Abstract
Reverse transcription-real time polymerase chain reaction (RT-qPCR) for the VP6 gene was used to study group A rotavirus shedding in children with symptomatic and asymptomatic rotavirus infection. Sequential stool samples (n=345) from ten children with rotavirus associated diarrhea and from five children (n=161) with asymptomatic rotavirus infection were collected over a period of two months. A RT-qPCR assay on the samples using a rotavirus VP6 plasmid standard demonstrated high reproducibility, with an inter-assay coefficient of variation (CV) of 1.40–2.97% and an intra-assay CV of 0.03–3.03%. The median duration of shedding was longer in children with diarrhea compared to asymptomatic children (24 days vs. 18 days) (p=0.066). The median quantitation cycle (Cq) at presentation in symptomatic children was 17.21 compared to 30.98 in asymptomatic children (p=0.086). The temporal pattern in symptomatic children consisted of a high initial viral shedding coinciding with the duration of diarrhea, followed by a rapid fall, and then a small increase in secondary shedding 21 days later. Compared to children with rotavirus diarrhea, those with asymptomatic infection shed lower quantities of virus throughout the observation period. No difference was noted between the G and P genotypes of samples collected at onset of infection and during the shedding period. Shedding was intermittent in a subset of children in both groups. RT-qPCR is a useful method to characterize shedding patterns.
Keywords: rotavirus, RT-qPCR, virus shedding, children
INTRODUCTION
Group A rotaviruses, which belong to the family Reoviridae, are the leading cause of childhood gastroenteritis accounting for over 453,000 deaths in children annually worldwide, with 95% of these occurring in the resource-poor countries [Tate et al., 2012]. The mature rotavirus virion is icosohedral and consists of a triple layered capsid encompassing a genome comprised of 11 segments of double-stranded RNA [Kapikian, 2001]. Rotaviruses are classified into various major groups based on the middle layer VP6 protein and further classification within the group is based on the outer coat VP7 and VP4 proteins [Estes, 1996].
Most rotavirus shedding patterns have been studied in the mouse model of rotavirus infection [Eydelloth et al., 1984; Ward et al., 1990; Burns et al., 1995]. In adult mice challenged with specific murine rotaviruses, a monophasic shedding pattern is noted starting at 1–2 days post administration and persisting till 6 days [Burns et al., 1995]. The viral inoculum needed to cause disease in this model was 2 × 103 plaque-forming units of murine rotavirus [Ward et al., 1990].
On the other hand, studies done on human volunteers demonstrated that just one plaque forming unit of human rotavirus (about 4. 6 × 104 rotavirus particles) could cause clinical disease [Ward et al., 1986]. Children with rotavirus infection have been reported to shed rotavirus prior to the onset of diarrhea and to continue to do so for 10 days from the onset of clinical symptoms [Nagayoshi et al., 1980; Vesikari et al., 1981]. In these studies, rotavirus was detected in feces by the traditional methods of immunoassay, immune electron microscopy, or polyacrylamide gel electrophoresis [Nagayoshi et al., 1980; Vesikari et al., 1981; Miotti et al., 1985]. However, these tests are relatively insensitive and can detect rotavirus only when it is present in quantities greater than 107 virions per gram of feces [Ward et al., 1986]. To detect smaller quantities of rotavirus, reverse transcription PCR (RT-PCR) has been found to be more sensitive, reducing the threshold of detection to as few as 1000 virus particles per ml [Xu et al., 1990; Wilde et al., 1991]. Additionally, PCR has been shown to detect rotavirus in the stool for up to 57 days after the onset of diarrhea, showing that a low level of viral excretion can occur even after abatement of symptoms [Richardson et al., 1998].
Recently, real time PCR has been used to quantify rotavirus infection [Schwarz et al., 2002]. This technique has been found to be more sensitive than both conventional RT-PCR and conventional nested PCR assays in detection of rotavirus infection [Pang et al., 2004]. Quantitation of rotavirus by this method in symptomatic and asymptomatic children has shown that the severity of illness correlates with the quantum of virus excreted [Kang et al., 2004]. In this study, qPCR was used to quantify rotavirus excretion in sequential samples from children who had rotavirus diarrhea or asymptomatic rotavirus infection in order to study the pattern of rotavirus shedding.
MATERIALS AND METHODS
Case recruitment and sample collection
The study was carried out at the Christian Medical College, Vellore. Fecal samples were collected from children less than 5 years of age who were admitted to the hospital with diarrhea. Fecal samples were also collected from asymptomatic children who were part of an ongoing rotavirus birth cohort study in an urban slum area in Vellore, where children were followed twice weekly with home visits by field workers to capture diarrhea and other morbidities. Surveillance fecal samples were collected every two weeks and diarrheal samples collected every time the child had an episode of diarrhea. Children selected for this study had not had a recorded diarrhea or prior asymptomatic infection for 6 months prior to inclusion. The details of recruitment and monitoring of this cohort have been described previously [Banerjee et al., 2006; Gladstone et al., 2008]. The fecal samples were tested for the presence of Group A rotavirus by an enzyme linked immunosorbent assay (Rota IDEIA, Thermo Fischer Scientific, United Kingdom) following the manufacturer’s instructions.
Sequential stool samples were collected from 10 symptomatic and 5 asymptomatic children who were positive for rotavirus. To maximize follow up and effective collection of stool samples, children residing within a six-kilometer radius from the hospital were recruited. To the extent feasible, a trained field worker made daily visits to the children’s homes to collect stool samples. Sample collection was continued for a maximum of sixty days after recruitment. All samples were transported in ice chests to the laboratory where they were logged in and tested immediately by enzyme linked immunosorbent assay (ELISA), weighed, mixed well and aliquotted for storage prior to RNA extraction.
Approval for the study was granted by the Institutional Ethics Committee of Christian Medical College, Vellore and written informed consent was obtained from the parent or guardian of each child.
Clinical assessment
All children admitted to the hospital received standard care for the management of diarrhea. Clinical examination was performed on each subject at the time of admission and a severity score was assigned according to the Vesikari scoring system [Ruuska and Vesikari, 1990].
RNA extraction, cDNA synthesis and genotyping PCR
The fecal samples were weighed and viral RNA was extracted from 200μl of the 10% of fecal suspension in balanced salt solution using guanidium isothiocyanate and silica method [Boom et al., 1990]. RNA was eluted in 50 μl of the RNase- free distilled water containing 40 units of ribonuclease inhibitor (RNAsin, Ambion). Complementary DNA (cDNA) was generated from 40 μl of the extracted RNA by reverse transcription (RT) in the presence of random primers (hexamers; Pd(N)6, Pharmacia Biotech), using Moloney murine leukemia virus reverse transcriptase enzyme (Invitrogen, Life Technologies). The resulting cDNA was the template for both VP7- and VP4-specific typing PCRs, using the oligonucleotide primers described earlier [Gouvea et al., 1990; Gentsch et al., 1992; Iturriza-Gomara et al., 2004].
Preparation of plasmid standards for quantitative PCR
The plasmid used as the standard was constructed by ligation of a 379 bp region of the rotavirus VP6 (inner capsid gene) PCR fragment in TOPO-TA 2.1 vector (Invitrogen). The rotavirus VP6 gene specific plasmid was propagated in Escherichia coli DH5α cells, purified by using a Gene Elute HP Plasmid Miniprep kit (Sigma-Aldrich, USA) and quantified by measuring OD260 using spectrophotometer. The quantity (copies/ml) of plasmid DNA standard was calculated according to a previously described formula [Yin et al., 2001]. Ten-fold dilutions were made in order to get 108–101 plasmid standards per 2μl sample for the qPCR. The dilutions were stored at −20 °C, while stock plasmid was stored at −70 °C.
Reverse transcription-qPCR using SYBR green dye
RT-qPCR using the SYBR green dye chemistry was performed using the real time PCR instrument Chromo4 system (MJ Research/Biorad, USA). The procedure was optimized for concentrations of primers, and denature/extension temperature (data not shown). The optimized reactions were carried out in a 25μl final reaction volume containing 0.5 μl of 20 picomoles forward and reverse VP6 gene specific primers, 12.5 μl of the kit supplied QuantiTect SYBR Green PCR master mix (Qiagen) (including HotStar Taq DNA polymerase, PCR reaction buffer, dNTP mix, SYBR Green I), 7.5 μl of RNAase free water and 2μl of the cDNA obtained by the method described above. The primers VP6-F (sense) (5′ GACGGVGCRACTACATGGT 3′) (nucleotides [nt] 747–766) and VP6-R (antisense) (5′ GTCCAATTCATNCCTGGTG 3′) (nt 1125–1106) amplified a 379-bp region of the VP6 gene. The thermal cycling protocol for the real time PCR was as follows: initial denaturation at 95°C for 15 minutes, followed by 40 cycles of denaturation at 94°C for 1 min, annealing temperature at 55°C for 1 min and extension temperature at 72°C for 1 min, followed by measurement of fluorescence. Serial dilutions of the plasmid containing the rotavirus VP6 gene were included in each run, and served as positive controls and used as normalization between the PCR runs. No template controls (NTC) were included along with the unknown samples in each run.
Melt profile analysis of the PCR product
Melt profile analysis was performed to measure the specificity of PCR product by the presence of single peak at the expected range of 79 to 81°C after PCR cycling products were heated from 45°C to 95°C at a linear transition rate of 0.1°C/10s. Fluorescence of the samples was monitored continuously while the temperature was increasing. The Opticon Monitor (version 3) software was used to calculate the Tm.
Data analysis
The measurement of rotavirus-specific RNA in the sample was an absolute quantitation, calculated by normalizing the quantitation cycle (Cq) values of fecal samples with that of internal standard controls using the Opticon Monitor (version 3) software. The threshold was defined automatically in the initial exponential phase, reflecting the highest amplification rate. For the Cq resulting from the amplification curves and this threshold, a direct relation between the cycle number and the log concentration of RNA molecules initially present in the RT-PCR reaction was evident. By linear regression analysis of these data, the Opticon Monitor (version 3) software set up a standard curve, which allowed the determination of the concentration of RNA present in the samples.
Validation of assays
The reproducibility of the assay with the plasmid derived standard curves was measured by calculating the intra-assay coefficient of variation (CV) by taking the mean CV for duplicate Cq for plasmid standards in all runs of the same assay. The inter-assay precision, measured as CV was determined by comparing the mean Cq for six independent experiments with sets of standard plasmid dilutions prepared prior to testing, with one set of dilutions used in each experiment. The sensitivity of the assay was determined by running 10-fold serial dilutions of a rotavirus positive cDNA with known copy numbers. The lowest dilution in the 10-fold dilution series, which amplified reliably, was defined as the detection limit. Further, a comparison experiment to determine the detection limit of the stool detection ELISA for rotavirus vs. qPCR of the VP6 gene was performed. A known amount of WC3 (G6P[5]) vaccine virus strain (3.4 × 107 fluorescent focused units-FFU/ml) was diluted as shown (Table III) and subjected to ELISA as per manufacturer’s instructions as well as RNA extraction, cDNA conversion and real time PCR was performed as described above.
TABLE III.
Comparison experiments to determine the stool detection limit of ELISA for rotavirus vs. real time PCR quantitation of the VP6 gene
| Standard plasmid copy | Cq values | FFU/ml (WC3 vaccine virus strain) | Cq values | Stool detection ELISA results |
|---|---|---|---|---|
| 7.90E+07 | 11.85 | 1.00E+07 | 12.04 | Positive |
| 7.90E+06 | 15.76 | 1.00E+06 | 16.96 | Positive |
| 7.90E+05 | 19.24 | 1.00E+05 | 19.77 | Positive |
| 7.90E+04 | 24.2 | 1.00E+04 | 23.52 | Negative |
| 7.90E+03 | 28.97 | 1.00E+03 | 29.8 | Negative |
| 7.90E+02 | 32.84 | 1.00E+02 | 33.01 | Negative |
Statistical analysis
Data were analyzed using STATA v. 10.1 (StataCorp, College Station, TX, USA) and R v. 2.15.1 (http://www.r-project.org/) software. Median duration of rotavirus excretion and the initial Cq values in symptomatic and asymptomatic children were compared using the Mann-Whitney U test. The overall pattern of virus shedding in symptomatic and asymptomatic children over time was assessed by fitting a smoothed curve of the Cq values using the LOESS (locally weighted least squares regression) smoothing technique [Gijbels and Prosdocimi, 2010].
RESULTS
Real–time PCR assay performance
The reproducibility of the real-time PCR method was assessed within (intra-assay) and between (inter-assay) runs of the assay on different days of testing. The Cq values obtained from the rotavirus plasmid standard were used for assessment, and the coefficient of variation (CV) was determined among the Cq value of the eight dilutions. Six independent assays performed for each dilution showed CVs in the range of 1.40–2.97%, indicating a high reproducibility of the assay (Table I). Similarly, the intra-assay variation, determined by tests carried out in duplicate in each run, had a CV in the range of 0.03–3.03%.
TABLE I.
Variations in Ct values of Real Time PCR in different runs with rotavirus plasmid standards
| Plasmid Dilutions | Cq values
|
Mean +/− SDa (CVb) | |||||
|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | Run 6 | ||
| 1 | 6.42 | 6.61 | 6.51 | 6.34 | 6.4 | 6.27 | 6.43 +/− 0.1211 (1.89) |
| 2 | 9.56 | 9.69 | 9.17 | 9.4 | 8.93 | 9.23 | 9.33 +/− 0.2768 (2.97) |
| 3 | 12.53 | 12.94 | 13.02 | 12.72 | 12.38 | 12.59 | 12.70 +/− 0.2465 (1.94) |
| 4 | 15.96 | 16.25 | 16.39 | 16.35 | 15.89 | 15.36 | 16.03 +/− 0.3883 (2.42) |
| 5 | 19.1 | 19.66 | 19.28 | 19.73 | 19.19 | 19.16 | 19.35 +/− 0.2719 (1.40) |
| 6 | 22.52 | 23.06 | 23.03 | 23.13 | 23.04 | 22.39 | 22.86 +/− 0.3196 (1.40) |
| 7 | 25.7 | 25.79 | 26.76 | 26.76 | 26.89 | 25.72 | 26.27 +/− 0.5869 (2.23) |
| 8 | 29.69 | 29.46 | 28.92 | 28.37 | 29.57 | 29.21 | 29.20 +/− 0.4923 (1.69) |
Standard deviation
Coefficient of variation (%)
Patient characteristics
Symptomatic children
A total of ten children with diarrhea were followed for 60 days. The baseline characteristics are summarized in Table II. The median age of the children was 8 months [Inter-quartile range (IQR), 6.5–11.0 months] and six were male. All of them had moderate to severe symptoms with a median Vesikari score of 11 (IQR, 11–13). The median duration of diarrhea in these children was 4 days (IQR, 2.5–6 days). Seven of these children were treated with IV fluids and oral rehydration solution whereas the rest were treated with oral rehydration solution alone. Five children were being breast fed at the time of presentation with symptoms. One child had an additional diagnosis of acyanotic heart disease and another had presented with exanthematous fever in addition to the diarrhea. A total of 345 samples of stool were collected from the ten symptomatic children and analyzed by real time PCR. The median number of stool samples collected from each child in this group was 34 (IQR, 26–39) samples.
TABLE II.
Clinical and viral characteristics of symptomatic and asymptomatic children
| ID no | Date of collection | Gender | Age (months) | Vesikari Score | Initial viral load (Cq values) | Duration of RV excretion(days) | Duration of diarrhea | RV genotype | |
|---|---|---|---|---|---|---|---|---|---|
| Symptomatic | |||||||||
| Case 1 | RV 338 | 20-Oct-03 | M | 6 | 11 | 14.36 | 31 | 3 | G1P[8] |
| Case 2 | CRI 13332 | 24-Nov-03 | F | 8 | 6 | 8.33 | 23 | 3 | G1P[8] |
| Case 3 | CRI 42538 | 23-Aug-06 | M | 7 | 13 | 23.96 | 33 | 4 | G1P[8] |
| Case 4 | ICRV 310 | 09-Aug-06 | M | 7 | 11 | 12.53 | 14 | 2 | G1P[8] |
| Case 5 | ICRV 329 | 29-Aug-06 | F | 9 | 11 | 17.28 | 25 | 4 | G2P[4] |
| Case 6 | ICRV 366 | 05-Oct-06 | F | 9 | 11 | 17.14 | 22 | 6 | G2P[4] |
| Case 7 | ICRV 372 | 10-Oct-06 | M | 13 | 12 | 25.84 | 36 | 6 | G2P[4] |
| Case 8 | ICRV 434 | 14-Dec-06 | F | 36 | 7 | 16.77 | 23 | 2 | G2P[4] |
| Case 9 | ICRV 460 | 23-Jan-07 | M | 3 | 16 | 21.03 | 17 | 4 | G2P[4] |
| Case 10 | ICRV 502 | 08-Mar-07 | M | 8 | 14 | 29.42 | 29 | 6 | G9P[UT] |
| Asymptomatic | |||||||||
| Case 1 | CRI 13129 | 18-Nov-03 | M | 10 | - | 7.61 | 25 | - | G1P[8] |
| Case 2 | CRI 14465 | 24-Dec-03 | M | 15 | - | 31.50 | 14 | - | G2P[UT] |
| Case 3 | CRI 14482 | 24-Dec-03 | F | 8 | - | 31.54 | 19 | - | G1P[UT] |
| Case 4 | CRI 14486 | 24-Dec-03 | M | 17 | - | 30.98 | 18 | - | G2P[4] |
| Case 5 | CRI 14756 | 02-Jan-04 | M | 12 | - | 29.38 | 8 | - | G2P[4] |
Asymptomatic children
A total of five asymptomatic children who were found to be positive for rotavirus in their surveillance stool samples were also followed up. The individual data has been represented in Table II. The median age of these children was 12 months (IQR, 9–16 months) and four were male. A total of 161 samples were collected, with a median of 31 (IQR, 27–39) samples.
Characterization of rotavirus shedding by Real Time PCR
Pattern of rotavirus shedding
Symptomatic children
The median duration of viral shedding of rotavirus in the children with diarrhea was 24 days (IQR, 22–31) days. All children excreted virus for at least 14 days and the maximal duration of shedding was 51 days. Detectable excretion of rotavirus ceased between 14–21 days in 2 patients, between 21–28 days in 4 patients and extended beyond 28 days in the remaining 4 patients. Rotaviruses excreted at the time of entry into the study were typed as G2P[4] in 5 patients, G1P[8] in 4 patients and G9P[UT] in the remaining patient. Samples collected in the second and third week from onset of the rotavirus episode were also genotyped and there was no change in either the G type or P type in any of these samples from that taken at the beginning of surveillance.
Asymptomatic children
The median duration of viral shedding of rotavirus in these children was 18 days (IQR, 14–19) days. These asymptomatic children excreted virus for at least 8 days with the maximal duration of shedding being 25 days. Rotavirus excretion ceased within 14 days in 1 child, between 14–21 days in 3 children and between 21–28 days in the remaining child. None of the asymptomatic children excreted virus beyond 28 days. Although the duration of viral shedding was less in asymptomatic children, the difference was not statistically significant (Mann-Whitney U test, p=0.066).
Viral quantitation
Symptomatic children
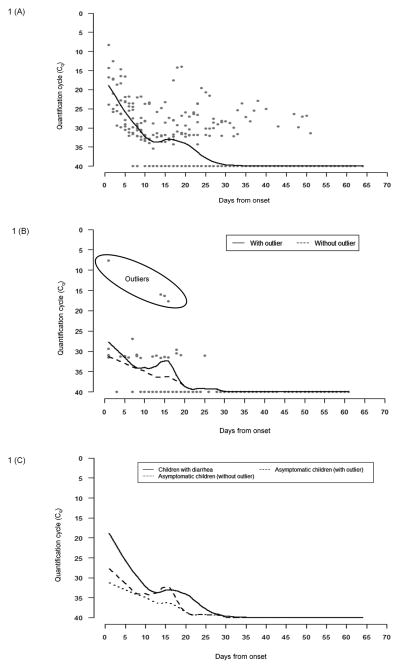
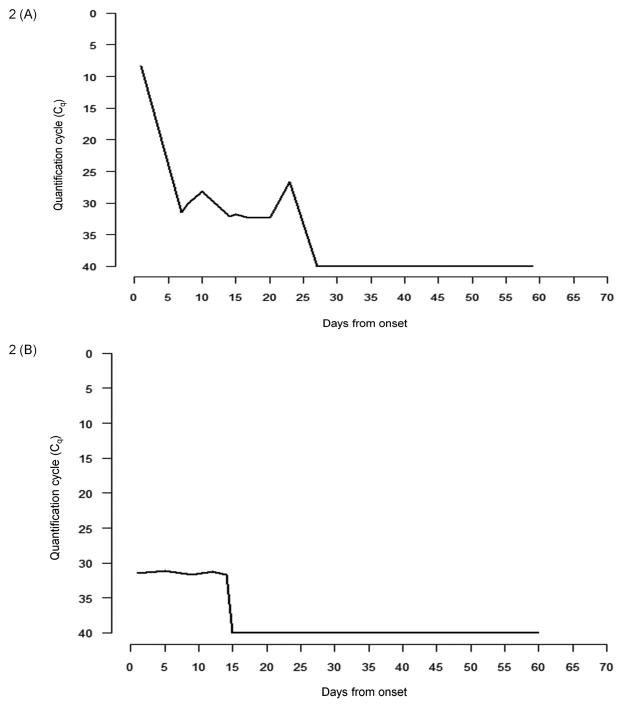
The initial median Cq value in symptomatic children was 17.21 (IQR, 14.36–23.96). The smoothed LOESS curve depicting the Cq values of all children at various time points is presented in Figure 1A and the Cq value in one child (CRI 13332) is depicted in Figure 2A, as an example. The LOESS curve shows very low Cq values at the onset of symptoms, which suggests a high rotavirus shedding (primary), followed by another small dip in Cq values at around 21 days suggesting a slight increase in the shedding (secondary) of the virus. The shedding pattern was continuous in four of ten symptomatic patients, where once shedding stopped, stool samples remained negative during follow-up. In the remaining patients there was initial shedding followed by intervening samples that were negative followed by positive stool samples subsequently, suggestive of intermittent rotavirus shedding.
Figure 1.
VP6 real-time PCR quantitation cycle (Cq) depicting the rotavirus shedding in (A) symptomatic and (B) asymptomatic children relative to days after onset of diarrhea or onset of surveillance, and (C) comparison of the smoothed curves between symptomatic and asymptomatic children. The dots represent the quantitation cycle (Cq) points, whereas the line shows the smoothed values obtained from the LOESS smoother.
Figure 2.
Quantitation cycle (Cq) for rotavirus shedding and the temporal shedding pattern in one (A) symptomatic (CRI 13332) and (B) asymptomatic (CRI 14465) child.
Asymptomatic children
The median Cq value in asymptomatic children was 30.98 (IQR, 29.38–31.50). The difference in initial Cq values between the symptomatic and asymptomatic group was not found to be statistically significant (Mann-Whitney U Test, p=0.086). However, there was one outlier in the asymptomatic group (CRI 13129, Table II) with a very high initial viral shedding compared to the rest of the asymptomatic cases. On removing this outlier, the difference between the initial shedding between symptomatic and asymptomatic samples was found to be statistically significant (Mann-Whitney U Test, p=0.007). When the shedding patterns in asymptomatic children were analyzed, two of the five children were found to have continuous rotavirus shedding while the other three shed intermittently. The pattern of virus shedding in asymptomatic children is presented as a smoothed curve in Figure 1B and the shedding pattern in one asymptomatic child (CRI 14465) is depicted in Figure 2B. The smoothed curve for asymptomatic children seemed to resemble the symptomatic shedding pattern, i.e. an initial high rotavirus shedding (depicted by low Cq values) followed by a rapid decline and then a small increase (secondary) approximately 15 days later. The increased secondary excretion was observed in only one child (CRI 14486, marked as an outlier in Figure 1B) and disappeared on removing the outliers. On comparing the smoothed curves of virus shedding over time, it was found that children with asymptomatic infection shed lower quantities of virus than those with symptomatic infection, throughout the period of observation (Figure 1C).
DISCUSSION
This study used qPCR as a tool for characterizing rotavirus shedding in symptomatic and asymptomatic children. Previous longitudinal studies utilizing less sensitive modes of detection of rotavirus had concluded that rotavirus excretion ceased within ten days of onset of symptoms [Nagayoshi et al., 1980; Vesikari et al., 1981]. In this study, the median duration of shedding in the symptomatic and asymptomatic children was found to be 24 days and 18 days, respectively. The majority of symptomatic children (90%) were found to shed virus beyond two weeks of onset of symptoms and a large proportion (40%) had viral excretion beyond 28 days. In contrast, none of the asymptomatic children shed virus beyond 28 days. However, it is important to note that in the surveillance program, children without diarrhea were sampled every two weeks, so the date of onset of asymptomatic infection is not known.
Four of the five asymptomatic children in this study had high initial Cqs, suggestive of lower virus shedding than in symptomatic children (Table II). This is consistent with the results of an earlier cross-sectional study, where the mean Cq for asymptomatic children was found to be higher than the symptomatic children [Kang et al., 2004]. However, one child had initial Cq values comparable to that of symptomatic children with prolonged virus shedding, which possibly suggests a ‘carrier’ state, since no history of diarrhea was obtained. A type of carrier state has also been described in mice, which excrete virus for prolonged periods of time [Kraft, 1961]. Such children may act as a potential source of infection for their households and communities.
Real time PCR also gives a useful insight into the pattern of viral shedding in human subjects. The peak shedding of rotavirus occurs in the first week after the onset of symptoms (Figure 1A). This coincides with the duration of diarrhea, which extended for a maximum of 6 days. Following this period, there is a sharp decline in virus excretion. Around the 21st day there was a slight increase in shedding (Fig. 1A), which was quite distinctive in some children. By genotyping there was no difference between the G and P types of the primary and secondary ‘infections’, thereby indicating that this was possibly a result of recrudescence of viral shedding rather than re-infection with a new viral strain. Intermittent shedding was seen in both symptomatic and asymptomatic children. Possible explanations for these findings, other than recrudescent shedding, could be false negativity of the qPCR due to very low viral load, reinfection with the same genotype, or physiological variations in rotavirus excretion following extrusion of infected villous cells in human subjects.
In a study in southern India, the gut transit time in healthy volunteers was estimated to be around 24 hours [Jayanthi et al., 1989]. The demonstration of viral excretion for a prolonged period of time (24 days symptomatic vs. 18 days for asymptomatic) indicates infection with prolonged viral replication rather than the slow clearance of a viral inoculum. Given the long duration of the shedding, lack of steady tapering and the secondary peak seen in some children, it is likely that these findings represent ongoing viral replication rather than a slow clearance of virus for the intestinal tract following an infection. Studies in children infected with rotavirus have shown that cycles of virus replication with alternating cycles of antibodies become stable over time, thus resulting in a low-concentration infection [Richardson et al., 1998]. The dogma of rotavirus being an exclusively enteric infection has been disproved by the finding of rotavirus RNA in blood and extraintestinal tissue of children presenting with rotavirus diarrhea [Blutt et al., 2003; Chiappini et al., 2005; Fischer et al., 2005]. Viraemia and/or biliary seeding by rotavirus could be a possible precursor to reinfection of enterocytes and explain the smaller secondary peak of rotavirus shedding. Presence of rotavirus antigen in the biliary epithelium has been confirmed in both animal models and humans suggesting that infected bile could be a mode of re-introduction of this pathogen to enterocytes [Qiao et al., 1999; Allen et al., 2007].
The occurrence of symptoms and viral excretion also depend on host factors. One of the key determinants of the host response is previous exposure to rotavirus infection. Cohort studies from Mexico, Guinea-Bissau and India have concluded that natural rotavirus infection conferred varying degrees of protection against subsequent infection [Velazquez et al., 1996; Fischer et al., 2002; Gladstone et al., 2011]. This protection increased with each new infection and reduced the severity of the diarrhea. It is likely that variations in rotavirus excretion among children with and without symptoms are determined by local or systemic immune responses, which in turn are modulated by previous exposure to rotavirus infection.
This study has some limitations. First, it was conducted on a small number of children, for whom appropriate serum and other specimens were not available. A larger group of symptomatic and asymptomatically infected children could have provided more robust estimates. Second, even though we did not find any difference in the genotype patterns between the initial and subsequent samples, the genotyping results could not confirm re-infection with the same strain. Sequence analysis of the samples would have provided confirmatory evidence of persistence of the same strain.
In conclusion, qPCR is a useful tool to detect and quantify virus in stools from children, both with and without diarrhea, acting as a possible surrogate for measurement of viral replication. Use of quantitation techniques on sequential samples in conjunction with prior exposure history and intensive investigation of antigenemia and antibody and cell-mediated immune response will enhance our understanding of the pathogenesis of rotavirus infection. In this study, the extended periods of viral excretion in these children could explain the large burden of rotavirus infection in the community, which appears to result in early exposure [Banerjee et al., 2007; Gladstone et al., 2011]. Future careful clinical and laboratory studies will further understanding of the natural history of rotavirus infection and the effect of a protective immune response induced by natural infection or vaccination on viral replication in children.
Acknowledgments
This work was supported by the Wellcome Trust under the Trilateral Cooperative Initiative for Research in Infectious Diseases in the Developing World (Grant No. 063144). RS, VKM, SB, AP and PR were supported by the Fogarty International Center Global Infectious Disease Research Training Program (Grant No. D43 TW007392).
Footnotes
Conflict of interests:
The authors declare that they have no conflict of interests.
Authors’ Contributions:
The contribution of each author towards the manuscript is as follows: GK, MIG and JJG conceived and designed the study; TVS and PDM helped in data collection and interpretation of the results; IM, VKM, SB, AP and PR conducted the laboratory assays for rotavirus testing; RS and TVS performed the statistical analysis. IM, RS, VKM and SB prepared the draft of the manuscript and MIG, JJG and GK provided critical inputs for revising the manuscript.
References
- Allen SR, Jafri M, Donnelly B, McNeal M, Witte D, Bezerra J, Ward R, Tiao GM. Effect of rotavirus strain on the murine model of biliary atresia. J Virol. 2007;81:1671–1679. doi: 10.1128/JVI.02094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I, Ramani S, Primrose B, Moses P, Iturriza-Gomara M, Gray JJ, Jaffar S, Monica B, Muliyil JP, Brown DW, Estes MK, Kang G. Comparative study of the epidemiology of rotavirus in children from a community-based birth cohort and a hospital in South India. J Clin Microbiol. 2006;44:2468–2474. doi: 10.1128/JCM.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I, Gladstone BP, Le Fevre AM, Ramani S, Iturriza-Gomara M, Gray JJ, Brown DW, Estes MK, Muliyil JP, Jaffar S, Kang G. Neonatal infection with G10P[11] rotavirus did not confer protection against subsequent rotavirus infection in a community cohort in Vellore, South India. J Infect Dis. 2007;195:625–632. doi: 10.1086/510853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutt SE, Kirkwood CD, Parreno V, Warfield KL, Ciarlet M, Estes MK, Bok K, Bishop RF, Conner ME. Rotavirus antigenaemia and viraemia: a common event? Lancet. 2003;362:1445–1449. doi: 10.1016/S0140-6736(03)14687-9. [DOI] [PubMed] [Google Scholar]
- Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JW, Krishnaney AA, Vo PT, Rouse RV, Anderson LJ, Greenberg HB. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143–153. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- Chiappini E, Azzari C, Moriondo M, Galli L, de Martino M. Viraemia is a common finding in immunocompetent children with rotavirus infection. J Med Virol. 2005;76:265–267. doi: 10.1002/jmv.20351. [DOI] [PubMed] [Google Scholar]
- Estes M. Rotaviruses and their replication. In: Knipe BNFDM, Howley PM, editors. Fields Virology. 3. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 1625–1655. [Google Scholar]
- Eydelloth RS, Vonderfecht SL, Sheridan JF, Enders LD, Yolken RH. Kinetics of viral replication and local and systemic immune responses in experimental rotavirus infection. J Virol. 1984;50:947–950. doi: 10.1128/jvi.50.3.947-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TK, Valentiner-Branth P, Steinsland H, Perch M, Santos G, Aaby P, Molbak K, Sommerfelt H. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, west Africa. J Infect Dis. 2002;186:593–597. doi: 10.1086/342294. [DOI] [PubMed] [Google Scholar]
- Fischer TK, Ashley D, Kerin T, Reynolds-Hedmann E, Gentsch J, Widdowson MA, Westerman L, Puhr N, Turcios RM, Glass RI. Rotavirus antigenemia in patients with acute gastroenteritis. J Infect Dis. 2005;192:913–919. doi: 10.1086/432549. [DOI] [PubMed] [Google Scholar]
- Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, Das BK, Bhan MK. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijbels I, Prosdocimi I. Loess. Wiley Interdiscip Rev Comput Stat. 2010;2:590–599. [Google Scholar]
- Gladstone BP, Muliyil JP, Jaffar S, Wheeler JG, Le Fevre A, Iturriza-Gomara M, Gray JJ, Bose A, Estes MK, Brown DW, Kang G. Infant morbidity in an Indian slum birth cohort. Arch Dis Child. 2008;93:479–484. doi: 10.1136/adc.2006.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, Desselberger U, Crawford SE, John J, Babji S, Estes MK, Kang G. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011;365:337–346. doi: 10.1056/NEJMoa1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, Fang ZY. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gomara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Jayanthi V, Chacko A, Gani IK, Mathan VI. Intestinal transit in healthy southern Indian subjects and in patients with tropical sprue. Gut. 1989;30:35–38. doi: 10.1136/gut.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Iturriza-Gomara M, Wheeler JG, Crystal P, Monica B, Ramani S, Primrose B, Moses PD, Gallimore CI, Brown DW, Gray J. Quantitation of group A rotavirus by real-time reverse-transcription-polymerase chain reaction: correlation with clinical severity in children in South India. J Med Virol. 2004;73:118–122. doi: 10.1002/jmv.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DMH, editor. Fields virology. 4. Philadelphia: Lippincott-Raven Publishers; 2001. pp. 1787–1833. [Google Scholar]
- Kraft LM. Responses of the mouse to the virus of epidemic diarrhea of infant mice. Neutralizing antibodies and carrier state. Proc Anim Care Panel. 1961;11:125–136. [Google Scholar]
- Miotti PG, Eiden J, Yolken RH. Comparative efficiency of commercial immunoassays for the diagnosis of rotavirus gastroenteritis during the course of infection. J Clin Microbiol. 1985;22:693–698. doi: 10.1128/jcm.22.5.693-698.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayoshi S, Yamaguchi H, Ichikawa T, Miyazu M, Morishima T, Ozaki T, Isomura S, Suzuki S, Hoshino M. Changes of the rotavirus concentration in faeces during the course of acute gastroenteritis as determined by the immune adherence hemagglutination test. Eur J Pediatr. 1980;134:99–102. doi: 10.1007/BF01846024. [DOI] [PubMed] [Google Scholar]
- Pang XL, Lee B, Boroumand N, Leblanc B, Preiksaitis JK, Yu Ip CC. Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction (RT-PCR) assay in stool specimens from children with diarrhea. J Med Virol. 2004;72:496–501. doi: 10.1002/jmv.20009. [DOI] [PubMed] [Google Scholar]
- Qiao H, Zhaori G, Jiang Z, Chen Y, Chen Y, Hou D. Detection of group C rotavirus antigen in bile duct and liver tissues of an infant with extrahepatic biliary atresia. Chin Med J (Engl) 1999;112:93–95. [PubMed] [Google Scholar]
- Richardson S, Grimwood K, Gorrell R, Palombo E, Barnes G, Bishop R. Extended excretion of rotavirus after severe diarrhoea in young children. Lancet. 1998;351:1844–1848. doi: 10.1016/S0140-6736(97)11257-0. [DOI] [PubMed] [Google Scholar]
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- Schwarz BA, Bange R, Vahlenkamp TW, Johne R, Muller H. Detection and quantitation of group A rotaviruses by competitive and real-time reverse transcription-polymerase chain reaction. J Virol Methods. 2002;105:277–285. doi: 10.1016/s0166-0934(02)00118-0. [DOI] [PubMed] [Google Scholar]
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Sarkkinen HK, Maki M. Quantitative aspects of rotavirus excretion in childhood diarrhoea. Acta Paediatr Scand. 1981;70:717–721. doi: 10.1111/j.1651-2227.1981.tb05774.x. [DOI] [PubMed] [Google Scholar]
- Ward RL, Bernstein DI, Young EC, Sherwood JR, Knowlton DR, Schiff GM. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis. 1986;154:871–880. doi: 10.1093/infdis/154.5.871. [DOI] [PubMed] [Google Scholar]
- Ward RL, McNeal MM, Sheridan JF. Development of an adult mouse model for studies on protection against rotavirus. J Virol. 1990;64:5070–5075. doi: 10.1128/jvi.64.10.5070-5075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J, Yolken R, Willoughby R, Eiden J. Improved detection of rotavirus shedding by polymerase chain reaction. Lancet. 1991;337:323–326. doi: 10.1016/0140-6736(91)90945-l. [DOI] [PubMed] [Google Scholar]
- Xu L, Harbour D, McCrae MA. The application of polymerase chain reaction to the detection of rotaviruses in faeces. J Virol Methods. 1990;27:29–37. doi: 10.1016/0166-0934(90)90143-4. [DOI] [PubMed] [Google Scholar]
- Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Putten KV, McCaughan GW, Eris JM, Bishop GA. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunol Cell Biol. 2001;79:213–221. doi: 10.1046/j.1440-1711.2001.01002.x. [DOI] [PubMed] [Google Scholar]