Abstract
When prolonged intense exercise is performed at high ambient temperatures, cardiac output must meet dual demands for increased blood flow to contracting muscle and to the skin. The literature has commonly painted this scenario as a fierce competition, wherein one circulation preserves perfusion at the expense of the other, with the regulated maintenance of blood pressure as the ultimate goal. This review redefines this scenario as commensalism, an integrated balance of regulatory control where one circulation benefits with little functional effect on the other. In young, healthy subjects, arterial pressure rarely falls to any great extent during either extreme passive heating or prolonged dynamic exercise in the heat. Nor does body temperature rise disproportionately due to a compromised skin blood flow. Rather, it often takes the superimposition of additional stressors – e.g., dehydration or simulated hemorrhage – upon heat stress to substantially impact blood pressure regulation.
Keywords: skin blood flow, exercise, heat stress, blood pressure, cardiac output
INTRODUCTION
Biologists define competition as “active demand by two or more organisms...for some ... resource in short supply” (Merriam-Webster on line). By definition, competition results in a winner and a loser. On the other hand, nature – and physiology -- is replete with examples of far more symbiotic interactions, including commensalism, defined by biologists as a mutualistic relation between two entities in which one benefits without substantially affecting the other.
In the 19th century, Dastre and Morat (1884) stated that when blood flow to one compliant circulation increases, blood flow to another compliant vascular bed must decrease to maintain ventricular filling pressure. In his book, Human Cardiovascular Control (Rowell 1993), Rowell states that “...prolonged high levels of exercise, with increased ambient temperature...forces humans to deal with the two most powerful competing regulatory demands they ever face: the competition between skin and muscle for large fractions of cardiac output” (p. 228). From observations such as these, the concept arose that during upright exercise in the heat, an intense competition occurs between skin and muscle for the available blood flow. That is, during prolonged exercise in hot ambient environments, one circulation must increase or be maintained at the clear expense of the other.
If cardiac output cannot meet the competing demands of cutaneous and other circulations, what are the functional consequences? During prolonged dynamic exercise in hot environments, if active muscle is the loser, exercise at a given intensity could not be sustained as metabolic demands outweigh supply. If skin is the loser, ineffective body cooling could lead to an increased rate of rise in core temperature and early termination of exercise. But is it as simple as a true competition between these vascular beds, with a winner and a loser?
Even without the added stress of dynamic exercise, extreme supine heating of resting humans can increase skin blood flow to 7-8 L/min, comprising 50% of the available cardiac output. Under such resting conditions, a similar competition may be envisioned among multiple vascular beds, including cerebral, coronary, splanchnic, and renal circulations. Does this scenario fit the definition of a “competition,” in which winners and losers are evident? What happens to blood pressure under these extreme conditions?
The scope of this paper is to discern and describe what happens when the integrated cardiovascular system must serve two masters, temperature regulation and blood pressure regulation, with the ultimate goal of maintaining filling pressure and ultimately, arterial blood pressure. The common denominator of both situations described above is a large increase in blood flow to the highly compliant cutaneous circulation. A complex and highly integrated series of central and peripheral cardiovascular adjustments are made to minimize changes in mean arterial pressure. Whether or not temperature regulation is compromised depends on the metabolic heat production and the environmental conditions; however, the notion that severe thermoregulatory consequences limit exercise performance is a clear overstatement under most conditions that have been investigated. There are certainly exercise-induced attenuations in skin blood flow, i.e., exercise skin blood flow is lower than resting flow at a given core temperature. But an argument can be made that the skin is actually over-perfused during passive heating and exercise reflexes that diminish cutaneous flow have little functional significance except in the most extreme circumstances. Therefore, in biological terms, the interrelation between the skin and active muscle for available cardiac output is commensalistic, rather than competitive. One thrives with little real impact on the other, and arterial pressure is rarely compromised.
Obviously, if enough strain is exerted on any physiological system, full homeostasis will ultimately be lost. In that vein, this review also examines the consequences of additive stressors superimposed upon hyperthermic situations. For example, when both skin and muscle blood flow have been elevated for prolonged periods of time without fluid relacement, dehydration may occur. Loss of plasma volume in dehydration further stresses the system because it magnifies the decreased filling pressure and increases the magnitude (and perhaps the number) of adjustments that must be made if arterial pressure is to be maintained. Similarly, real or simulated hemorrhage during passive heat stress has the same effect. And finally, although most of the data in the literature has been collected on young, healthy men and women, ageing may alter the ability of the system to make such adjustments. Does the nature of the “competition” change for older individuals who typically have a compromised skin vasodilatory response?
PASSIVE HEATING
First, let us consider the situation of intense passive heating in resting humans. There have been several recent reviews on this topic (Crandall and Gonzalez-Alonso 2010; Wilson and Crandall 2011; Crandall 2008; Johnson 2010) that have elegantly integrated the classic and contemporary studies in this area; therefore the central and peripheral adjustments to extreme passive heat stress will be briefly reviewed to construct our argument for commensalism among the various affected regional circulations. While the primary focus of this review is exercise and heat stress, investigations into the central and peripheral cardiovascular adjustments during passive heat stress provide a basis for examining hyperthermic influences on regional circulations in the absence of increased metabolism and its resultant requirement for active muscle blood flow. Determining if arterial pressure is the primary regulated variable under these passive conditions helps frame the context for whether blood flow to the skin or other regional circulations is truly compromised under extreme conditions, and if so, what happens to arterial pressure?
Studies investigating cardiovascular control during passive heat stress have relied upon various methods of increasing, then clamping, skin temperature at ~38-39°C, including water-perfused suits, lower limb warm water immersion, and exposure to high environmental heat in an environmental chamber. Under such conditions, the core-to-skin temperature gradient is reversed and as warmed blood flow returns from the cutaneous circulation core temperature increases. During pronounced supine passive heat stress skin blood flow may increase from ~300 ml/min to upwards of 7-8 L/min (Minson et al. 1998) (Rowell 1986; Rowell et al. 1968; Wyss et al. 1974). In order to support this large increase in blood flow and volume directed toward the cutaneous circulation both central (increased cardiac output) and peripheral (redistribution of blood flow from other circulations) adjustments occur, minimizing changes in mean arterial pressure.
Central Mechanisms
With a large flow and volume of blood directed to the compliant cutaneous circulation during passive whole body heating, resting cardiac output essentially doubles (Rowell 1974; Rowell 1986, 1993; Rowell 1984). This increase occurs primarily through an increase in heart rate, with a slight increase in stroke volume despite significant reductions in central venous pressure (CVP). Many studies have demonstrated that CVP can approach 0 mmHg depending on the severity of the heat stress (Minson et al. 1998). Paralleling the decreased CVP, left ventricular filling pressure (indexed by pulmonary capillary wedge pressure) is also reduced (Wilson et al. 2009; Wilson et al. 2007). Yet despite these profound reductions in cardiac filling pressure, cardiac output increases, stroke volume is well maintained or even rises a little, and mean arterial pressure is well maintained (Fig. 1).
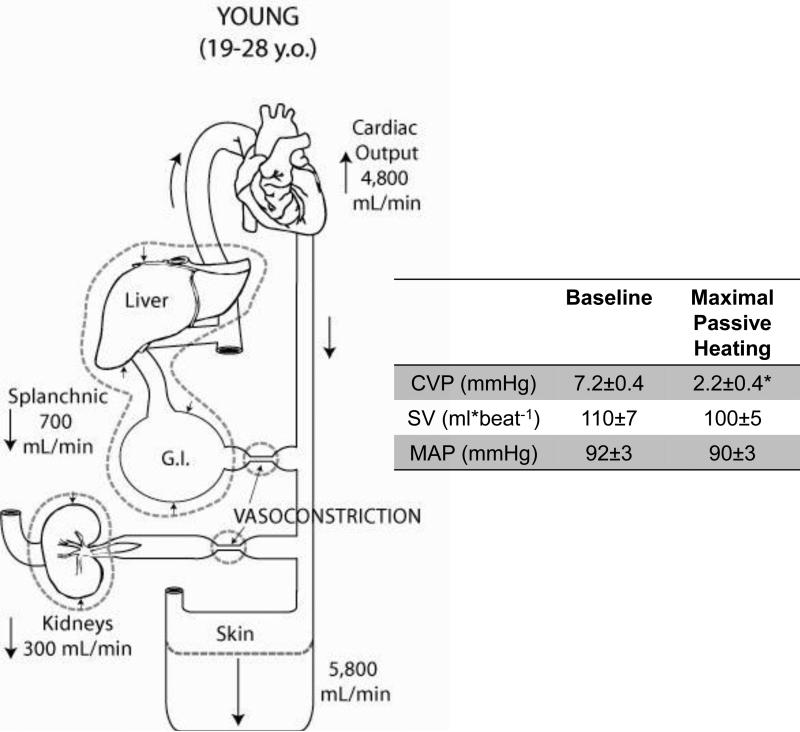
Figure 1. The increase in cardiac output and the redistribution of blood volume in young humans during passive heat stress to the limits of thermal tolerance.
Cardiac output increases and blood is redistributed from the renal and splanchnic vascular beds to the cutaneous vascular bed. The table inlet shows a significant reduction in central venous pressure (CVP) while stroke volume (SV) and mean arterial pressure (MAP) are maintained during passive supine heating to the limit of thermal tolerance. Data are redrawn from Minson et al. J Appl Physiol 1998.
Over a series of studies, Crandall et al. showed that although passive whole body heat stress was accompanied by an increase in ejection fraction (Crandall et al. 2008), it had no effect on echocardiographic indices of diastolic function (Brothers et al. 2009). However, indices of systolic function (peak septal and lateral mitral annular systolic velocities and isovolumetric acceleration) were increased. Even with a reduction in left ventricular filling pressure stroke volume is well maintained, suggesting that heat stress induces a leftward shift in the operating point on the Frank-Starling curve (Wilson et al. 2009). Along this steeper portion of the curve, small changes in filling pressure result in relatively large changes in stroke volume (Wilson et al. 2009). The functional relevance of this operating point shift is highlighted when the additive stresses of upright posture or simulated hemorrhage are superimposed and stroke volume falls. In the absence of such additional influences, however, mean arterial pressure is not compromised despite substantial cutaneous vasodilatation.
Baroreflex Control During Heat Stress
An area that has been extensively explored in the regulation of blood pressure during heat stress has been the impact of heat stress on baroreflex control of heart rate and sympathetic nerve activity. Numerous studies have consistently demonstrated that baroreflex control of heart rate, and the sensitivity of the heart rate-blood pressure response is not affected by whole body heat stress (Crandall 2000; Yamazaki and Sone 2000 ,Cui, 2002 #242; Wilson et al. 2001; Cui et al. 2002). Baroreflex control of muscle sympathetic nerve activity (MSNA) may be increased (Cui et al. 2002; Keller et al. 2006). Additionally, peripheral skeletal muscle adrenergic sensitivity to the increase in MSNA (Keller et al. 2010) is relatively unaffected by increased temperature, suggesting that the skeletal muscle vasculature retains its ability to vasoconstrict, thus aiding in the maintenance of mean arterial pressure.
Peripheral Circulatory Adjustments
The integrated peripheral circulatory adjustments and hemodynamic responses to severe whole body heat stress were elegantly measured in the classic studies by Rowell and colleagues (Rowell 1974; Rowell et al. 1965; Rowell et al. 1968). High skin and rising core temperatures induced a redistribution of blood flow from the renal and splanchnic vascular beds to the cutaneous circulation. In the supine posture, this redistribution of blood flow coupled with an increase in cardiac output results in little to no reduction in arterial pressure (Fig. 1). Figure 1 shows data from a later series of studies conducted by Minson et al. (Minson et al. 1998) that illustrates the redistribution of blood flow with prolonged supine whole body heat stress, and the accompanying central cardiovascular changes all measured concurrently in the same subject.
More recently, Crandall et al. (2008) utilized advanced imaging techniques including technitium-99m labeling of red blood cells and gamma camera imaging to reinforce and extend findings from the classic studies of Rowell et al. (Minson et al. 1998). While Rowell and Minson et al. calculated blood flow using dye dilution techniques to partition out the regions circulations, Crandall et al. showed that during pronounced passive heat stress thoracic blood volume, heart blood volume, and the blood volume in the central vascular structures all decrease (Crandall et al. 2008). This was accompanied by a decrease in liver and splenic blood volume and a modest reduction in mean arterial pressure 87±2 to 81±2 mmHg. Taken together, these studies demonstrate that when humans are passively heated to their tolerance limits, blood flow is elegantly redistributed and mean arterial pressure is fairly well maintained in the face of falling central venous pressure.
Because of the propensity for orthostatic hypotension to occur in hyperthermic circumstances, there has been considerable interest in the impact of heat stress on cerebral blood flow. In the supine posture, cerebral perfusion and cerebral vascular conductance decrease by as much as 50% during supine passive heat stress (Wilson et al. 2002; Low et al. 2008). Carbon dioxide is a potent vasodilator stimulus in the cerebral circulation, yet the magnitude of the reduction in cerebral perfusion with heat stress cannot be fully explained by reductions in CO2 accompanying temperature-induced hyperventilation (Nelson et al. 2011). One mechanism for the reduction in cerebral perfusion that has been confirmed in animal models is an increase in cerebral sympathetic nerve activity. Increases in muscle and skin sympathetic nerve activity are clearly evident in humans (Low et al. 2011; Hagbarth et al. 1972; Normell and Wallin 1974); thus it is plausible that a cerebral sympathetic nerve activity-mediated reduction in cerebral perfusion similarly occurs. Considering that muscle adrenergic responsiveness is not altered with increasing heat-stressed (Keller et al. 2010) this sets up a scenario where cerebral vascular resistance decreases while skin vascular conductance increases, again without significantly altering arterial pressure.
In summary, during passive supine heat stress central and peripheral circulatory adjustments are able to compensate for the increased skin blood flow and capacitance to the extent that mean arterial pressure is well maintained. It is only with the addition of upright tilting, simulated hemorrhage, and/or a pathology-induced inability to adjust to the cardiovascular demands of heat-stress that mean arterial pressure falls significantly. Of course, this occurs in the absence of the “competing” need to pump a substantial fraction of the cardiac output to active skeletal muscle as discussed below.
Upright Posture/Simulated Hemorrhage/Lower Body Negative Pressure
Compared to normothermia, hyperthermic subjects develop earlier syncopal symptoms and onset of hypotension during upright tilting and/or application of lower body negative pressure (LBNP, i.e., simulated hemorrhage) (Johnson et al. 1973). When upright tilting or LBNP are superimposed on hyperthermia the fall in central venous pressure is exacerbated, stroke volume declines, and the magnitude of the increase in total peripheral resistance is blunted compared to thermoneutral conditions. These changes contribute to a fall in mean arterial pressure and ensuing orthostatic intolerance.
In cool or thermoneutral conditions the splanchnic and cutaneous circulations serve as a reservoirs during orthostatic challenges -- vascular resistance increases through sympathetic adrenergic mechanisms liberating blood volume to help maintain cardiac filling pressure. During hyperthermia, despite a heat-stress induced increase in resistance, the splanchnic circulation maintains the ability to further constrict to help maintain CVP (Minson et al. 1999) and arterial pressure. In the cutaneous circulation upright tilting or LBNP during hyperthermia results in a withdrawal of active vasodilator activity (as opposed to increased sympathetic adrenergic activity) (Crandall et al. 1996); however a significant volume of blood remains in this compliant circulation, contributing to the fall in CVP, SV, and ultimately MAP. Interestingly, recent work from Pearson et al. has identified a role for elevated in skin temperature in modulating vasoconstrictor responsiveness in human skin (Pearson et al. 2012). During a hemorrhagic challenge (LBNP) cutaneous vasoconstriction is severely attenuated when skin temperature exceeds ~38°C. A possible mechanism for the lack of a reduction in cutaneous vascular conductance under such conditions may be the influence of nitric oxide (NO), which is required for full expression of reflex cutaneous vasodilatation (Kellogg et al. 1998; Shastry et al. 1998) and also attenuates vasoconstrictor responsiveness (Shibasaki et al. 2008; Wingo et al. 2009). Unlike supine passive heating alone, when skin temperature exceeds ~38°C and further reductions in central blood volume are elicited by gravitational challenges, mean arterial pressure is challenged.
An additional mechanism contributing to impaired tolerance to upright tilting or LBNP during hyperthermia is a reduction in plasma volume and total body water through sweating. Tolerance to simulated hemorrhage (LBNP) is preserved in heat stressed subjects following either rapid plasma volume expansion (Keller et al. 2009) or slow fluid administration of intravascular volume sufficient to offset fluid losses due to sweating (Lucas et al. 2012). With these ameliorating influences, cardiac output and stroke volume are bolstered by ~20%, which contributes to a greater cardiac output reserve during LBNP and the maintenance of mean arterial pressure.
Finally, cerebral perfusion and vascular conductance further decline with the addition of orthostatic stress to passive heat stress. Syncope will eventually and inevitably occur when cerebral perfusion becomes inadequate in the face of falling MAP. Recently, Lee et al. demonstrated that large reductions in cerebral perfusion during the early stages of LBNP can occur despite maintained MAP in individuals with decreased orthostatic tolerance (Lee et al. 2012). While inter-individual differences in the regulation of cerebral perfusion during heat stress exist, one potential countermeasure for maintaining MAP and thus cerebral perfusion is rapid skin cooling (Wilson et al. 2002).
In summary, even during extreme supine heating of resting humans, arterial blood pressure is well maintained despite a tremendous increase in skin blood flow. Only when further challenges are superimposed on the system does mean arterial pressure fall.
Aging
Thus far, the central and peripheral physiological adjustments during passive heat stress have focused on measurements obtained in young healthy subjects. With primary human aging in the absence of overt pathologies there are significant alterations in the regional control of blood flow during passive whole body heat stress (Minson et al. 1998). While mechanisms associated with the peripheral thermoregulatory responses with aging have been recently reviewed (Holowatz and Kenney 2010; Holowatz et al. 2010; Holowatz et al. 2007), the physiological responses of the healthy elderly population similarly demonstrate the elegant regulatory control of central and peripheral mechanisms during profound passive heat stress.
In a series of studies in the late 1990s, we demonstrated that when passively heated, subjects with a mean age of 70 years (1) exhibited substantially lower skin blood flows, and (2) redistributed less blood flow from the renal and splanchnic vascular beds to the cutaneous circulation (Minson et al. 1998). Despite similar decreases in central venous pressure between the age groups, there was a much smaller increase in cardiac output in the older subjects. This reduced rise in cardiac output was accompanied by a significant reduction in stroke volume (absolute heart rate was similar between groups). Despite a ~4 L/min (75%) lower cardiac output, total peripheral resistance was higher in the older subjects and mean arterial pressure was well maintained (Table 1). Considering the age difference in skin blood flow, one might expect greater increases in core temperature during passive heat stress in the older subjects. Yet core and skin temperatures were similar between young and older subjects. Thus, at least during passive heating it does not appear that mean arterial pressure is maintained at the expense of thermoregulatory control or function.
Table 1.
Thermoregulatory, central cardiovascular and peripheral blood flow adjustments, and mean arterial pressure in young and older subjects at thermoneutral baseline and after whole body heating (water-perfused) suit to the limit of their thermal tolerance.
| Young | Older | |||
|---|---|---|---|---|
| Baseline | Heating | Baseline | Heating | |
| Tes (°C) | 36.7±0.2 | 38.4±0.2 | 36.5±0.2 | 38.3±0.2 |
| Tsk (°C) | 34.2±0.4 | 39.7±0.2 | 34.2±0.3 | 40.0±0.3 |
| Qc (L*min-1) | 6.5±0.4 | 11.2±1.0 | 5.6±0.3 | 7.3±0.6* |
| HR (beats*min-1) | 60±5 | 120±10 | 64±4 | 110±12 |
| SV (ml*min-1) | 110±7 | 100±5 | 92±8 | 70±8* |
| FBF (ml*100ml-1*min-1) | 5.0±1.2 | 30.0±2.5 | 5.0±1.0 | 18.0±2.0* |
| SBF (ml*min-1) | 1450±50 | 725±25 | 1130±70 | 675±20 |
| RBF(ml*min-1) | 1145±110 | 855±50 | 875±80* | 700±25* |
| MAP (mmHg) | 95±5 | 93±7 | 101±3 | 101±5 |
| TPR (mmHg*min*L-1) | 13.5±0.5 | 8.6±0.5 | 16.8±0.8* | 13.2±1.0* |
Tes, esophageal temperature; Tsk, mean skin temperature; Qc, cardiac output; HR, heart rate; SV, stroke volume; FBF, forearm blood flow; SBF, splanchnic blood flow; RBF, renal blood flow; MAP, mean arterial pressure; TPR, total peripheral resistance. Adapted from Minson et al. J Appl Physiol 1998.
From a clinical standpoint it is clear that the biggest risk to the aged during extreme prolonged heat stress is cardiovascular in nature due to excess cardiovascular disease related deaths during heat waves (Basu and Samet 2002) result. Despite the ability of healthy fit older subjects to tolerate prolonged supine heating as a result of integrated cardiovascular adjustments, older individuals have a greater relative strain on a potentially compromised left ventricle and rely on a greater percentage of their heart rate reserve to increase cardiac output to increase skin flow. With increasing cardiovascular co-morbidities and medications that may prevent the necessary central and peripheral adjustments to passive heat stress comes an increased risk of concomitant failure of both cardiovascular and thermoregulatory control systems. However, investigation of the alterations in cardiovascular control and blood flow distribution during passive heat stress in healthy aged populations suggests that despite age-related changes in thermoregulatory cardiovascular adjustments, aged subjects do not experience greater core or skin temperatures when compared to their young counterparts. These findings lend further support to a commensalistic relationship among the regional circulations impacted by, and adjusting to, severe passive heat stress.
EXERCISE AND HEAT STRESS
The Nature of the “Competition”
Dynamic exercise presents further challenges to the regulatory processes that strive to maintain blood pressure and temperature homeostasis. With exercise, there is a resetting of the baroreflex response curve such that a higher mean arterial pressure is maintained throughout exercise. Despite this resetting to a higher mean arterial pressure during exercise, blood pressure regulation is challenged as peripheral vasodilatation occurs in active skeletal muscle to deliver oxygen to meet the heightened metabolic demand. This decrease in muscle vascular resistance with increasing exercise intensity creates a challenge to systemic blood flow delivery that is met by increases in both cardiac output and vascular resistance to non-exercising tissues. In fact, even in thermoneutral conditions the capacity of the muscle alone to vasodilate has the potential to outstrip the pumping capacity of the heart (Andersen and Saltin 1985).
Additionally, the increase in metabolic activity for energy production in the working muscle increases heat production, which in turn impacts temperature regulation. During dynamic exercise in the heat the combined demands of increased nutritive blood flow to metabolically active muscles and the tremendous capacity of the skin to vasodilate in response to elevated body and skin temperatures far outstrip the pumping capacity of the heart. Yet, within a wide range of exercise intensities and environmental conditions, mean arterial pressure is closely defended and the relative perfusion of muscle and cutaneous vascular beds is more a compromise -- or commensalism -- than a true competition, without a winner or a loser. Impressively, not only is arterial pressure not negatively impacted to any great extent, but functional metabolic and thermoregulatory demands are met as well.
Functional Control of Metabolism and Thermoregulation
Dynamic exercise results in substantial metabolic heat production that in turn requires thermoregulatory skin blood flow to increase in order to mitigate the elevation in core temperature. During heavy exercise, the metabolic rate of active muscle can increase about 100 fold or 70 W•kg-1 of muscle (Nadel 1986). Both an increased skin blood flow and evaporative sweating are essential for removal of the heat produced by the active muscle to prevent severe hyperthermia as the former convects heat from muscle to the periphery while the latter provides the majority of heat loss to the environment.
The elevated core temperature during exercise results in an increase in vascular conductance due to the heightened thermal drive for skin vasodilatation. During dynamic exercise in warm ambient conditions, mean arterial pressure is higher than at rest and, at least in compensable environments - where thermoregulatory mechanisms are capable of dissipating sufficient metabolic heat - core temperature can be maintained within functional tolerance limits for prolonged periods of time (Rowell 1974). This maintenance of arterial pressure requires a compensatory increase in vascular resistance elsewhere, yet active muscle blood flow is not compromised (Table 2) (Mack et al. 1994; Taylor et al. 1990; Taylor et al. 1988; Kellogg et al. 1991a, b). The effect of hyperthermia on active muscle blood flow has been examined in several studies during mild to moderate exercise in the heat (Gonzalez-Alonso 1998; Gonzalez-Alonso et al. 1998; Nielsen et al. 1990; Savard et al. 1988; Pearson 2011) with the consistent conclusion that under euhydrated conditions, even during extreme hyperthermia (core temperatures exceeding 39°C), active muscle blood flow is not compromised. Direct measures of muscle blood flow by Savard et al (Savard et al. 1988) clearly demonstrate this concept (illustrated by the open circles in Fig. 2).
Table 2.
Four studies are highlighted that examined the effects of increasing thermal stress during one-legged knee extensor exercise or treadmill walking (Nielsen et al, 1990) on mean arterial pressure, skin blood flow, and muscle blood flow. In these studies, the arrows represent relative changes from resting conditions.
| Study | Tsk or Tdb | Tc | Mean Arterial Pressure | Skin Blood Flow | Muscle Blood Flow | Outcomes |
|---|---|---|---|---|---|---|
| Pearson et al, 2011 | C: ↔ | C:↔ | C: ↑↑↑ | C: ↑ | C: ↑ | ↑ Tsk and/or Tc from whole body heating, resulted in ↑ skin and muscle blood flow vs C. Mean arterial pressure was reduced vs. C with ↑ thermal stress. |
| ↑ | ↔ | ↑ ↑ | ↑ ↑ ↑ | ↑ ↑ | ||
| ↑ | ↑ | ↑ | ↑ ↑ ↑ | ↑ ↑ ↑ | ||
| ↑ ↑ | ↑ ↑ | ↑ | ↑ ↑ ↑ | ↑ ↑ ↑ | ||
| Savard et al, 1988 | C: ↔ | C: ↔ | C: ↑↑ | C: ↑ | C: ↑↑↑ | ↑ Tsk and Tc from whole body heating resulted in ↑ skin blood flow while muscle blood flow remained ↔ vs. C. |
| ↑ | ↑ | ↑ ↑ | ↑ ↑ | ↑ ↑ ↑ | ||
| Gonzalez-Alonso et al, 1998 | ↑ | ↑ | ↑ ↑ | NA | ↑ ↑ | During exercise in the heat D ↓ skin blood flow and muscle blood vs. euhydrated exercise in the heat. |
| D: ↑ | D: ↑↑ | ↑ | NA | ↑ | ||
| Nielsen et al, 1990 | C: ↑ | C: ↑ | ↑ | NA | ↑ ↑ ↑ | ↑Tdb, Tsk, and Tc during exercise in the heat did not significantly alter mean arterial pressure or muscle blood flow vs. C. |
| ↑ ↑ | ↑ ↑ | ↑ | NA | ↑ ↑ ↑ |
In the control site (C) exercise alone increased mean arterial pressure, skin blood flow, and muscle blood flow. When thermal stress such as increasing skin temperature (Tsk), dry bulb temperature (Tdb), or core temperature (Tc) was added to exercise, muscle blood flow was unchanged or slightly elevated, and skin blood flow increased further. These data suggest that neither muscle blood flow or skin blood flow was compromised during moderate exercise with increasing thermal strain. However, under dehydrated conditions (D) when mean arterial pressure was challenged, the decrease in perfusion pressure caused a concomitant decrease in muscle blood flow.
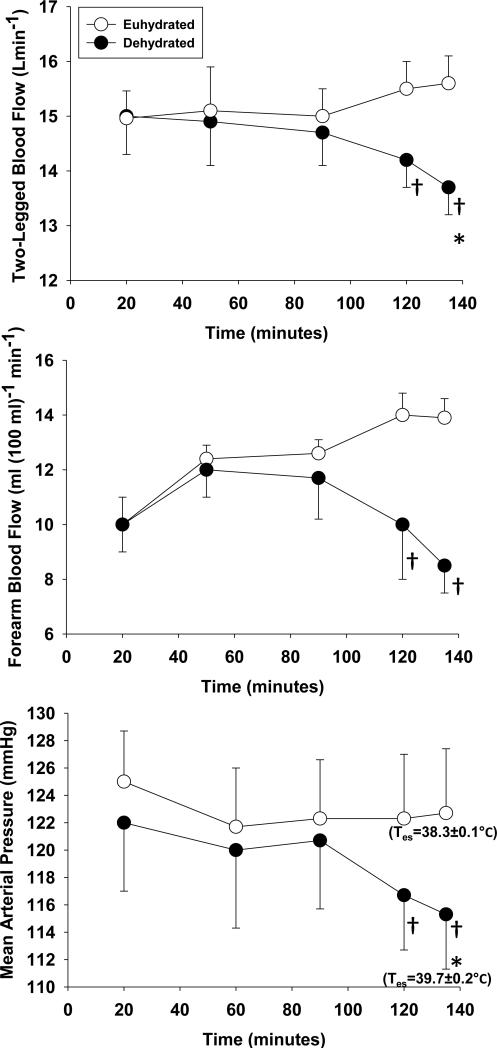
Figure 2. The effects of continuous exercise in the heat on muscle blood flow, skin blood flow and mean arterial pressure during -○- euhydrated and -●- dehydrated trials.
During continuous cycle exercise (208 ± 21 W) in the heat (35°C, 40-50% relative humidity) muscle blood flow (two-legged blood flow), skin blood flow (forearm blood flow), and mean arterial pressure is preserved when euhydrated. Inadequate fluid replacement that results in significant dehydration (3.9 ± 0.3% body weight) and reductions in mean arterial pressure is accompanied by a concomitant decrease in both muscle and skin blood flow, suggesting that mean arterial pressure is the regulated variable. In addition, the dehydrated trial resulted in significantly higher esophageal temperatures (Tes) at the end of exercise, which increases the core-to-skin temperature gradient during times of reduced skin blood flow. Redrawn from Gonzàlez-Alonso et al (1998).
Therefore, when exercise is performed in the heat and a “competition” between nutritive muscle blood flow and thermoregulatory skin blood flow arises, thermoregulatory demands do not divert flow away from active muscle. Changes in conductance within non-exercising tissues, including the skin, are responsible for the preservation of mean arterial pressure during dynamic exercise in the heat.
Rather than divert flow away from active muscle, cardiovascular reflexes associated with exercise attenuate the absolute magnitude of both splanchnic and skin blood flow for a given core temperature during exercise in the heat. When environmental heat stress is added to dynamic exercise, splanchnic and renal vasoconstriction increase such that there is an increased resistance and lower perfusion of these beds at a given exercise intensity compared to thermoneutral conditions (Fig. 3). One exception where skin blood flow is not fully reduced during the onset of exercise is during sustained high (>40°C) local skin temperatures (Taylor et al. 1984), which increase skin blood flow in a mechanistically distinct manner from reflex mechanisms. Approximately 70% of the increase in skin blood flow during sustained local heating to 42°C is due to nitric oxide-dependent mechanisms (Minson et al. 2001). Endogenous and exogenous nitric oxide has been shown to reduce cutaneous vasoconstrictor responsiveness in vivo (Durand et al. 2005; Hodges et al. 2007; Shibasaki et al. 2007; Shibasaki et al. 2008). The ability for local temperature to modulate skin blood flow may be important during extremely high skin temperatures, such as those achieved during extreme thermal stress or while wearing thermal protective gear. Sustained high local skin temperature may act redundantly to maintain skin blood flow during times when there is high sympathetic activity (Pearson et al. 2013), such as during exercise. Further studies are needed to examine the extent to which high local skin temperature attenuates non-thermoregulatory control of skin blood flow and blood pressure regulation.
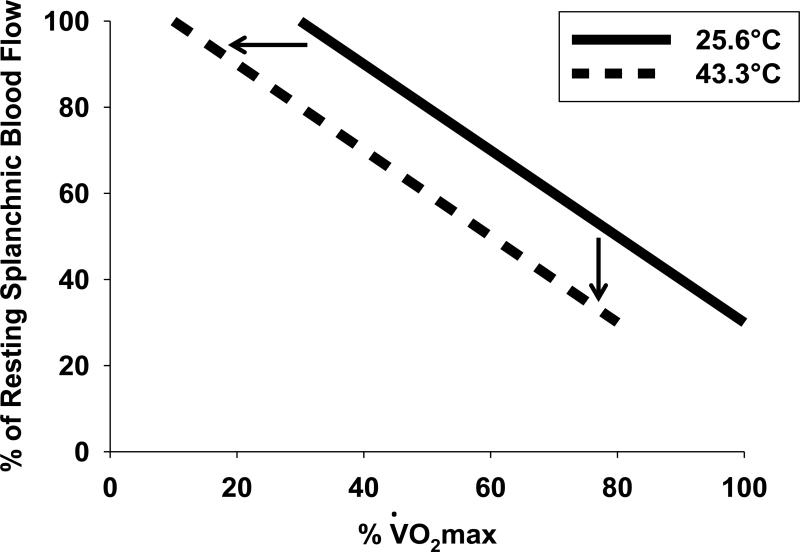
Figure 3. Schematic illustrating the effect of heat stress on splachnic blood flow during graded exercise.
Splachnic blood flow is reduced at a given intensity (% O2max) during heat stress (43.3°C) compared to thermoneutral conditions (25.6°C) allowing for a redistribution of blood flow to the skin for thermoregulatory purposes. Redrawn from Rowell et al (1965).
Dynamic exercise alters control of the skin blood flow-core temperature relation in several essential ways. First, the onset of moderate-to-high intensity dynamic exercise is associated with an initial cutaneous vasoconstriction (Fig. 4) (Gagge and Gonzalez 2010; Bevegard and Shepherd 1966; Johnson and Park 1982), even under conditions of elevated core and skin temperatures (Johnson and Park 1982). This transient vasoconstriction is dependent on an intact adrenergic vasoconstrictor system (Kellogg et al. 1991a) and partially related to cardiopulmonary baroreceptor unloading (Nishiyasu et al. 1993), illustrating that exercise-related reflexes alter thermoregulatory increases in skin blood flow via vasoconstrictor adjustments in the cutaneous vascular bed. Further, during dynamic exercise intensities exceeding 100 W, the threshold for the onset of cutaneous vasodilatation is shifted to a higher core temperature, resulting in a lower skin blood flow for the same core temperature relative to rest (Fig. 4) (Smolander and Holmer 1991; Taylor et al. 1988). This reduction in cutaneous vascular conductance during hyperthermia is primarily mediated by an initial vasoconstriction at the beginning of exercise and an attenuated active vasodilator activity as exercise progresses (Kellogg et al. 1991a, b; Kenney et al. 1991). Finally, dynamic exercise seems to place an “upper limit” on skin blood flow that restricts cutaneous blood flow to ~50% of the maximum perfusion observed at rest when core temperature exceeds 38°C (Fig. 3) (Brengelmann et al. 1977; Gonzalez-Alonso et al. 1999b; Kenney et al. 1991)). Collectively, these non-thermoregulatory exercise-induced changes in the magnitude of cutaneous vascular conductance reduce skin blood flow for a given core temperature during exercise in the heat compared to the same core temperature at rest.
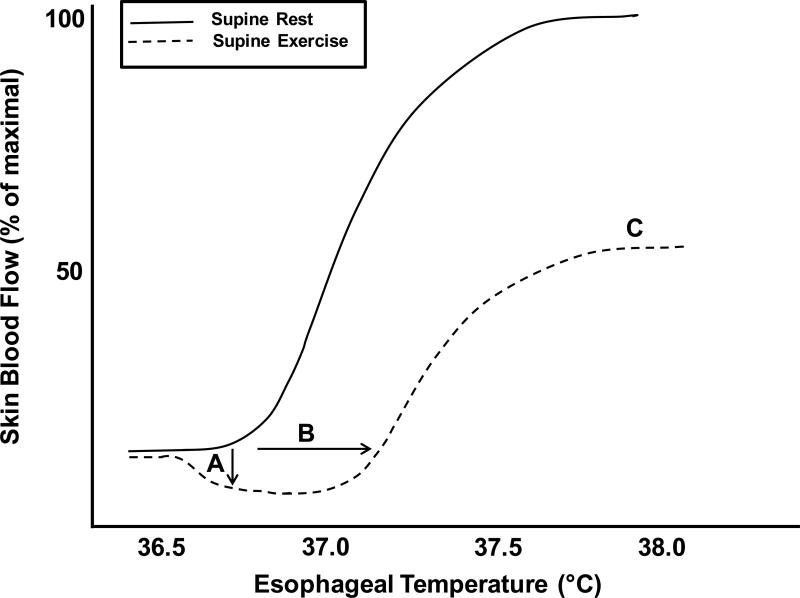
Figure 4. Influence of dynamic exercise on the skin blood flow response to hyperthermia.
During dynamic exercise skin blood flow is reduced during (A) an initial adrenergic vasoconstriction associated with the onset of exercise, (B) shift in the threshold for vasodilation toward higher internal temperatures, and (C) plateau at ~50% of maximal skin blood flow compared to rest.
Further support for the theory that skin blood flow is controlled in ways designed to preserve arterial pressure during exercise in the heat comes from studies examining the effect of exercise and hyperthermia on skin blood flow during additional challenges to central blood volume and consequently, blood pressure. Dehydration, which leads to reduced plasma volume and attenuated venous filling pressure attenuates skin blood flow and reduces the maximal skin blood flow achieved during prolonged exercise in the heat (Kenney et al. 1990; Nadel 1980). Similarly, upright posture imposes a great orthostatic stress, particularly during hyperthermia, when the highly compliant vessels of the skin dilate and become a relative “sink” for blood volume (Nielsen et al. 1984; Rowell 1986). During moderate intensity cycle exercise, upright posture increases the threshold for active vasodilatation, resulting in an attenuated skin blood flow for a given core temperature (Johnson et al. 1974). Roberts and Wenger found that the rightward shift in forearm blood flow-core temperature relation was related to the extent to which upright exercise lowered stroke volume (Roberts and Wenger 1980). Mack et al. later found that during mild, steady state exercise the unloading of cardiopulmonary baroreceptors with lower body negative pressure played an integral role in maintaining MAP by increasing forearm vascular resistance (Mack et al. 1988).
Cardiopulmonary reflexes are activated during reductions in central vascular pressure, including right atrial transmural pressure, which -- without an increase in cardiac contractility -- result in a lower stroke volume. Cardiopulmonary reflexes prevent MAP from falling by decreasing skin blood flow at times when cardiac output is reduced, including upright exercise and dehydration-induced hypovolemia. In contrast, when supine exercise is performed, stroke volume is restored and the plateau in skin blood flow response to rising core temperature is not observed (Gonzalez-Alonso et al. 1999a; Nadel et al. 1979). The restoration of skin blood flow is also observed when cardiopulmonary baroreceptors are loaded in cases such as water immersion (Nielsen et al. 1984), negative-pressure breathing (Nagashima et al. 1998), or central volume infusion (Nose et al. 1990). These latter perturbations help maintain mean arterial pressure by increasing cardiac output and stroke volume and are accompanied by a continuous rise in skin blood flow that does not display the characteristic plateau observed when exercise is performed in hot ambient conditions (Nielsen et al. 1984).
Considering that mean arterial pressure is well maintained under these conditions of added stress suggests that the attenuation in skin blood flow acts to preserve mean arterial pressure when central venous volume or pressure is compromised. Collectively, while muscle blood flow is maintained and metabolic demands for nutritive flow are met to a point limited only by cardiac output and perfusion pressure (Mortensen et al. 2005), skin blood flow is reduced during dynamic exercise compared to rest and it is this increase in peripheral resistance that at least partially protects mean arterial pressure during dynamic exercise in the heat. But is this a clear “win” for the muscle and does a reduced flow compared to rest necessarily mean that skin – or thermoregulation -- is “losing”?
Is the skin overperfused during exercise?
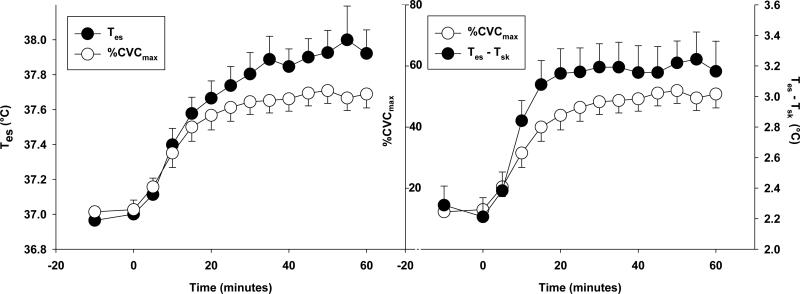
Despite the clear observation of an attenuated skin blood flow during dynamic exercise even as sustained heat production is driving core temperature upward, this reduction in convective transfer of heat from the core to the skin is not detrimental to thermoregulation during exercise. That is, if the plateau in skin blood flow that is observed during exercise in the heat compromised thermoregulation, one would expect core temperature to increase at a greater rate beyond that point. Such is not the case, as shown in Fig. 5.
Figure 5. Continuous exercise in compensable heat stress results in a thermoregulatory homeostasis.
During continuous cycling exercise (60% O2peak) in compensable heat stress (30°C, 40% relative humidity) a thermoregulatory homeostasis can be achieved and is marked by plateaus in esophageal temperature (Tes), skin blood flow (%CVCmax), and core-to-skin temperature gradients (Tes-Tsk). During exercise skin blood flow reaches a plateau at about 50% maximum dilation, yet core temperature does not begin to rise at a greater rate beyond this plateau (left) due to increased heat loss from the core-to-skin gradient (right). Redrawn and unpublished findings from Bruning et al (2012).
Overall convective heat transfer is determined by (1) skin blood flow, (2) the specific heat of the blood, and (3) the core-to-skin temperature gradient. Nadel (1986) argued that the relatively lower skin blood flow during exercise is partially offset by the larger thermal gradient produced by high core temperatures during exercise (Nadel 1986). For example, during moderate intensity exercise in the heat, hypovolemic subjects displayed a 30% attenuation in skin blood flow that resulted in less than a 10% difference in heat transfer compared to normovolemia (Nadel 1986, 1980). In support of this finding, Brengelmann demonstrated that increasing skin blood flow was accompanied by a decrease in the core-to-skin gradient, aiding in convective heat transfer (Johnson et al. 1986). Additionally, Rowell makes a compelling argument for the utility of attenuated cutaneous volume in thermoregulation: Under conditions of increased skin blood flow, this increase must be met with a proportional decrease in mean transit time in order to maintain venous return to the heart from the cutaneous vascular beds. As mean transit time decreases, heat loss per unit of blood may decrease, potentially creating a heat dissipation/cutaneous perfusion mismatch (Rowell 1986).
Perturbations that limit the rise in, or directly decrease, skin blood flow are rarely are accompanied by elevated core temperatures. Additional evidence to this support this notion comes from studies of older individuals who, despite a lower cutaneous vascular conductance during exercise in a hot environment, do not store more heat than young subjects who exhibit significantly higher skin blood flows (Kenney et al. 1990; Kenny et al. 2010).
In addition to increasing skin blood flow and/or widening of the core-to-skin gradient through reflex cutaneous vasodilatation, the role of thermoregulatory sweat production and evaporative cooling in the maintenance of homeostatic core temperatures cannot be discounted. Increased exercise intensity is associated with an earlier onset of thermoregulatory sweating and higher sweating rates which facilitate evaporative cooling in environments where the capacity of the environment to take up water vapor exceeds the requisite evaporative heat loss. However, for a given exercise intensity, regulating thermal balance is only possible within a certain range of environmental conditions termed the “prescriptive zone” (Lind 1963) in which the core-to-skin gradient can be maintained. In environmental conditions where high ambient heat and humidity preclude adequate evaporation of thermoregulatory sweat, skin temperature is driven upward and the core to skin gradient is reversed. Increasing blood flow to the skin in those conditions results in net heat gain through convective transfer from the skin to the body core (Rowell 1974; Nielsen 1996). Therefore, in these extreme conditions where the core-to-skin thermal gradient is minimized or reversed, increasing skin blood flow can in fact be detrimental to thermoregulation.
It should also be mentioned that, similar to the cutaneous circulation, active muscle may be relatively overperfused. When mean arterial pressure is compromised causing a drop in perfusion pressure, active muscles increase oxygen extraction. Brief periods of maximal exercise in the heat does not reduce maximal aerobic capacity compared to what can be achieved in cooler environments, even with prior hyperthermia elicited from submaximal exercise (Rowell 1986). During maximal, whole body exercise, about 50% of the skeletal muscle is active (Rowell 1993), which if maximally perfused could overwhelm the pumping capacity of the heart (Andersen and Saltin 1985). Therefore, during extremely heavy whole body exercise in the heat, metabolic rather than thermoregulatory demands may limit exercise performance.
Collectively, the evidence suggest that during dynamic exercise the reduction in skin blood flow for a given core temperature is not detrimental to thermoregulation, and may in fact support the dissipation of metabolic heat by widening the core-to-skin gradient and increasing mean transit time through the cutaneous circulation. In the case of exercise in high ambient heat and humidity, attenuated skin blood flow may limit convective heat gain in the short term, until metabolic heat production drives core temperatures upward and a critical core temperature is reached. Within compensable environments, evidence that shows significant reductions in skin blood flow with little increase in heat storage (Nadel 1986; Kenney, 1990; Havenith et al. 1995) suggesting that the attenuated cutaneous vascular conductance and widening of the core-to-skin gradient with exercise allows for simultaneous metabolic heat dissipation and maintenance of a higher mean arterial pressure compared to rest.
Similarly, whole body maximal exercise is limited by the pumping capacity of the heart to deliver adequate blood flow to the exercising muscle. This inability of either cutaneous or skeletal muscle blood flow to increase limitlessly sets functional limits for both of these circulations, while preventing a fall in MAP. This supports the point that the skin circulation does not “lose” to the muscle, but rather that the integrated system of thermoregulatory and non-thermoregulatory mechanisms governing cutaneous flow in fact benefit the cutaneous beds such that they are able to serve their thermoregulatory purpose without compromising systemic blood flow delivery.
SUMMARY AND CONCLUSIONS
Commensalism derives from the Latin cum mensal, which means sharing the same table. In the context of dynamic exercise in a warm environment, that “table” is the available cardiac output. Rowell stated that “...skin and muscle ‘compete’ for blood flow and their combined needs can easily exceed the pumping capacity of the heart” (Rowell, 1986, p. 363). That argument has been extended in the literature and in exercise physiology textbooks to conclude that, if taken to high exercise intensity, body temperature, or both, one circulation must win, i.e., be maintained or increase at the expense of the other, and one must lose. Attenuated muscle blood flow would result in cessation of exercise or a forced diminution of intensity; diminished skin blood flow would result in elevated core temperatures and the same effect on the ability to continue exercise. But neither happens in young, healthy humans.
Rowell describes the phenomenon of “cardiovascular drift”, in which arterial pressure declines as a consequence of increased blood flow directed to vasodilated skin, illustrating that the compensatory control envisioned by Dastre and Morat is not always complete(Rowell 1993). However, in the absence of dehydration or other superimposed stresses, mean pressure is well maintained even under extreme conditions.
Alternately, Johnson (Johnson 2010) has argued that this “conflict between regulatory systems” limits prolonged exercise by compromising central temperature regulation rather than reducing blood pressure. With the exception of the pathological condition of clinical heat stroke, temperature regulation proceeds relatively unimpaired when adjustments are made to limit the rise in skin blood flow. For example, during prolonged exercise in warm environments, skin blood flow rises then plateaus as core temperature approaches 38°C. Yet, this plateau is not accompanied by a nonlinear increase in core temperature as exercise continues as one might expect if thermoregulation was compromised.
While the ensuing argument eschewing a “competition” between skin and muscle blood flows may be considered by some to be semantic, the elegance of control should be highlighted over a simple battle for supremacy. While there is no doubting Rowell's contention that “prolonged ... exercise with increased ambient temperature...forces humans to deal with the two most powerful competing regulatory demands they ever face” (p. 228) (Rowell 1986), in fact those demands are met without compromising either blood pressure or temperature regulation.
Footnotes
Conflicts of Interest
The authors of have no conflicts of interest to report.
REFERENCES
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. The Journal of physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiologic reviews. 2002;24(2):190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- Bevegard BS, Shepherd JT. Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. Journal of applied physiology. 1966;21(1):123–132. doi: 10.1152/jappl.1966.21.1.123. [DOI] [PubMed] [Google Scholar]
- Brengelmann GL, Johnson JM, Hermansen L, Rowell LB. Altered control of skin blood flow during exercise at high internal temperatures. Journal of applied physiology. 1977;43(5):790–794. doi: 10.1152/jappl.1977.43.5.790. [DOI] [PubMed] [Google Scholar]
- Brothers RM, Bhella PS, Shibata S, Wingo JE, Levine BD, Crandall CG. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol. 2009;296(4):H1150–1156. doi: 10.1152/ajpheart.01069.2008. doi:01069.2008 [pii] 10.1152/ajpheart.01069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG. Carotid baroreflex responsiveness in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2000;279(4):H1955–1962. doi: 10.1152/ajpheart.2000.279.4.H1955. [DOI] [PubMed] [Google Scholar]
- Crandall CG. Heat stress and baroreflex regulation of blood pressure. Medicine and science in sports and exercise. 2008;40(12):2063–2070. doi: 10.1249/MSS.0b013e318180bc98. doi:10.1249/MSS.0b013e318180bc98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG, Gonzalez-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf) 2010;199(4):407–423. doi: 10.1111/j.1748-1716.2010.02119.x. doi:10.1111/j.1748-1716.2010.02119.x APS2119 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG, Johnson JM, Kosiba WA, Kellogg DL., Jr. Baroreceptor control of the cutaneous active vasodilator system. Journal of applied physiology. 1996;81(5):2192–2198. doi: 10.1152/jappl.1996.81.5.2192. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. The Journal of physiology. 2008;586(1):293–301. doi: 10.1113/jphysiol.2007.143057. doi:jphysiol.2007.143057 [pii] 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002;282(1):R252–258. doi: 10.1152/ajpregu.00337.2001. doi:10.1152/ajpregu.00337.2001. [DOI] [PubMed] [Google Scholar]
- Durand S, Davis SL, Cui J, Crandall CG. Exogenous nitric oxide inhibits sympathetically mediated vasoconstriction in human skin. The Journal of physiology. 2005;562(Pt 2):629–634. doi: 10.1113/jphysiol.2004.075747. doi:10.1113/jphysiol.2004.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagge AP, Gonzalez RR. Comprehensive Physiology. John Wiley & Sons, Inc.; 2010. Mechanisms of Heat Exchange: Biophysics and Physiology. doi:10.1002/cphy.cp040104. [Google Scholar]
- Gonzalez-Alonso J. Separate and combined influences of dehydration and hyperthermia on cardiovascular responses to exercise. International journal of sports medicine. 1998;19(Suppl 2):S111–114. doi: 10.1055/s-2007-971972. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. The Journal of physiology. 1998;513(Pt 3):895–905. doi: 10.1111/j.1469-7793.1998.895ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Mora-Rodriguez R, Coyle EF. Supine exercise restores arterial blood pressure and skin blood flow despite dehydration and hyperthermia. The American journal of physiology. 1999a;277(2 Pt 2):H576–583. doi: 10.1152/ajpheart.1999.277.2.H576. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. Journal of applied physiology. 1999b;86(3):1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Havenith G, Inoue Y, Luttikholt V, Kenney WL. Age predicts cardiovascular, but not thermoregulatory, responses to humid heat stress. European journal of applied physiology and occupational physiology. 1995;70(1):88–96. doi: 10.1007/BF00601814. [DOI] [PubMed] [Google Scholar]
- Hodges GJ, Kosiba WA, Zhao K, Alvarez GE, Johnson JM. The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol. 2007;293(5):H3187–3192. doi: 10.1152/ajpheart.00815.2007. doi:10.1152/ajpheart.00815.2007. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. Journal of applied physiology. 2010;109(5):1538–1544. doi: 10.1152/japplphysiol.00338.2010. doi:10.1152/japplphysiol.00338.2010 japplphysiol.00338.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Frontiers in bioscience : a journal and virtual library. 2010;15:718–739. doi: 10.2741/3642. doi:3642 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev. 2007;35(3):119–125. doi: 10.1097/jes.0b013e3180a02f85. doi:10.1097/jes.0b013e3180a02f85 00003677-200707000-00006 [pii] [DOI] [PubMed] [Google Scholar]
- Johnson JM. Exercise in a hot environment: the skin circulation. Scand J Med Sci Sports. 2010;20(Suppl 3):29–39. doi: 10.1111/j.1600-0838.2010.01206.x. doi:10.1111/j.1600-0838.2010.01206.x. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Brengelmann GL, Hales JR, Vanhoutte PM, Wenger CB. Regulation of the cutaneous circulation. Federation proceedings. 1986;45(13):2841–2850. [PubMed] [Google Scholar]
- Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. Journal of applied physiology. 1973;35(6):798–803. doi: 10.1152/jappl.1973.35.6.798. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Park MK. Effect of heat stress on cutaneous vascular responses to the initiation of exercise. Journal of applied physiology. 1982;53(3):744–749. doi: 10.1152/jappl.1982.53.3.744. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Rowell LB, Brengelmann GL. Modification of the skin blood flow-body temperature relationship by upright exercise. Journal of applied physiology. 1974;37(6):880–886. doi: 10.1152/jappl.1974.37.6.880. [DOI] [PubMed] [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. The Journal of physiology. 2006;573(Pt 2):445–451. doi: 10.1113/jphysiol.2006.108662. doi:jphysiol.2006.108662 [pii] 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. The Journal of physiology. 2009;587(Pt 5):1131–1139. doi: 10.1113/jphysiol.2008.165118. doi:jphysiol.2008.165118 [pii] 10.1113/jphysiol.2008.165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Sander M, Stallknecht B, Crandall CG. alpha-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. The Journal of physiology. 2010;588(Pt 19):3799–3808. doi: 10.1113/jphysiol.2010.194506. doi:jphysiol.2010.194506 [pii] 10.1113/jphysiol.2010.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL, Jr., Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. Journal of applied physiology: respiratory, environmental and exercise physiology. 1998;85(3):824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr., Johnson JM, Kosiba WA. Competition between cutaneous active vasoconstriction and active vasodilation during exercise in humans. The American journal of physiology. 1991a;261(4 Pt 2):H1184–1189. doi: 10.1152/ajpheart.1991.261.4.H1184. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr., Johnson JM, Kosiba WA. Control of internal temperature threshold for active cutaneous vasodilation by dynamic exercise. Journal of applied physiology. 1991b;71(6):2476–2482. doi: 10.1152/jappl.1991.71.6.2476. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. Journal of applied physiology. 1990;68(5):1902–1908. doi: 10.1152/jappl.1990.68.5.1902. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Tankersley CG, Newswanger DL, Puhl SM. Alpha 1-adrenergic blockade does not alter control of skin blood flow during exercise. Am J Physiol Heart Circ Physiol. 1991;260(3 Pt 2):H855–861. doi: 10.1152/ajpheart.1991.260.3.H855. [DOI] [PubMed] [Google Scholar]
- Kenny GP, Gagnon D, Dorman LE, Hardcastle SG, Jay O. Heat balance and cumulative heat storage during exercise performed in the heat in physically active younger and middle-aged men. European journal of applied physiology. 2010;109(1):81–92. doi: 10.1007/s00421-009-1266-4. doi:10.1007/s00421-009-1266-4. [DOI] [PubMed] [Google Scholar]
- Lee JF, Harrison ML, Brown SR, Brothers RM. The magnitude of heat-stress induced reductions in cerebral perfusion does not predict heat-stress induced reductions in tolerance to a simulated hemorrhage. Journal of applied physiology. 2012 doi: 10.1152/japplphysiol.00878.2012. doi:japplphysiol.00878.2012 [pii] 10.1152/japplphysiol.00878.2012. [DOI] [PubMed] [Google Scholar]
- Lind AR. A physiological criterion for setting thermal environmental limits for everyday work. Journal of applied physiology. 1963;18:51–56. doi: 10.1152/jappl.1963.18.1.51. [DOI] [PubMed] [Google Scholar]
- Low DA, Keller DM, Wingo JE, Brothers RM, Crandall CG. Sympathetic nerve activity and whole body heat stress in humans. Journal of applied physiology. 2011;111(5):1329–1334. doi: 10.1152/japplphysiol.00498.2011. doi:10.1152/japplphysiol.00498.2011 japplphysiol.00498.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. Journal of applied physiology. 2008;104(4):976–981. doi: 10.1152/japplphysiol.01040.2007. doi:01040.2007 [pii] 10.1152/japplphysiol.01040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RA, Ganio MS, Pearson J, Crandall CG. Sweat loss during heat stress contributes to subsequent reductions in lower-body negative pressure tolerance. Exp Physiol. 2012 doi: 10.1113/expphysiol.2012.068171. doi:expphysiol.2012.068171 [pii] 10.1113/expphysiol.2012.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack G, Nose H, Nadel ER. Role of cardiopulmonary baroreflexes during dynamic exercise. Journal of applied physiology. 1988;65(4):1827–1832. doi: 10.1152/jappl.1988.65.4.1827. [DOI] [PubMed] [Google Scholar]
- Mack GW, Nose H, Takamata A, Okuno T, Morimoto T. Influence of exercise intensity and plasma volume on active cutaneous vasodilation in humans. Medicine and science in sports and exercise. 1994;26(2):209–216. doi: 10.1249/00005768-199402000-00011. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. Journal of applied physiology. 2001;91(4):1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. Journal of applied physiology. 1998;84(4):1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. The American journal of physiology. 1999;276(1 Pt 2):R203–212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, Gonzalez-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. The Journal of physiology. 2005;566(Pt 1):273–285. doi: 10.1113/jphysiol.2005.086025. doi:10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel ER. Circulatory and thermal regulations during exercise. Federation proceedings. 1980;39(5):1491–1497. [PubMed] [Google Scholar]
- Nadel ER. Non-thermal influences on the control of skin blood flow have minimal effects on heat transfer during exercise. The Yale journal of biology and medicine. 1986;59(3):321–327. [PMC free article] [PubMed] [Google Scholar]
- Nadel ER, Cafarelli E, Roberts MF, Wenger CB. Circulatory regulation during exercise in different ambient temperatures. Journal of applied physiology. 1979;46(3):430–437. doi: 10.1152/jappl.1979.46.3.430. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Nose H, Takamata A, Morimoto T. Effect of continuous negative-pressure breathing on skin blood flow during exercise in a hot environment. Journal of applied physiology. 1998;84(6):1845–1851. doi: 10.1152/jappl.1998.84.6.1845. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Haykowsky MJ, Stickland MK, Altamirano-Diaz LA, Willie CK, Smith KJ, Petersen SR, Ainslie PN. Reductions in cerebral blood flow during passive heat stress in humans: partitioning the mechanisms. The Journal of physiology. 2011;589(Pt 16):4053–4064. doi: 10.1113/jphysiol.2011.212118. doi:jphysiol.2011.212118 [pii] 10.1113/jphysiol.2011.212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B. Olympics in Atlanta: a fight against physics. Medicine and science in sports and exercise. 1996;28(6):665–668. doi: 10.1097/00005768-199606000-00004. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Rowell LB, Bonde-Petersen F. Cardiovascular responses to heat stress and blood volume displacements during exercise in man. European journal of applied physiology and occupational physiology. 1984;52(4):370–374. doi: 10.1007/BF00943365. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and muscle metabolism during exercise and heat stress. Journal of applied physiology. 1990;69(3):1040–1046. doi: 10.1152/jappl.1990.69.3.1040. [DOI] [PubMed] [Google Scholar]
- Nishiyasu T, Shi X, Mack GW, Nadel ER. Forearm vascular responses to baroreceptor unloading at the onset of dynamic exercise. Journal of applied physiology. 1993;75(2):979–985. doi: 10.1152/jappl.1993.75.2.979. [DOI] [PubMed] [Google Scholar]
- Normell LA, Wallin BG. Sympathetic skin nerve activity and skin temperature changes in man. Acta Physiol Scand. 1974;91(3):417–426. doi: 10.1111/j.1748-1716.1974.tb05696.x. [DOI] [PubMed] [Google Scholar]
- Nose H, Mack GW, Shi XR, Morimoto K, Nadel ER. Effect of saline infusion during exercise on thermal and circulatory regulations. Journal of applied physiology. 1990;69(2):609–616. doi: 10.1152/jappl.1990.69.2.609. [DOI] [PubMed] [Google Scholar]
- Pearson J. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flow. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(3):R663–673. doi: 10.1152/ajpregu.00662.2010. doi:10.1152/ajpregu.00662.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Lucas RA, Crandall CG. Elevated local skin temperature impairs cutaneous vasoconstrictor responses to a simulated hemorrhagic challenge while heat stressed. Exp Physiol. 2012 doi: 10.1113/expphysiol.2012.068353. doi:expphysiol.2012.068353 [pii] 10.1113/expphysiol.2012.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Lucas RA, Crandall CG. Elevated local skin temperature impairs cutaneous vasoconstrictor responses to a simulated haemorrhagic challenge while heat stressed. Exp Physiol. 2013;98(2):444–450. doi: 10.1113/expphysiol.2012.068353. doi:10.1113/expphysiol.2012.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MF, Wenger CB. Control of skin blood flow during exercise by thermal reflexes and baroreflexes. Journal of applied physiology. 1980;48(4):717–723. doi: 10.1152/jappl.1980.48.4.717. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiological reviews. 1974;54(1):75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Reflex control of regional circulations in humans. J Auton Nerv Syst. 1984;11(2):101–114. doi: 10.1016/0165-1838(84)90069-9. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation: Regulation During Physical Stress. Oxford University Press; New York: 1986. [Google Scholar]
- Rowell LB. Human Cardiovascular Control. Oxford University Press; New York: 1993. [Google Scholar]
- Rowell LB, Blackmon JR, Martin RH, Mazzarella JA, Bruce RA. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. Journal of applied physiology. 1965;20(3):384–394. doi: 10.1152/jappl.1965.20.3.384. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Blackmon JR, Twiss RD, Kusumi F. Splanchnic blood flow and metabolism in heat-stressed man. Journal of applied physiology. 1968;24(4):475–484. doi: 10.1152/jappl.1968.24.4.475. [DOI] [PubMed] [Google Scholar]
- Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. Journal of applied physiology. 1988;64(2):649–657. doi: 10.1152/jappl.1988.64.2.649. [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. Journal of applied physiology: respiratory, environmental and exercise physiology. 1998;85(3):830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Durand S, Davis SL, Cui J, Low DA, Keller DM, Crandall CG. Endogenous nitric oxide attenuates neutrally mediated cutaneous vasoconstriction. The Journal of physiology. 2007;585(Pt 2):627–634. doi: 10.1113/jphysiol.2007.144030. doi:10.1113/jphysiol.2007.144030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Low DA, Davis SL, Crandall CG. Nitric oxide inhibits cutaneous vasoconstriction to exogenous norepinephrine. Journal of applied physiology. 2008;105(5):1504–1508. doi: 10.1152/japplphysiol.91017.2008. doi:91017.2008 [pii] 10.1152/japplphysiol.91017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolander J, Holmer I. Individual response to physical work in the heat in relation to sweating and skin blood flow. International archives of occupational and environmental health. 1991;63(3):225–226. doi: 10.1007/BF00381573. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA. Roles of absolute and relative load in skin vasoconstrictor responses to exercise. Journal of applied physiology. 1990;69(3):1131–1136. doi: 10.1152/jappl.1990.69.3.1131. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Graded cutaneous vascular responses to dynamic leg exercise. Journal of applied physiology. 1988;64(5):1803–1809. doi: 10.1152/jappl.1988.64.5.1803. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, O'Leary DS, Park MK. Modification of the cutaneous vascular response to exercise by local skin temperature. Journal of applied physiology. 1984;57(6):1878–1884. doi: 10.1152/jappl.1984.57.6.1878. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank-Starling relations in humans. The Journal of physiology. 2009;587(Pt 13):3383–3392. doi: 10.1113/jphysiol.2009.170381. doi:jphysiol.2009.170381 [pii] 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39(1):12–17. doi: 10.1097/JES.0b013e318201eed6. doi:10.1097/JES.0b013e318201eed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. The Journal of physiology. 2001;536(Pt 2):615–623. doi: 10.1111/j.1469-7793.2001.0615c.xd. doi:PHY_12483 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. Journal of applied physiology. 2002;93(1):85–91. doi: 10.1152/japplphysiol.01043.2001. doi:10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. The Journal of physiology. 2007;585(Pt 1):279–285. doi: 10.1113/jphysiol.2007.137901. doi:jphysiol.2007.137901 [pii] 10.1113/jphysiol.2007.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M, Crandall CG. Effect of elevated local temperature on cutaneous vasoconstrictor responsiveness in humans. Journal of applied physiology. 2009;106(2):571–575. doi: 10.1152/japplphysiol.91249.2008. doi:91249.2008 [pii] 10.1152/japplphysiol.91249.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss CR, Brengelmann GL, Johnson JM, Rowell LB, Niederberger M. Control of skin blood flow, sweating, and heart rate: role of skin vs. core temperature. Journal of applied physiology. 1974;36(6):726–733. doi: 10.1152/jappl.1974.36.6.726. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Sone R. Modulation of arterial baroreflex control of heart rate by skin cooling and heating in humans. Journal of applied physiology. 2000;88(2):393–400. doi: 10.1152/jappl.2000.88.2.393. [DOI] [PubMed] [Google Scholar]