower fluorescence intensity. Such a transition can be observed as a protein unfolds(Pfefferkorn et al.). Specifically for Trp residues, there are strong stoke shifts dependent on the solvent, indicating that the maximum emission wavelength of Trp is contingent upon the Trp environment. A red-shift to higher wavelength is attributed to the exposure of Trp to the solvent as it emerges from the hydrophobic core of the protein(Pfefferkorn et al.).
To assess protein unfolding, an excitation wavelength of 295 nm was employed (for the selective intrinsic Trp excitation, where the contributions from Tyr and Phe fluorescence emissions are weak) and the emission fluorescence between 320 nm and 500 nm at 25°C was recorded. A maximum emission peak at 367 nm was observed for both collagens I and II upon excitation at 295 nm (Figures 3c and 3d). A significant loss in the relative fluorescence intensity (about 70% and 60% for collagen I and II respectively) at the maximum 367 nm emission peak was observed for both proteins after 3h in the Fenton oxidizing reaction, suggesting a substantial loss in the tertiary structure upon unfolding (Figures 3c and 3d). Moreover, the loss in the fluorescence signal at the maximum emission peak was accompanied by a red shift from 367 nm to 405 nm after 12h of incubation in the Fenton reaction, supporting an increase in the Trp exposure to the solvent in the unfolded proteins (Pfefferkorn et al.). The intrinsic Trp fluorescence emission spectra of the native protein controls in presence of just one of the reagents of the Fenton reaction (either in presence of [EDTA+FeCl2] or in presence of just H2O2) didn’t display significant changes in the tertiary structure and folding (data not shown).
Exposure to hydroxyl radicals (·OH) increases carbonyl content and MMP sensitivity of collagens I and II
In the next set of experiments we aimed at determining whether collagen oxidation and unfolding would increase its susceptibility to non-enzymatic and enzymatic degradation. To this end we induced collagen I and II oxidation using the Fenton reaction. The carbonyl content (mainly carbonylated Pro, Arg, Lys and Thr residues) of the ·OH oxidized collagen I and II was determined spectrophotometrically, following the derivatization of the carbonylated amino acids residues of oxidized proteins using DNPH. The final product of the carbonyl-derivatization reaction, (2,4 Dinitrophenylhydrazone) has a maximum absorption at 375 nm which can be used to quantify the amount of carbonyl/protein. The ·OH induced oxidation of each collagen protein is accompanied by at least 200 fold increase in the carbonyl content in the modified proteins as compared to the background (no collagens, just the solution with 2,4-DNPH) and to the controls proteins (collagens in the absence of the oxidizing agents (Figure 4a). Denaturing SDS-PAGE analysis demonstrated extensive oxidative cleavage of the protein backbone of oxidized collagens as indicated by the presence of lower molecular weight bands (Figure 4B).
Figure 4. ROS exposure increases carbonyl content and MMP sensitivity of purified collagen I and II.
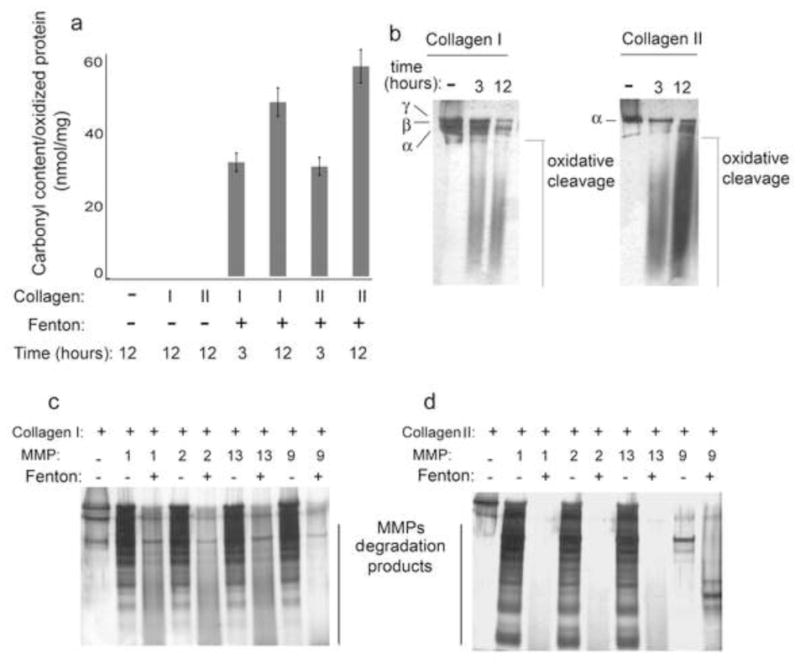
a) Bar graph and SD of spectrophotometric determination of the carbonyl content (as nmol carbonyl/mg protein) in Collagens I and II following exposure to Fenton reaction mixture. b) Silver stained SDS-PAGE analysis of native and ROS exposed collagens. Non enzymatic oxidative cleavage was seen in purified collagens I and II following oxidation. c,d) Metalloproteases assay on native and oxidized collagens I and II. Oxidized collagens exhibit increased sensitivity to MMP cleavage as compared to native (non-oxidized) collagens.
Degradative enzymes, such as A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) and matrix metalloproteinases (MMPs), play key roles in development of osteoarthritis (OA). We hypothesized that during aging and in certain metabolic conditions ROS promotes unfolding of major protein components of the IVD matrix making them vulnerable to proteolytic enzymes. To explore the relationship between the oxidation, unfolding, and the increased sensitivity to metalloprotease- mediated degradation of collagens I and II exposed to the Fenton reaction, an enzymatic assay was performed in which native or ·OH oxidized collagens were incubated with recombinant metalloproteases (MMP) 1, 2, 9, and 13 for 1 hour at 37°C. All tested MMPs were more active in degrading collagens I and II exposed to ·OH in the Fenton reaction as compared to the native proteins (Figures 4c and 4d).
Increased protein carbonylation in IVD exposed to ·OH ex vivo
Although it is generally accepted that the breakdown of articular cartilage in OA is mechanically driven, the mechanisms whereby protein oxidation promotes vulnerability of cartilaginous structures to injury during physical stress have not been worked out. To address our hypothesis that degeneration and protein unfolding seen in aging mice can be partially attributed to oxidative stress, IVD were isolated from young (3 month old) mice and either left untreated (in PBS) or exposed to ·OH generated by the Fenton reaction for 12, 24, or 48 hours. Proteins were extracted using NaCl and guanidine hydrochloride and incubated in DNPH. DNPH derivatized samples were run on a gradient SDS-PAGE, blotted, and incubated with a primary antibody specifically recognizing DNP moiety of the proteins. Western blot analysis indicates a significant increase in carbonylated proteins from IVD exposed to ·OH as compared to untreated IVD (Figure 5a). To further confirm these results, IVD were isolated from control or paraquat exposed young mice and immunogold labeling of carbonyl groups was performed on paraffin sections from these IVD. Immunohistochemical analysis detected the presence of carbonyl groups in the cartilaginous endplates of both the control and paraquat treated young mice. However, there was a much a higher density of immunogold labeled carbonyl groups observed in the cartilaginous endplates isolated from paraquat exposed mice (Figure 5b). Following the extensive increase in protein carbonylation observed in IVD following exposure to the Fenton reaction, we aimed to investigate whether oxidative damage to the cartilage structure could result in the release of cartilage structural proteins. To this extent, the supernatants of the control, 24 hour, and 48 hour Fenton reactions were collected. Following protein determination, 5μg from each sample were separated by gradient SDS-PAGE. Silver stain analysis of SDS-PAGE exhibits increased fragmentation and protein degradation in the supernatant from the 24 and 48 hour Fenton exposure time points as compared to the control supernatants (Figure 5c). Normalized spectral count analysis of the supernatants collected following ·OH induced oxidation revealed degenerative alterations to the collagen-proteoglycan network of Fenton reaction exposed IVD. Significant differences in protein expression of the supernatants were observed between the control and ·OH exposed IVD (Figure 5d). The prominent loss of several cartilage structural proteins (including collagens type I, III, VI, XI, and XII), proteoglycans (aggrecan, versican, decorin, and biglycan), and disc-matrix proteins (fibronectin) from oxidatively stressed IVD was exhibited by the detection of these proteins in the supernatants (Figures 5d and 5e). Next we aimed to investigate whether the observed disruption to the disc matrix composition would affect the biological performance of the fibrocartilage. Control and ·OH exposed IVD, adjacent to the discs used for the above study, were subjected to compressive forces and Young’s modulus was measured. There was a significant loss of elasticity and increased stiffness observed in the cervical and upper thoracic IVD in the treatment group as compared to controls (Figure 5f). However, there was no significant difference in viscoelastic properties detected in the lower thoracic and lumbar IVD between control and treatment groups (Figure 5f). Together, these results indicate that the alterations observed following the induction of oxidative stress in fibrocartilage from young mice mirrored the biochemical and biophysical degenerative modifications characteristic of aging IVD.
Figure 5. Increased protein carbonylation of IVD exposed to ROS ex vivo.
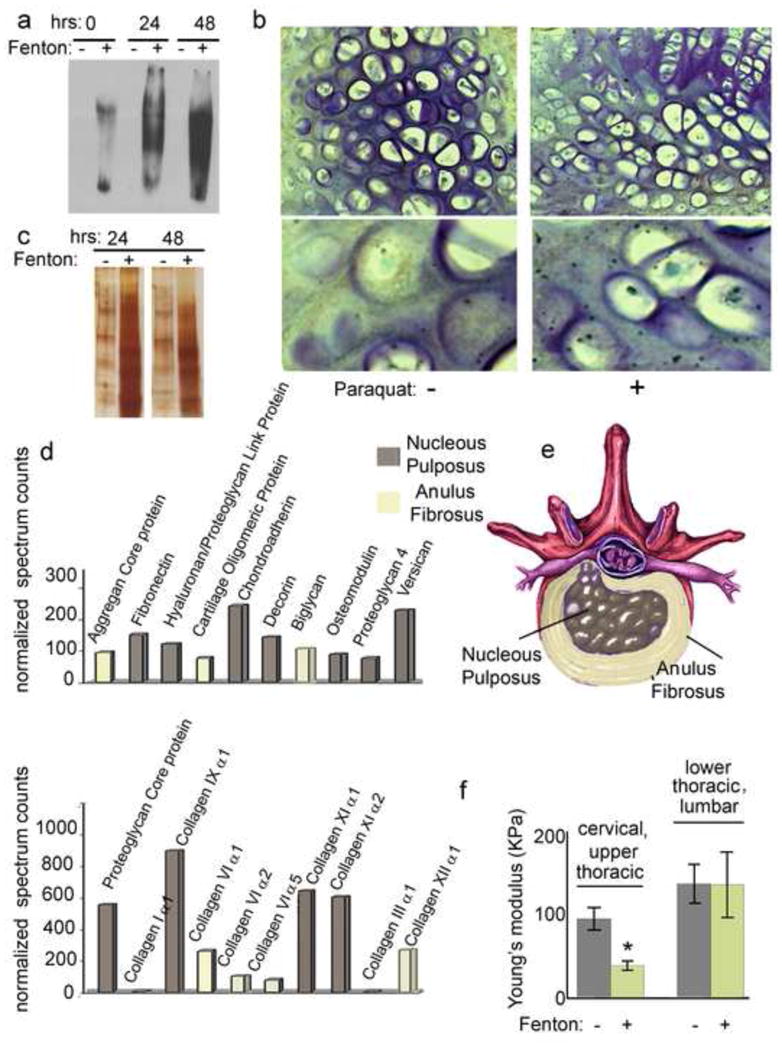
a) Western blot analysis of carbonylated proteins detected in IVD following incubation in the Fenton reaction solution. Lanes marked as “−” indicate non-derivatized proteins (derivitization control) and “+” indicate derivatized proteins. b) Immunohistological analysis of immunogold labeled carbonylsin IVD isolated from control and paraquat exposed 3 month old mice c) Silver stained SDS-PAGE analysis of reaction mixture from untreated and exposed IVD ex vivo. Increased fragmentation and degradation is observed in the supernatant from the 48 hour exposure time point as compared to the control. 5 μg of each mixture was analyzed. d) Normalized spectral counts following MS/MS analysis of the supernatants collected from untreated and 48 hour Fenton reaction exposed IVD. f)Viscoelastic properties of cervical, thoracic, and lumbar IVD isolated from control and paraquat treated 3 month old mice.
Discussion
The extracellular matrix of the IVD is comprised of collagens, proteoglycans, and non-collagenous proteins that form a dynamic network conferring resistance to mechanical loading while permitting both rotational and planar mobility. Each of these proteins is conformationally arranged in a manner that is maximally adapted for biomechanical functions. Understanding how this tissue is affected by age and environmental factors is essential for addressing the enormous burden of spine disease that affects our society (Indrakanti et al., 2012). The present study characterizes the changes that occur in IVDs of C57BL/6 mice as they age. As expected from earlier studies (Roughley, 2004; Schollmeier et al., 2000), these mice were found to progressively lose proteoglycans and collagen II from within the NP resulting in loss of elastic properties of this tissue. The goal of the present study was to gain a better understanding of the mechanisms whereby oxidative stress contributes to these changes. The results demonstrate a spontaneous, age related, accumulation of ROS within the axial skeleton that is associated with an increase in carbonylated proteins of the IVD which enhances susceptibility to proteolytic attack and impairs mechanical function.
To understand how such oxidative damage contributes to IVD degeneration we exposed purified collagen I and II to the Fenton reaction and assessed the resulting structural modifications. This treatment disrupted the triple helical structure of these proteins exposing internal amino acids to the solvent and enhancing susceptibility to both proteolytic cleavage and further oxidative damage. The mechanical and molecular compromise of the IVD associated with age was reproduced in ex vivo preparations of cervical and thoracic IVD from young mice through exposure to oxidative stress. Thus oxidative damage to structural elements of the IVD is shown to directly enhance susceptibility to degradative processes and leads to alterations in mechanical properties in ways that contribute to IVD failure.
ROS are short-lived free radicals. It is known that aging is associated with an increase in their abundance (Kregel and Zhang, 2007), as also observed in the older mice studied here. It has been proposed that at physiologic levels, ROS are essential for mediating a stress response to tissue damage (Hekimi et al., 2011). Indeed ROS are a stimulus for activation of specific stress responses that act to enable DNA repair, proteostasis, and mitochondrial metabolism as these critical processes are challenged in aging tissues (Lewis et al., 2012). However, it is also clear that higher, or sustained levels of ROS, damage a broad variety of molecules including lipids, nucleic acids, and proteins (Blumberg, 2004; Lotz and Loeser, 2012). Release of oxygen and nitrogen radicals, following mechanical cartilage damage by excessive shear stress, have been implicated previously in chondrocytes cell death by several mechanisms including apoptosis and preterm senescence (Healy et al., 2005). Chondrocyte apoptosis is induced directly by ROS and RNS-induced cytotoxicity (Blanco et al., 1998; Wu et al., 2007) as well as indirectly, by activation of the apoptosis signal-regulating kinase (ASK) 1 which induces c-Jun N-terminal kinases (JNK) and p38 Mitogen-activated protein (MAP) kinases. Both kinases amplify the ASK1 activation pathway, thus inducing cell death even when ROS concentrations are not sufficient to initiate apoptosis (Kim et al., 2008; Pan et al., 2009).
The underlying biochemical and biophysical mechanisms involved in ROS and RNS damage to cartilage proteins have never been addressed. In our analysis, we demonstrated for the first time the complex level of oxidative modifications induced on cartilage proteins by aging-related oxidative stress. The most commonly found post-translational oxidative modification was the introduction of carbonyl groups (aldehydes or ketones) to amino acid side chains. Carbonylation was most notably observed on aggrecan, fibronectin, and collagen type IV, alpha 1. This is in agreement with previous studies showing that lysine, arginine, and proline residues in aging cells are the residues mostly oxidized (Requena et al., 2001). Known effects of protein carbonylation include impairment of protein function, irreversible aggregation, increased exposure of hydrophobic residues and unfolding with heightened susceptibility to degradation, and elevated ubiquitination, targeting the protein for degradation(Cannizzo et al.; Cannizzo et al.; Dalle-Donne et al., 2003; David et al.; De la Fuente and Miquel, 2009; Dunlop et al., 2009; Haigis and Yankner; Oliveira et al.; Tyedmers et al.).
In our analysis we also found that oxidatively damaged collagen I and Collagen II lost their native primary and secondary structure. Importantly the loss of native structure and unfolding in collagen facilitated protein cleavage. Protein oxidation can cleave amino acid side chains or directly the polypeptide backbone and induce protein fragmentation(Stadtman and Berlett, 1997). ·OH attack of aspartyl, glutamyl, and prolyl side chains have previously been shown to facilitate peptide bond cleavage(Stadtman and Berlett, 1997).
The formation of protein-protein cross-linkages is another modification emanating from ROS generated oxidative stress. Cross-linkages can occur via disulfide bond formation between two oxidized cysteines or by Schiff base formation from the reaction of an amine group with a carbonyl (aldehyde or ketone) group of another protein (Oliver et al., 1987; Stadtman, 1992, 2004). Our analysis of IVD age-related modifications indicated an increase in protein aggregation.
The significance of our findings is in the demonstration that protein oxidation could be sufficient to induce early but significant biochemical modifications of cartilage structural proteins leading to early degenerative modifications and subsequent alterations of the intervertebral disc biodynamics. The demonstration of protein fragmentation in IVD of young animals undergoing oxidative stress clearly shows that oxidation of healthy cartilage promotes changes in protein folding/conformation as well as oxidative cleavage and increased susceptibility to MMPs processing. These results can explain age-related degenerative modifications in IVD in absence of inflammatory conditions. Increased levels of free radicals, commonly present in aging, could induce the oxidative post-translational modifications, which we comprehensively mapped in this study, affecting cartilage structural integrity and, in the long run its mechanical functions.
Experimental Procedure
Materials
Highly purified mouse type I and II collagens I (Cat.# 1066 and Cat.# 20061, respectively) were purchased from Chondrex, Inc. (Redmond, WA) as lyophilized powder. The collagen powders were reconstituted in acetic acid (0.05M, pH 3.5) according to the manufacturer’s instructions. The final concentration was 2.5 mg/ml for collagen I and 2.0 mg/ml for collagen II. Immediately before the Fenton reaction, an aliquot from each collagen solution was diluted with 2X phosphate buffer saline (PBS, pH 7.4 and 0.15 M NaCl) to obtain a neutral solution suitable for the oxidation assay. Hydrogen peroxide (H2O2) (Cat# 124K3644), ferrous chloride, EDTA, dithiothreitol (DTT), iodoacetamide, ammonium bicarbonate, and guanidine hydrochloride were of the highest grade available from Sigma-Aldrich (St. Louis, MO). Trifluoroacetic acid, acetonitrile, acetic acid, formic acid, and methanol (99% purity, HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA, USA). Porcine trypsin (20 ug, specific activity > 5000 units/mg seq. grade modified) was purchased from Promega (Madison, WI, USA). Collagenase class II (215U/mg) and hyaluronidase (30KU/mg) were from Worthington (Cat# 4174 and 5474, respectively). Complete TM Proteinase inhibitor cocktail was purchased from Santa Cruz Biotechnology (Santa Cruz, CA., USA).
Methods
Mice and tissue preparation
C57BL/6 mice (3, 12, and 22 months old) were obtained from Harlan, a supplier of age-controlled mouse colonies for the National Institute on Aging (NIA). Animal euthanasia and tissue harvesting were conducted according to a protocol approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine. In some experiments, mice were injected intraperitoneally once with 10mg/25g body weight of paraquat 24 hours prior to intervertebral disc collection. Spinal columns from 3, 12 and 22 month old male C57Bl/6 mice were harvested after euthanization by decapitation under isoflurane anesthesia. Spines were then transferred to 100 mm petri dishes with cold PBS and cleared of attached muscles and connective tissues. Cervical, thoracic and lumbar spinal segments containing the intervertebral discs flanked by vertebral bone were isolated using a number 10 scalpel blade and a dissecting microscope. Intervertebral discs were then carefully cleared of adjacent bone using a number 15 scalpel blade and fine tweezers.
Histological analysis of murine intervertebral lumbar discs
The spines were fixed in methacarn (methanol:chloroform:acetic acid 60:30:10 by vol) for 1 week and decalcified in 10% EDTA pH 7.4 for 1 week. All decalcified spines underwent lateral plain radiograph before processing to histological preparation for verification of completion of the procedure. They were dehydrated in graded alcohols and xylene, embedded in paraffin and serially cut into 4 μm sagittal sections. Mid-sagittal sections were stained with hematoxylin-eosin, alcian blue pH 2.5, and safranin O/fast green and three sections per spine were used for each stain to minimize occurrence of folding artifact. Sections were evaluated and histological pictures were taken at the L1/L2 level. For immunohistochemical analysis of protein carbonyls, adjacent sections permeabilized with 0.1% Triton X-100 in dPBS for 15 minutes. Sections were rinsed three times with dPBS. Sections were incubated with 1mg/ml DNPH in 2N HCL for 30 minutes (Millipore, USA), washed several times with dPBS, blocked with 1% FBS, 1% BSA in dPBS, and incubated overnight with rabbit anti-DNPH antibody (1:150; Millipore). Following three washes with dPBS, sections were incubated with ultrasmall gold-conjugated goat anti- rabbit IgG (Electron Microscopy Sciences, Hatfield, PA). Ultrasmall gold conjugates were enhanced with the Aurion R-Gent SE-LM and sections were counterstained with toluidine blue. Stained histological sections were viewed with a Zeiss Axioskop 40 microscope (Carl Zeiss MicroImaging, Thornwood, NY) and images captured digitally using a ProgRes® camera (Jena, Germany).
Western Blot Analysis
Proteins were sequentially extracted from IVD using NaCl and guanidine hydrochloride as described earlier. For the immunoblot detection of carbonyl groups introduced into proteins by oxidative reactions, proteins extracted from IVD were derivatized using the Oxyblot Protein Oxidation Detection Kit (Millipore, USA). Samples were separated on 4–15% SDS-PAGE and transferred membranes were incubated with the following antibodies: Rabbit polyclonal anti-DNP antibody (Millipore), goat polyclonal anti Collagen I, Collagen II, and β-actin (Santa Cruz Biotechnology). Secondary antibodies used were goat anti-rabbit IgG-HRP (Millipore) and bovine anti-goat IgG-HRP (Santa Cruz Biotechnology). Proteins were visualized by chemiluminescence and densitometric values were determined using Image J Software (NIH).
Mechanical properties
Mouse intervertebral discs were measured in unconfined compression using custom-made mechanical testing device (Soltz and Ateshian, 1998). Intervertebral discs were placed in testing chamber and equilibrated under creep tare load of 0.5 g for 30 minutes. Stress-relaxation tests were conducted to 10% strain at the ramp velocity of 1 μm/s. The equilibrium Young’s modulus (EY) was determined from the equilibrium stress-strain data and initial cross-section area.
Chemical modification of collagens I and II by Fenton reaction
Hydroxyl radicals were generated by the Fenton reaction conducted at neutral pH (7.4) in PBS (0.15 M NaCl)(Shahab et al.). Under these conditions a molar ratio of 2:1:1:1 (H2O2: FeCl2: EDTA: protein) was shown to be suitable to generate enough free ·OH radicals for the protein oxidation at 37°C. In the case of collagen oxidation using Fenton reaction, a solution of 0.13–0.15 mg/ml oxidized with the FeCl2/EDTA/H2O2 (1:1:2) mixture was shown to induce significant changes in the secondary and tertiary structure (Shahab et al.). Equal concentrations (0.22 mg/ml) for both collagen I and II were used to conduct the Fenton reaction at neutral pH in the presence of FeCl2/EDTA/H2O2 (1:1:2) (0.05mM FeCl2/0.05mM EDTA/0.1mM H2O2), in a final volume of 200 μL. The oxidation reaction was maintained for 0.5, 2, 3, 6 and 12 hours intervals at 37°C. The controls included each collagen solution by itself, or each in combination with FeCl2, FeCl2+EDTA, and H2O2. All the controls were incubated for the same time period at 37°C to maintain identical experimental conditions. Structural changes in the collagens (both at the level of secondary and tertiary structure) were ascertained by circular dichroism, intrinsic Trp fluorescence and SDS-PAGE.
Circular dichroism analysis of secondary structure changes in collagens I and II upon oxidation
The circular dichroic (CD) spectra of protein solutions provide information about the secondary structure of proteins. Far UV CD spectrum of native and modified CII exhibit a maxima at 221 nm and a minima at 198 nm, which are characteristic features of the collagen triple helix (Schaub et al.). The global secondary structure of native and ·OH modified collagens I and II was analyzed with a Jasco 815 CD spectropolarimeter. Ten different scans were acquired and averaged for each protein between 190 and 320 nm using the following parameters: bandwidth 4nm, interval 0.5 nm, 2mm path length cell and 10 liters/min nitrogen flow rate. The CD in millidegrees (mdeg) is plotted against the wavelength (nm) for all collagens having the same final concentration of 0.22 mg/ml.
Intrinsic Trp fluorescence analysis of tertiary structure changes of collagens I and II upon oxidation
Intrinsic Trp/Tyr fluorescence emission profiles of collagens I and II were recorded to analyze the degree of changes in the tertiary structure and unfolding upon oxidation with free ·OH radicals generated by Fenton reaction. In a hydrophobic environment (buried within the core of the protein), Tyr and Trp have a high quantum yield and therefore a high fluorescence intensity(Pfefferkorn et al.). In contrast, in a hydrophilic environment (exposed to solvent) their quantum yield decreases leading to low fluorescence intensity, as in the case of protein unfolding (Pfefferkorn et al.). Using an excitation wavelength of 295 nm (for intrinsic Trp excitation) the emission fluorescence between 320 nm and 500 nm was monitored. The spectrofluorimeter main parameters for the scanning mode included: bandwidths of 3 nm for excitation and of 4 nm for emission, and a response time of 0.3 sec. Data were recorded at 0.2 nm intervals. At least eight scans were average for each scan reading. The data are reported as RFU (relative fluorescence units) as a function of wavelength (nm) after correction for the background (which included the buffer emission in absence of collagens and in the presence of oxidizing agents, when present).
Denaturing SDS-PAGE analysis of the native and ·OH modified collagens
SDS-PAGE gel analysis was performed under denaturing conditions using pre-cast 4–20% Mini-PROTEAN TGX gels (Bio Rad, CA, USA). Equal amounts (8 μg) of protein from both the native and the ·OH -oxidized collagens I and II were boiled for 5 minutes at 95°C in loading buffer containing SDS and β-mercaptoethanol. The samples were spun at 14,000 rpm in a microcentrifuge prior to gel loading. The gels were run at constant 125 V for 50 minutes and subsequently silver stained using a silver-staining kit (Thermo Scientific, Rockford, IL USA).
Spectrophotometric determination of carbonyl content in collagen I and II following oxidation
The carbonyl content (mainly carbonylated Pro, Arg, Lys, and Thr residues) of the ·OH oxidized collagen I and II was determined spectrophotometrically with the OxiSelect Protein Carbonyl Spectrophotometric Assay kit (Cell Biolabs, CA, USA). The absorbance of the DNPH-derivatized carbonyl groups was determined at 375 nm. A 1 cm-width cuvette was used for all readings, and the final results were normalized to the total protein in each sample.
Metalloproteases assays on native (unfolded) and chemically oxidized collagens I and II
To determine the relationship between the oxidation, unfolding, and the increased sensitivity to metalloproteases degradation for the collagens exposed to Fenton reaction, an in vitro enzymatic assay was conducted in which native or ·OH oxidized collagens were incubated with recombinant metalloproteases (MMP) 1, 2, 7 and 9 for 1 hour at 37°C. 3 μg of collagen I and II were incubated for 3 hours at 37°C in the Fenton reaction. Performed in parallel, the control native collagens, not exposed to any oxidizing agent, were incubated with 20 ng of each recombinant MMP 1, 2, 9 and 13 in 25 μl of neutral buffer for metalloproteases (50 mM Tris-HCl, pH 7.4 containing 150 mM NaCl and 5 mM CaCl2) for 1 hour at 37°C. The reaction was stopped via the addition of 5 μl of 5x loading buffer to each sample. The products of the enzymatic digestion of collagen-I and II were evaluated using 7.5% SDS-PAGE on Mini-PROTEAN TGX gels (Bio Rad, CA, USA). The gels were then silver-stained (Thermo Scientific, Rockford, IL USA).
Protein extraction from IVD cartilage from 3, 12 and 22 months old mice
IVD from 3, 12, and 22 months old mice were equilibrated in 200 μL of sterile phosphate buffer saline (PBS) for 15 min at 37°C. The samples were further subjected to 0.1% hyaluronidase (3 units per sample) in PBS/ for 30 minutes at 37° to remove surface hyaluronate. Samples were spun at 14,000 rpm for 30 seconds on a benchtop centrifuge to remove the digested hyaluronate. The remaining pieces of IVD were resuspended in 200 μL sterile PBS and further subjected to collagenase treatment using 8 units of bacterial collagenase type II (Worthington Biochemical Corporation, Lakewood, NJ, USA) overnight at 37°C for each sample. Protease inhibitors were then added to each sample the total protein solution from each IVD sample was further subjected to the protein extraction with NaCl and guanidine hydrochloride.
Sequential protein extractions from IVD with NaCl and guanidine hydrochloride
We developed a protocol for protein extraction from the hyaluronidase/collagenase treated IVD using as reference the method employed by (Wilson et al.) for the proteomic analysis of the articular cartilage. The total protein solution retrieved after overnight collagenase treatment was subjected to 9 freeze/thaw cycles in liquid nitrogen/37° C water bath. The samples were further incubated in the buffer for protein extraction with NaCl (1M final NaCl in 100 mM Tris/acetate pH 8.0) during 18 hours at 4°C on the shaker. After NaCl extraction, the samples were spun at 14,000 rpm for 30 minutes, supernatant collected, and labeled E1 (extraction E1). The pellet from E1 extraction (representing proteins not soluble in 1M NaCl) was resuspended in 100 uL 4M guanidine HCL, 65 mM DTT (dithiothreitol), 10 mM EDTA in 50 mM sodium acetate, pH 5.8 for 18 hours at 4°C on the shaker to complete the protein extraction. The guanidine hydrochloride (GdnHCl) treated samples (extraction E2) were further spun at 14,000 rpm for 30 minutes in the microcentrifuge and the supernatant was collected and labeled E2 (protein extracted with GdnHCl). The same extraction procedure was repeated one more time for the pellet and the second supernatant was combined with the first E2 supernatant representing the total E2 extracted proteins with GdnHCl.
For proteomic analysis on the IVD extracted proteins, the proteins from both the E1 and E2 samples were precipitated with 5 volumes of acetone (ON, at −20°C). The pellets from acetone precipitation were collected by centrifugation at 14,000 rpm for 30 minutes in a microcentrifuge, and further washed three times with 50% acetone. The final pellets were resuspended in 100 μL solubilization buffer (7M Urea, 2M Thiourea, 4% CHAPS in 30 mM Tris, pH 8.0). Aliquots from the solubilized E1 and E2 extractions were used to determine the total protein concentration using the BCA method (Thermo Scientific, Rockford, IL USA).
IVD proteome analysis
1D SDS PAGE and in-gel trypsin digestion
The NaCl (E1) and GdnHCl (E2) extracted proteins (about 10 ug each), from 3, 12 and 22 months old mice were fractionated using 1D SDS-PAGE on 4%–20% pre-casted gels from Biorad. The gels were stained using a silver-staining kit (Thermo Scientific, Rockford, IL USA). 10 gel bands were cut across each sample lane. In gel tryptic digestion was carried out at 37°C overnight.
NanoLC-ESI-MS/MS analysis of tryptic peptides
Each gel digest was analyzed by nano LC/MS/MS with a Waters NanoAcquity HPLC system interfaced to a ThermoFisher Q Exactive. The mass spectrometer was operated in data-dependent mode, with the Orbitrap operating at 60,000 FWHM and 17,500 FWHM for MS and MS/MS respectively. The fifteen most abundant ions were selected for MS/MS. Raw data files were converted to mgf files using Proteome Discoverer 1.3 (Thermo Fisher Scientific).
Redox proteomic analysis of the IVD cartilage
All mgf files were searched against the house mouse (Mus musculus) (16,230 sequences) in SwissProt 57.15 (515,203 total sequences; 181,334,896 total residues) with Mascot, in-house, (Matrix Science, London, UK; version 2.3.02). The following parameters were used for all searches: trypsin; 1 missed cleavage; fixed modification of carbamidomethylation (for Cys); monoisotopic masses: peptide precursor mass tolerance of 10 ppm; and product ion mass tolerance of 0.8 Da. To characterize the oxidized proteome we used as variable modifications the amino acids oxidations reported in the Mascot, mainly: monooxidations on Arg, Cys (sulfenic acid), Phe, Lys, Met (Met-sulfoxide), Trp, Tyr; diooxidations on Arg, Cys (sulfinic acid), Met (Met-sulfone), Lys, Phe, Pro, Trp (N-formyl-kynurenine/dihydroxy Trp/dioxindolylalnine), Tyr; trioxidations on Cys (cysteic acid); Arg-GluSa (glutamic semialdehyde concersion of Arg); Pyrrolidinone (Pro). The proteins were considered identified having at least one bold red (BR) significant peptide with an ion score cutoff of 24 or greater (corresponding to p< 0.05 and a FDR proteins <1.0).
Validation of the posttranslational (PTM) modifications and normalizing spectral counts
Scaffold (version 3, Proteome Software, Portland, OR, USA) was used to validate MS/MS based peptide and protein identifications together with their corresponding post-translational oxidations mentioned above. All Mascot DAT files, for each IVD cartilage sample (i.e. 10 bands each from 3, 12 and 22 months) were loaded together as one “biological sample” within Scaffold. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm. Protein identifications were accepted if they could be established at greater than 96.0% probability and contained at least one identified unique peptide.
Ex vivo imaging
Spine samples were collected from CellROX® (oxidative stress reagent, excitation 640 nm / emission 664 nm, Molecular Probes, CA, USA) intravenously injected animals and imaged using the In-Vivo F PRO imaging system (Bruker BioSpin Molecular Imaging, CT, USA). Samples were imaged for 3 min at excitation 610 nm/ emission 700 nm using build-in cooled CCD camera automated with high precision 10X zoom lens in a closed optical path imaging chamber. Images were threshold with respect to background intensity and different levels of fluorescence intensity were displayed using pseudo rainbow color scheme analyzed using build-in Carestream Molecular Imaging Software. Fluorescence intensity scale ranges from pink (lowest level) to red (highest level).
Highlights.
Matrix proteins in aging intervertebral discs are carbonylated and glycated
Oxidatively modified proteins unfold and have increased susceptibility to enzymatic degradation
Oxidation of intervertebral disc matrix protein is an early event inducing tissue degenerative changes
Significance.
Our study highlight for the first time how aging-related oxidative posttranslational modifications of IVD matrix proteins affect the tissue structural integrity. The significant biochemical modification that we mapped by redox proteomic can promote protein unfolding, oxidative cleavage and increased susceptibility to MMPs processing. Altogether, leading to early degenerative modification of the IVD and subsequent alterations of the disc biodynamics.
Acknowledgments
This work was supported by the NIH grant (HL076485) and the generous donations from Arnold Penner and the Jabez and Helen Hardin Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998;250:418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009;276:5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. The Journal of clinical investigation. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Whiteman M, Mattey DL, Halliwell B. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis. 2001;60:627–631. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann Rheum Dis. 70:1354–1356. doi: 10.1136/ard.2010.146399. [DOI] [PubMed] [Google Scholar]
- Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blumberg J. Use of biomarkers of oxidative stress in research studies. The Journal of nutrition. 2004;134:3188S–3189S. doi: 10.1093/jn/134.11.3188S. [DOI] [PubMed] [Google Scholar]
- Broadley SA, Hartl FU. Mitochondrial stress signaling: a pathway unfolds. Trends in cell biology. 2008;18:1–4. doi: 10.1016/j.tcb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Cannizzo ES, Clement CC, Morozova K, Valdor R, Kaushik S, Almeida LN, Follo C, Sahu R, Cuervo AM, Macian F, et al. Age-related oxidative stress compromises endosomal proteostasis. Cell Rep. 2:136–149. doi: 10.1016/j.celrep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 74:2313–2323. doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente M, Hernanz A, Guayerbas N, Alvarez P, Alvarado C. Changes with age in peritoneal macrophage functions. Implication of leukocytes in the oxidative stress of senescence. Cellular and molecular biology. 50 Online Pub. 2004:OL683–690. [PubMed] [Google Scholar]
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15:3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- Dufour E, Boulay J, Rincheval V, Sainsard-Chanet A. A causal link between respiration and senescence in Podospora anserina. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4138–4143. doi: 10.1073/pnas.070501997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop RA, Brunk UT, Rodgers KJ. Oxidized proteins: mechanisms of removal and consequences of accumulation. IUBMB Life. 2009;61:522–527. doi: 10.1002/iub.189. [DOI] [PubMed] [Google Scholar]
- Fay J, Varoga D, Wruck CJ, Kurz B, Goldring MB, Pufe T. Reactive oxygen species induce expression of vascular endothelial growth factor in chondrocytes and human articular cartilage explants. Arthritis research & therapy. 2006;8:R189. doi: 10.1186/ar2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Yankner BA. The aging stress response. Mol Cell. 40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Healy ZR, Lee NH, Gao X, Goldring MB, Talalay P, Kensler TW, Konstantopoulos K. Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci U S A. 2005;102:14010–14015. doi: 10.1073/pnas.0506620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends in cell biology. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- Homma Y, Tsunoda M, Kasai H. Evidence for the accumulation of oxidative stress during cellular ageing of human diploid fibroblasts. Biochem Biophys Res Commun. 1994;203:1063–1068. doi: 10.1006/bbrc.1994.2290. [DOI] [PubMed] [Google Scholar]
- Indrakanti SS, Weber MH, Takemoto SK, Hu SS, Polly D, Berven SH. Value-based care in the management of spinal disorders: a systematic review of cost-utility analysis. Clinical orthopaedics and related research. 2012;470:1106–1123. doi: 10.1007/s11999-011-2141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine. 1995;20:674–684. doi: 10.1097/00007632-199503150-00008. [DOI] [PubMed] [Google Scholar]
- Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. The spine journal : official journal of the North American Spine Society. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kim MO, Heo JS, Kim JS, Han HJ. Acetylcholine inhibits long-term hypoxia-induced apoptosis by suppressing the oxidative stress-mediated MAPKs activation as well as regulation of Bcl-2, c-IAPs, and caspase-3 in mouse embryonic stem cells. Apoptosis. 2008;13:295–304. doi: 10.1007/s10495-007-0160-y. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Lewis KN, Andziak B, Yang T, Buffenstein R. The Naked Mole-Rat Response to Oxidative Stress: Just Deal with It. Antioxidants & redox signaling. 2012 doi: 10.1089/ars.2012.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine. 2000;25:166–169. doi: 10.1097/00007632-200001150-00005. [DOI] [PubMed] [Google Scholar]
- McAlindon TE, Jacques P, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Levy D, Felson DT. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39:648–656. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira BF, Nogueira-Machado JA, Chaves MM. The role of oxidative stress in the aging process. ScientificWorldJournal. 10:1121–1128. doi: 10.1100/tsw.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J Gastroenterol. 2009;15:1702–1707. doi: 10.3748/wjg.15.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, Currie MG, Martel-Pelletier J. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000;43:1290–1299. doi: 10.1002/1529-0131(200006)43:6<1290::AID-ANR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn CM, McGlinchey RP, Lee JC. Effects of pH on aggregation kinetics of the repeat domain of a functional amyloid, Pmel17. Proc Natl Acad Sci U S A. 107:21447–21452. doi: 10.1073/pnas.1006424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharna HK, Monnier V, Boja B, Moskowitz RW. Lysyl oxidase and Maillard reaction-mediated crosslinks in aging and osteoarthritic rabbit cartilage. J Orthop Res. 1995;13:13–21. doi: 10.1002/jor.1100130105. [DOI] [PubMed] [Google Scholar]
- Pokharna HK, Phillips FM. Collagen crosslinks in human lumbar intervertebral disc aging. Spine (Phila Pa 1976) 1998;23:1645–1648. doi: 10.1097/00007632-199808010-00005. [DOI] [PubMed] [Google Scholar]
- Preynat-Seauve O, Coudurier S, Favier A, Marche PN, Villiers C. Oxidative stress impairs intracellular events involved in antigen processing and presentation to T cells. Cell stress & chaperones. 2003;8:162–171. doi: 10.1379/1466-1268(2003)008<0162:osiiei>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena JR, Chao CC, Levine RL, Stadtman ER. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci U S A. 2001;98:69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life sciences. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- Schaub LJ, Campbell JC, Whitten ST. Thermal unfolding of the N-terminal region of p53 monitored by circular dichroism spectroscopy. Protein Sci. 21:1682–1688. doi: 10.1002/pro.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schollmeier G, Lahr-Eigen R, Lewandrowski KU. Observations on fiber-forming collagens in the anulus fibrosus. Spine. 2000;25:2736–2741. doi: 10.1097/00007632-200011010-00004. [DOI] [PubMed] [Google Scholar]
- Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264:21597–21602. [PubMed] [Google Scholar]
- Shahab U, Ahmad S, Moinuddin, Dixit K, Habib S, Alam K, Ali A. Hydroxyl radical modification of collagen type II increases its arthritogenicity and immunogenicity. PLoS One. 7:e31199. doi: 10.1371/journal.pone.0031199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Role of oxidant species in aging. Curr Med Chem. 2004;11:1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kushida K, Ohishi T, Kawana K, Hoshino H, Uchiyama A, Inoue T. Quantitative analysis of crosslinks pyridinoline and pentosidine in articular cartilage of patients with bone and joint disorders. Arthritis Rheum. 1994;37:724–728. doi: 10.1002/art.1780370517. [DOI] [PubMed] [Google Scholar]
- Terman A. Catabolic insufficiency and aging. Annals of the New York Academy of Sciences. 2006;1067:27–36. doi: 10.1196/annals.1354.005. [DOI] [PubMed] [Google Scholar]
- Tiku ML, Gupta S, Deshmukh DR. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic Res. 1999;30:395–405. doi: 10.1080/10715769900300431. [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Urban MR, Fairbank JC, Bibby SR, Urban JP. Intervertebral disc composition in neuromuscular scoliosis: changes in cell density and glycosaminoglycan concentration at the curve apex. Spine. 2001;26:610–617. doi: 10.1097/00007632-200103150-00010. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 40:323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Diseberg AF, Gordon L, Zivkovic S, Tatarczuch L, Mackie EJ, Gorman JJ, Bateman JF. Comprehensive profiling of cartilage extracellular matrix formation and maturation using sequential extraction and label-free quantitative proteomics. Mol Cell Proteomics. 9:1296–1313. doi: 10.1074/mcp.M000014-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Annals of the rheumatic diseases. 2011;70:844–850. doi: 10.1136/ard.2010.132720. [DOI] [PubMed] [Google Scholar]
- Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem. 2007;101:1520–1531. doi: 10.1002/jcb.21268. [DOI] [PubMed] [Google Scholar]


