Abstract
Seizures in newborns are associated with a high risk for subsequent epilepsy and adverse neurodevelopmental consequences. Understanding the mechanisms by which neonatal seizures adversely disturb the immature brain is important in developing therapeutic strategies. Using the convulsant agent flurothyl to mimic repetitive neonatal seizures we show that early-life seizures result in long-term alteration in the maintenance phase of long-term potentiation (LTP) in layer IV to layer II/III synapses of the somatosensory cortex without alteration of basal synaptic transmission, the induction phase of LTP and short-term depression. Such alterations may have a role in functional deficits seen following neonatal seizures.
Keywords: early seizures, plasticity, somatosensory cortex
1. INTRODUCTION
Seizures are one of the most common neurological emergencies occurring in newborns and are associated with a considerable risk of long-term sequelae, including epilepsy, cognitive and behavioral issues (Holmes, 2004, Ronen et al., 2007). While the etiology of the neonatal seizures is the most important factor in outcome, there is increasing data from humans that seizures independently contribute to long-term adverse consequences (Glass et al., 2009; 2011, but see Kwon et al., 2011).
To elucidate the effect of neonatal seizure on neuronal plasticity in the present study we used flurothyl model of repetitive seizures. Flurothyl is a volatile convulsant that produces well controlled generalized seizures with no apparent direct drug effect which makes it widely used in basic epilepsy research (Velíšková et al., 2005; Khan et al., 2010). Using the flurothyl model of repetitive seizures on immature rats we previously showed that neonatal seizures produce a long-term increase of seizure susceptibility and alteration in excitation/inhibition balance of synaptic transmission in layer II/III neurons of the somatosensory cortex (Isaeva et al., 2009; 2010). As the cerebral cortex is involved in encoding and processing of sensory information and has been shown to express different forms of activity-dependent synaptic plasticity (Castro-Alamancos et al., 1995) here we explored the hypothesis that early life seizures can modify synaptic plasticity in the somatosensory cortex.
2. MATERIAL AND METHODS
All experiments were performed in accordance with the guidelines set by the National Institute of Health and Dartmouth Medical School for the humane treatment of animals. Sprague-Dawley rats (N=8) were subjected to 75 flurothyl-induced seizures using previously described method (Isaeva et al., 2010). To elucidate the effect of neonatal seizure on neuronal plasticity in our animal model we chose the age range from postnatal day 0 to 15 which corresponds to the last trimester gestational period and first year of life in humans (Avishai-Eliner, et al. 2002). Untreated littermate pups (N=9) were used as controls. Brain slices were prepared from P46-P60 rats. The rats were deeply anesthetized with isoflurane and decapitated. Slices (400 μm) were cut in the coronal plane transferred to an incubation chamber where they rested for at least 2 hrs before recordings in oxygenated artificial cerebrospinal fluid (ACSF) of the following composition (mM): NaCl 126, KCl 3.5, CaCl2 2.0, MgCl2 1.3, NaHCO3 25, NaH2PO4 1.2 and glucose 11 (pH 7.3-7.4).
Field potential (FP) recordings were made from LII/III of somatosensory cortex using electrodes filled with ACSF (2-4mΩ). 2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl) pyridazinium bromide (SR95531) was included in the recording pipette (50 μM) to block gamma-aminobutyric acid (GABA) A receptors. Synaptic responses were evoked by stimulation of LIV of somatosensory cortex with 100 μsec pulses of 30-80 μA through a concentric bipolar stimulating electrode using a stimulus isolator. Baseline responses were obtained at 0.05 Hz using a stimulation intensity that produced half-maximal response for each recording. To induce LTP we used a primed burst (PB) potentiation protocol repeated 5 times at intervals of 10 sec consisting of a single priming pulse followed 170 ms later by a burst of 10 stimuli at 100 Hz (Diamond et al., 1988). Data were analyzed using the Mini Analysis (version 6.0.3; Synaptosoft, Decatur, GA), Clampfit (Axon Instruments Inc, Union City, CA) and Origin 7.0 (Microcal Software, Northampton, MA) software. Statistical comparison was performed using unpaired Student’s t-test. Results in the text and in the figures are expressed as the mean ± SEM.
3. RESULTS
Stimulation of LIV of somatosensory cortex evoked FPs in LII/III in all slices from flurothyl-treated and control groups of animals. The maximal rising slope of the FP as a measure of synaptic efficiency was not significantly different between groups (0.55 ± 0.11 mV/ms (n=7 animals/16 slices) in control vs 0.41 ± 0.05 mV/ms (n = 6 animals/14 slices) in flurothyl-treated group, p = 0.24). We next examined the effect of repetitive flurothyl seizures on synaptic plasticity using the PB potentiation protocol. In the presence of SR 95531 in the recording electrode the delivery of the PB potentiation protocol to the LIV of somatosensory cortex consistently induced prolonged enhancement of the evoked FP in LII/III in controls as well as flurothyl-treated group. Application of the NMDA receptor antagonist D-2-amino-5-phosphonovaleric acid blocked the induction of LTP in both groups. LTP maintenance phase was increased significantly in the flurothyl-treated group when compared with controls (FP changes 50 min after PB stimulation: 153.3 ± 18.6 %, n = 6 animals/7 slices vs 94.4 ± 7.4%, n = 6 animals/ 9 slices, p = 0.01). This increase in LTP maintenance occurred without modifications in the induction phase of LTP (Fig. 1). The average maximal response was not significantly different between groups: control (n=6 animals/ 9 slices): 231.8 ± 23.5 % of baseline and flurothyl-treated group (n= 6 animals/7 slices): 212.4 ± 12.3 %, p = 0.54. During high-frequency stimulus trains FP exhibit a strong depression. This form of short-term synaptic plasticity has been observed in different cortical areas (Castro-Alamancos et al., 1995, Hernan et al., 2013) and is thought to provide the synapse specific gain control of cortical circuits (Abbott et al., 1997). In our study FP slope decreased to 12.8 ± 3.1% (n=6 animals/10 slices) of its initial value over the course of a 100 Hz train in control, and to 8.0 ±1.8% (n=6 animals/ 9 slices) in the flurothyl-treated group. Figure 2 demonstrates that there was no difference in response to repetitive stimulation between the flurothyl and control groups.
Figure 1.
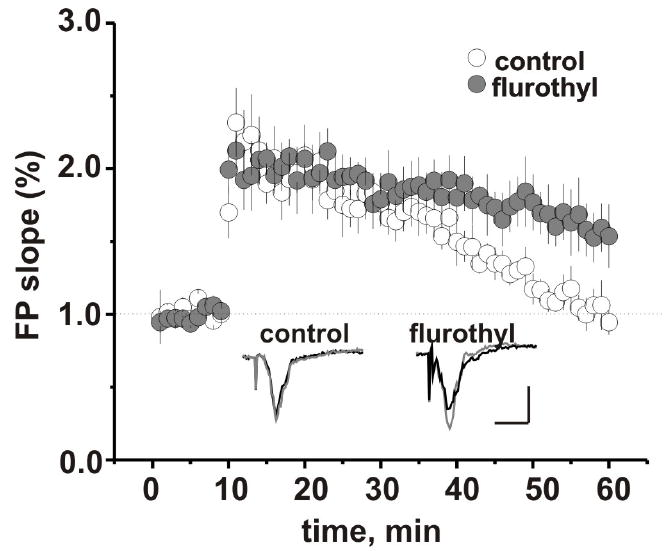
Effect of flurothyl induced seizures on the expression and maintenance of LTP in the somatosensory cortex. The baseline FP in control (white) and flurothyl-treated (grey) group was recorded for 10 min, then the primed burst potentiation protocol was applied and the recording was continued for another 50 min. All data were normalized to baseline. Insert: examples of FPs recorded in LII/III before (black) and 50 min after conditioning stimulation of LIV of somatosensory cortex (grey). Traces scale bars are 0.3 mV by 10 ms. Values are mean ± SEM.
Figure 2.
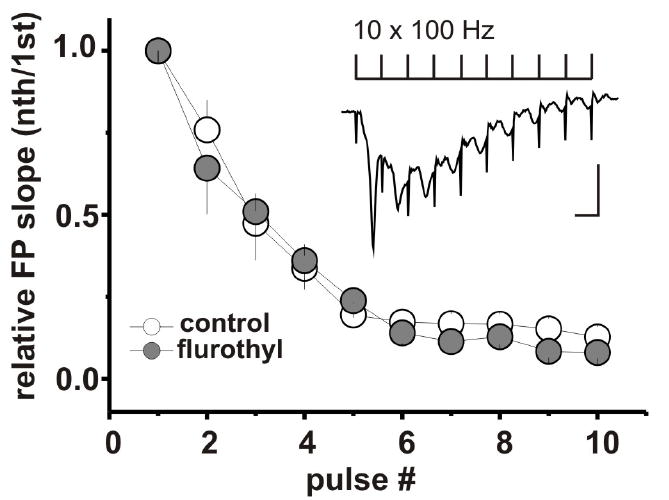
Repetitive neonatal seizures do not affect the short-term depression of synaptic responses during conditioning stimulation. FP slopes were normalized to the FP slope in response to first pulse and graphed versus pulse number. Pulses 1–10 were averaged individually. Insert: trace represent average of five consecutive FP recordings during the conditioning stimulation consisted of 10 pulses delivered at frequency 100 Hz. Traces scale bars are 0.3 mV by 10 ms. All measurements are reported as mean ± SEM.
3. DISCUSSION
We previously reported that neonatal seizures resulted in enhanced excitability in somatosensory cortex which can be due to the disruption of the excitation-inhibition balance in sensory pathways in the flurothyl group (Isaeva et al., 2010). In the present study the postsynaptic response in LIV to LII/III network in somatosensory cortex was not changed in the flurothyl-treated group compared to controls, indicating that the basal evoked synaptic transmission in this vertical pathway is unaltered in rats experiencing recurrent neonatal seizures.
In our study neither short-term depression nor induction phase of LTP was modified in the flurothyl-treated group. However, the maintenance phase of LTP was significantly altered. Expression of LTP at layer IV to layer II/III synapses in the somatosensory cortex depends on changes in the postsynaptic NMDA receptors (Bender et al., 2006). Lack of an effect of early life seizures on the induction phase of LTP is in agreement with our previous study where it was shown that amplitude of miniature NMDA-dependent excitatory postsynaptic currents (as indicator of postsynaptic alteration) was not altered in layer II/III synapses in somatosensory cortex after early life seizures (Isaeva et al., 2010).
During high frequency stimulation Ca2+ entry through the postsynaptic NMDA receptors activates different Ca2+ dependent second messengers which are crucial for the maintenance of LTP. Most factors with established molecular mechanisms responsible for the maintenance phase of LTP interfere with these cascades (Malinow et al., 1988, Matthies et al., 1991). Several studies show that the maintenance phase of LTP could be disrupted by seizures or altered in seizure-prone animals (Hesse and Teyler, 1976, Schubert et al., 2005). However, the lowered threshold for epileptiform activity is not always associated with loss or impairment of LTP and alteration of LTP rather depends on the cellular mechanism underlying the increase in neuronal activity (Luthi et al., 1997).
In summary, the results from the present study demonstrate that neonatal seizures have long-term effects on synaptic plasticity in the somatosensory cortex. Understanding the molecular mechanism of these synaptic changes and determining whether alterations in plasticity reported here interfere with sensory processing in patients with a history of neonatal seizures requires further investigation.
Acknowledgments
NIH (NINDS): NS073083, Emmory R. Shapses Research Fund; State Foundation of Fundamental Research of Ukraine F46.2/001.
ABBREVIATIONS
- FP
field potential
- P
postnatal day
- LIV
layer 4
- LII/III
layer 2/3
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate
- GABA
gamma-aminobutyric acid
Footnotes
CONFLICT OF INTEREST: None of the authors has a conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero) Trends in Neurosciences. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. The Journal of Neuroscience. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. The Journal of Neuroscience. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Dunwiddie TV, Rose GM. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. The Journal of Neuroscience. 1988;8:4079–4088. doi: 10.1523/JNEUROSCI.08-11-04079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. The Journal of Pediatrics. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Hong KJ, Rogers EE, Jeremy RJ, Bonifacio SL, Sullivan JE, Barkovich AJ, Ferriero DM. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatric Research. 2011;70:535–540. doi: 10.1203/PDR.0b013e31822f24c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse GW, Teyler TJ. Reversible loss of hippocampal long-term potentiation following electroconvulsive seizures. Nature. 1976;264:562–564. doi: 10.1038/264562a0. [DOI] [PubMed] [Google Scholar]
- Hernan AE, Holmes GL, Isaev D, Scott RC, Isaeva E. Altered short-term plasticity in the prefrontal cortex after early life seizures. Neurobiology of Disease. 2013;50:120–126. doi: 10.1016/j.nbd.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL. Effects of early seizures on later behavior and epileptogenicity. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:101–105. doi: 10.1002/mrdd.20019. [DOI] [PubMed] [Google Scholar]
- Isaeva E, Isaev D, Khazipov R, Holmes GL. Long-term suppression of GABAergic activity by neonatal seizures in rat somatosensory cortex. Epilepsy Research. 2009;87:286–289. doi: 10.1016/j.eplepsyres.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva E, Isaev D, Savrasova A, Khazipov R, Holmes GL. Recurrent neonatal seizures result in long-term increases in neuronal network excitability in the rat neocortex. European Journal of Neuroscience. 2010;31:1446–1455. doi: 10.1111/j.1460-9568.2010.07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, O’Shea TM, Goldberg RN, Donovan EF, Fanaroff AA, Poole WK, Higgins RD, Walsh MC. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. Journal of Child Neurology. 2011;26(3):322–328. doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, Van der Putten H, Botteri FM, Mansuy IM, Meins M, Frey U, Sansig G, Portet C, Schmutz M, Schröder M, Nitsch C, Laurent JP, Monard D. Endogenous serine protease inhibitor modulates epileptic activity and hippocampal long-term potentiation. The Journal of Neuroscience. 1997;17:4688–4699. doi: 10.1523/JNEUROSCI.17-12-04688.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Madison DV, Tsien RW. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988;335:820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Matthies H, Jr, Behnisch T, Kase H, Matthies H, Reymann KG. Differential effects of protein kinase inhibitors on pre-established long-term potentiation in rat hippocampal neurons in vitro. Neuroscience Letters. 1991;121:259–262. doi: 10.1016/0304-3940(91)90699-t. [DOI] [PubMed] [Google Scholar]
- Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- Schubert M, Siegmund H, Pape HC, Albrecht D. Kindling-induced changes in plasticity of the rat amygdala and hippocampus. Learning & Memory. 2005;12:520–526. doi: 10.1101/lm.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan OI, Zhao Q, Miller F, Holmes GL. Interictal spikes in developing rats cause long-standing cognitive deficits. Neurobiology of Disease. 2010;39:362–371. doi: 10.1016/j.nbd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velísková J, Miller AM, Nunes ML, Brown LL. Regional neural activity within the substantia nigra during peri-ictal flurothyl generalized seizure stages. Neurobiology of Disease. 2005;20:752–759. doi: 10.1016/j.nbd.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


