Abstract
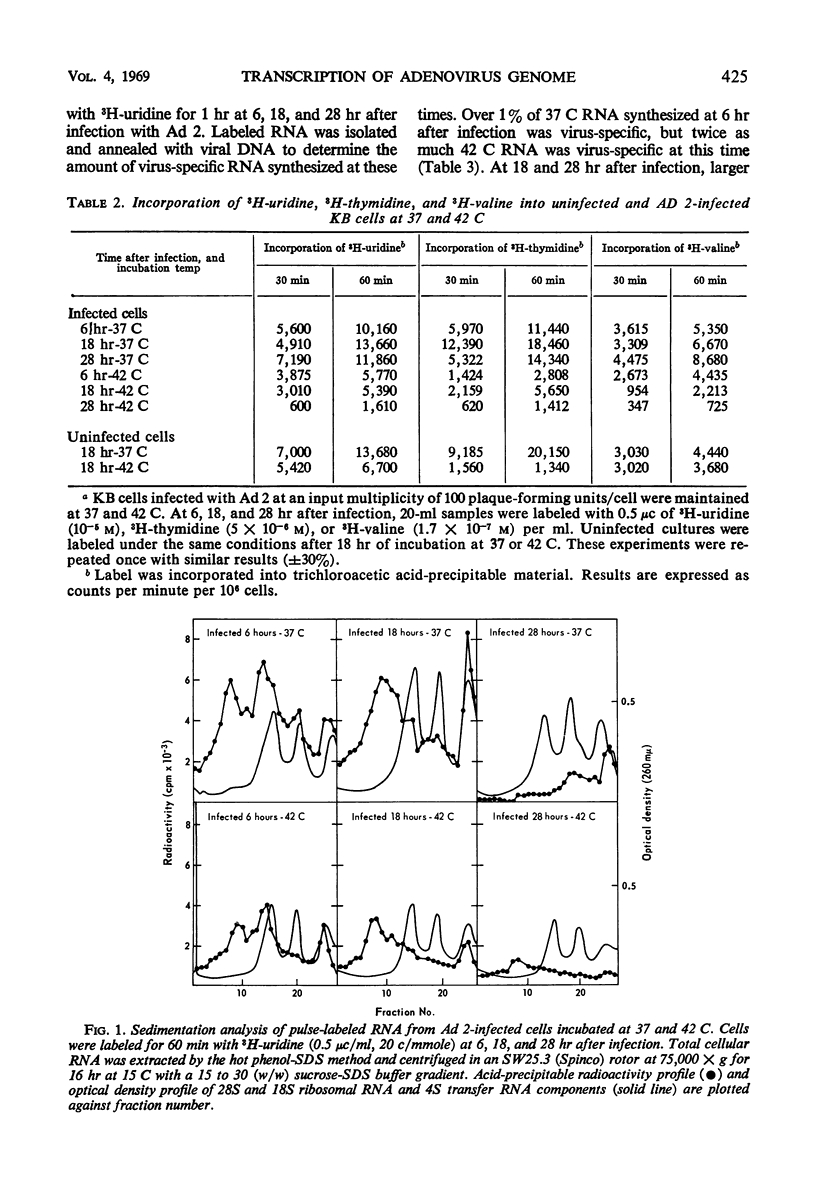
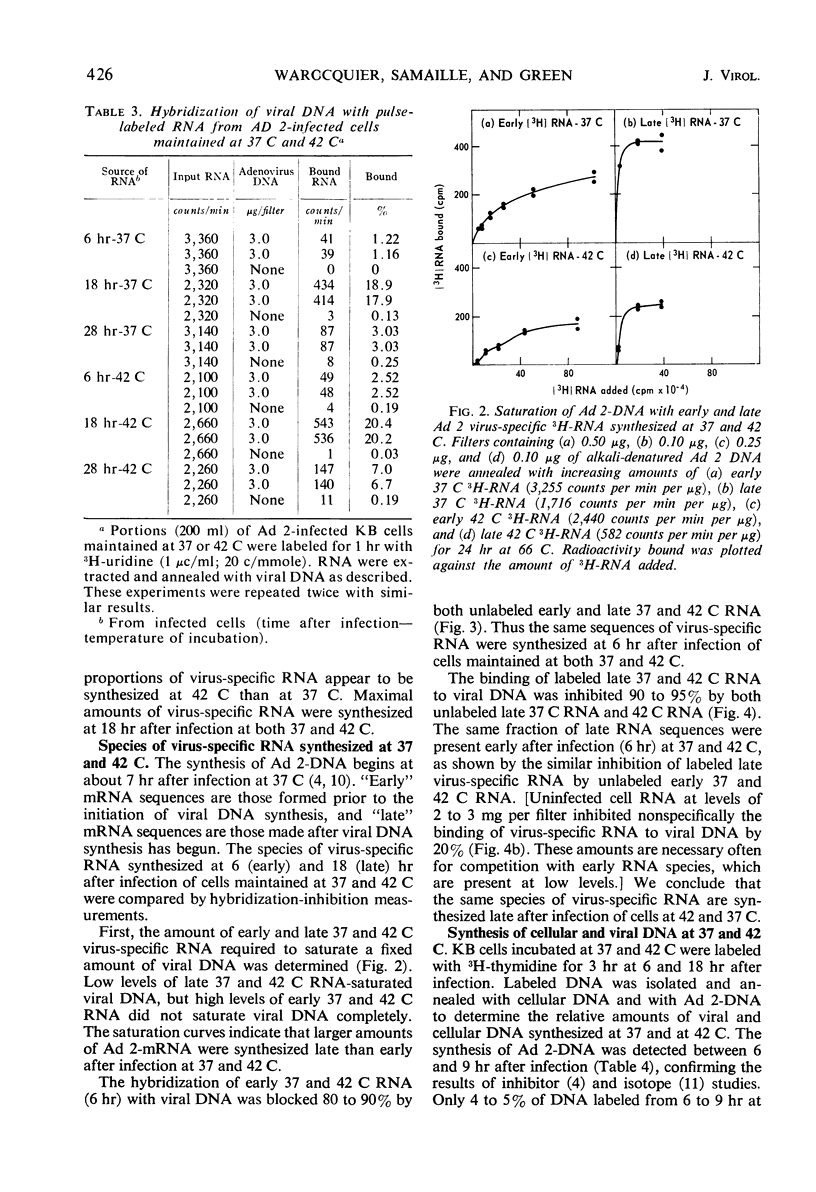
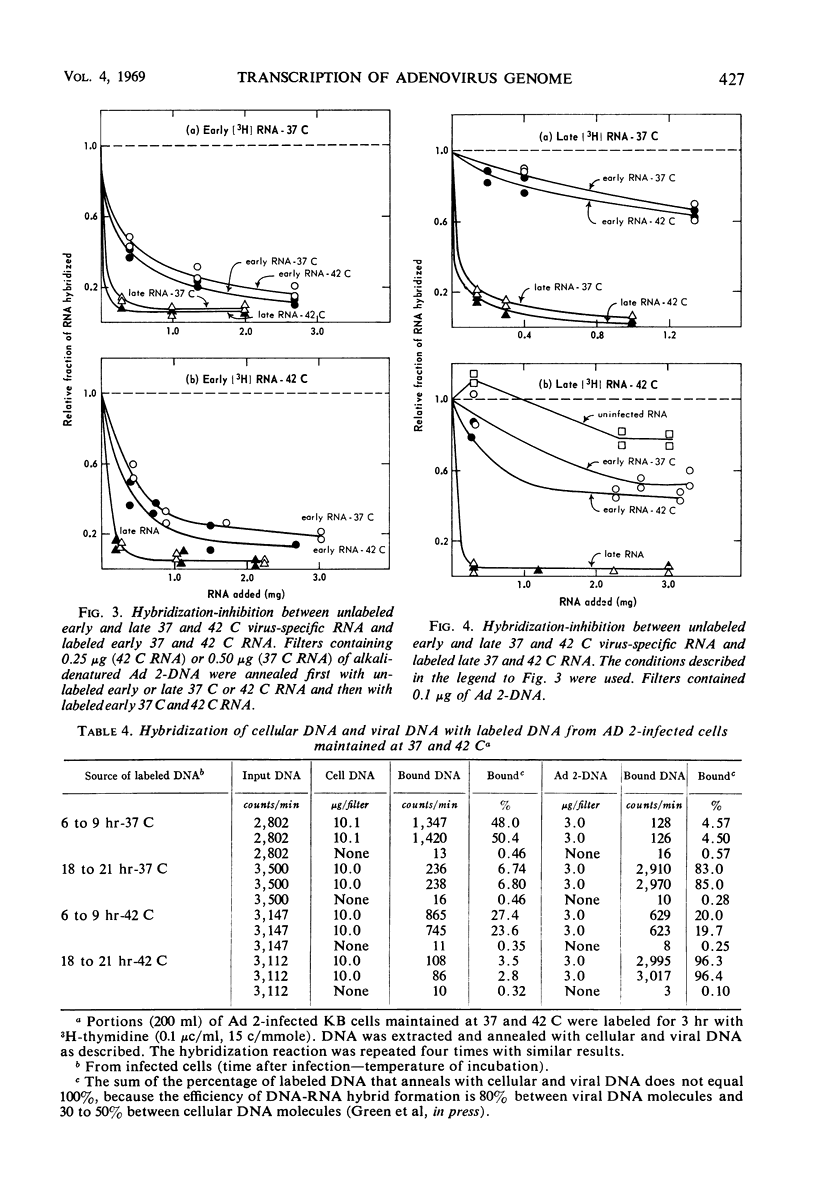
The synthesis of infectious virus is reduced by 99% in adenovirus type 2-infected KB cells maintained at 42 C in suspension. Studies to delineate the steps in virus biosynthesis blocked at 42 C revealed the following. (i) The inhibition of ribosomal ribonucleic acid (RNA) synthesis and the block in the conversion of ribosomal RNA precursors to ribosomal RNA which normally occur in infected cells at 37 C was accentuated at 42 C. (ii) The same species of early and late viral messenger RNA were synthesized at 37 and 42 C. (iii) The shift from host cell deoxyribonucleic acid (DNA) synthesis to viral DNA replication occurred earlier at 42 C than at 37 C. These findings indicate that the thermosensitive block(s) in virion formation does not occur at the level of viral DNA replication or transcription of viral mRNA but probably involves the synthesis of late viral proteins or the maturation of the virion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. V. Properties of purified viral-specific RNA from human adenovirus-induced tumor cells. J Mol Biol. 1968 Jan 14;31(1):63–73. doi: 10.1016/0022-2836(68)90054-5. [DOI] [PubMed] [Google Scholar]
- GIRARD M., PENMAN S., DARNELL J. E. THE EFFECT OF ACTINOMYCIN ON RIBOSOME FORMATION IN HELA CELLS. Proc Natl Acad Sci U S A. 1964 Feb;51:205–211. doi: 10.1073/pnas.51.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. Biochemical studies on adenovirus multiplication. III. Requirement for DNA synthesis. Virology. 1962 Dec;18:601–613. doi: 10.1016/0042-6822(62)90063-6. [DOI] [PubMed] [Google Scholar]
- GREEN M., DAESCH G. E. Biochemical studies on adenovirus multiplication. II. Kinetics of nucleic acid and protein synthesis in suspension cultures. Virology. 1961 Feb;13:169–176. doi: 10.1016/0042-6822(61)90051-4. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M., Rapp F. Replication and complementation of human adenoviruses and simian papovavirus at an elevated temperature. J Virol. 1968 Jul;2(7):670–677. doi: 10.1128/jvi.2.7.670-677.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A. The thermosensitive critical event of the viral cycle. Cold Spring Harb Symp Quant Biol. 1962;27:159–174. doi: 10.1101/sqb.1962.027.001.018. [DOI] [PubMed] [Google Scholar]
- POLASA H., GREEN M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. 8. ANALYSIS OF PROTEIN SYNTHESIS. Virology. 1965 Jan;25:68–79. doi: 10.1016/0042-6822(65)90253-9. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Samaille J., Warocquier R. Influence des températures supra-optimales sur le cycle de multiplication de l'adénovirus 5. Ann Inst Pasteur (Paris) 1966 Feb;110(2):244–251. [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]
- Warocquier R., Samaille J. Action de l'actinomycine D, de la puromycine et de la p-fluorophénylalanine sur la phase thermosensible du cycle de multiplication de l'adénovirus 5. C R Acad Sci Hebd Seances Acad Sci D. 1968 Jun 24;266(26):2513–2515. [PubMed] [Google Scholar]


