Abstract
OBJECTIVE
6-mercaptopurine (6-MP) is efficacious in the treatment of inflammatory bowel disease (IBD). However, about one-third of patients respond poorly to therapy. This study aimed to characterize the inherent differences in 6-MP transport that may contribute to the differences in treatment responses.
METHODS
Intracellular 6-MP accumulation was assayed in Epstein–Barr virus (EBV)-transformed lymphocytes from IBD patients, using 14C-radiolabeled 6-MP. Cell proliferation was determined by methyl thiazolyl tetrazolium (MTT) assay. Apoptosis was assayed based on the activation of caspase 3. The expressions of 15 potential 6-MP transporters were evaluated by reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS
Intracellular 6-MP accumulation, varying significantly among patients, was carrier-dependent and partially sodium-dependent. 6-MP cytotoxicity was, at least in part, due to apoptosis and correlated with intracellular drug accumulation. The efflux transporters did not appear to contribute to the variability of intracellular drug accumulation between patients, since none correlated with drug accumulation or cyto-toxicity. Rather, differential expression of five influx/uptake transporters might be a key contributor to the difference in the accumulation of and susceptibility to the drug.
CONCLUSIONS
The heterogeneity of the drug transporters may be the reason for the therapeutic sensitivity of 6-MP in IBD patients. As the 6-MP uptake is a carrier-mediated and partially sodium-dependent process, future studies are necessary to evaluate the role of the putative transporters and their correlation with drug sensitivity in patients.
Keywords: 6-mercaptopurine/azathioprine, Crohn’s disease, drug resistance, drug transport/uptake, inflammatory bowel disease
INTRODUCTION
6-mercaptopurine (6-MP) and its prodrug azathioprine (AZA) are nucleobase analogues used frequently in the treatment of inflammatory bowel disease (IBD). Although 6-MP and AZA have proven efficacy in IBD, only about two-thirds of patients receiving 6-MP treatment respond to the drug.1–4 Since it is known to act intracellularly, variations in drug transport or intracellular accumulation may contribute to differences in clinical response.
AZA is rapidly converted to 6-MP by a non-enzymatic reaction in the liver.5 6-MP is taken up by a variety of cells and tissues, including erythrocytes as well as T and B lymphocytes.6 The cellular uptake of 6-MP is believed to be a rapid process. Study of oral administration of AZA in mice showed peak plasma concentrations of AZA and metabolites as early as 10 minutes after drug administration, followed by a rapid decline.7 In a pharmacokinetic study of 6-MP in renal transplant patients, plasma concentrations of 6-MP were low and unquantifiable by high pressure liquid chromatography (HPLC) in any patient 8 hours after oral AZA administration.8
Once inside the cells, 6-MP is metabolized to its active metabolites, the 6-thioguanine nucleotides (6-TGNs). 6-TGNs, because of their structural similarity to the endogenous base-pair guanine, are incorporated into DNA of leukocytes and cause strand breakage.9,10 One 6-TGN, 6-thioguanine triphosphate, has been shown to increase apoptosis in both peripheral blood and intestinal lamina propria T cells via the inhibition of Rac1, a glutamyl transpeptidase that inhibits apoptosis.11 6-MP has also been shown to induce apoptosis in lipopolysaccharide (LPS)-activated splenic B lymphocytes.12 Physiological nucleobase and nucleosides and their analogues are hydrophilic and are thought to require transport proteins to cross the cell membrane at physiologically sufficient rates.13,14 Little is known about the specifics of nucleobase transport and thus, the process of 6-MP transport is poorly understood. Transport of nucleobases by nucleoside transporters has been described.15 Multiple uptake transporters expressed in human tissues may contribute to intracellular accumulation of 6-MP, including equilibrative nucleoside transporter (ENT)-1, -2, -3, -4 and concentrative nucleoside transporter (CNT)-1, -2, -3.16–18 CNT-1 and -2 depend on sodium as a co-transported cation, whereas CNT-3 may co-transport sodium but also lithium or hydrogen. The equilibrative transporters are not sodium-dependent.19
Inherent variation in the intracellular accumulation of 6-MP may lead to differences in susceptibility to drug cytotoxicity. Thus, we evaluated intracellular accumulation of 6-MP and susceptibility to 6-MP cytotoxicity in a panel of transformed human lymphocytes derived from control and IBD patients. The expression of 15 potential nucleoside transporters in these cell lines was examined in an attempt to identify the responsible transporters by correlating them with intracellular 6-MP accumulation.
MATERIALS AND METHODS
Cell lines
Under a Johns Hopkins Medicine institutional review board-approved protocol, peripheral blood mono-nuclear cells (PBMCs) were isolated from 10 patients with IBD (Table 1). The informed consents were obtained prior to their enrollment in the study. Thiopurine S-methyltransferase genotype or enzyme activity was not available for most of the patients. The cells from 5 mL blood (approximately 1 × 106 PBMCs/mL) were Epstein–Barr virus (EBV)-transformed according to the standard protocol at the Johns Hopkins Cell Center.20,21 The transformed lymphocytes were cultured in suspension in RPMI 1640 medium supplemented with 10% fetal bovine serum and 0.5% penicillin/streptomycin in a 5% carbon dioxide to 95% oxygen atmosphere at 37°C.
Table 1.
Summary of the characteristics of the patients from whom Epstein–Barr virus-transformed lymphocytes were derived
| Patient | Age (years) | Age at diagnosis (years) | Gender | Race | Disease type | Disease location | Type of disease | Exposure to 6-MP | Surgery history |
|---|---|---|---|---|---|---|---|---|---|
| B | 49 | 12 | M | W | CD | Ileocolon | Penetrating | No | No |
| C | 29 | 14 | F | W | CD | Perianal location | Penetrating | Yes | Yes |
| D | 52 | 23 | M | W | CD | Ileocolon | Stricturing/penetrating | NA | Yes |
| E | 22 | 6 | F | W | CD | Ileocolon, Stomach | Inflammatory | Yes | No |
| F | 46 | 25 | F | W | UC | Colon | Inflammatory | NA | No |
| G | 61 | 29 | F | W | CD | Ileocolon | Penetrating | No | Yes |
| H* | 34 | 11 | F | W | CD | Colon, small bowel | Stricturing | Yes | Yes |
| J | 50 | 32 | F | W | CD | Ileocolon | Penetrating | No | Yes |
| K* | 75 | 36 | F | W | CD | Ileocolon | Penetrating | Yes | No |
| L | 18 | 12 | F | W | CD | Colon, stomach | Inflammatory | Yes | No |
B to L in the first column refer to different patients.
M, male; F, female; W, white/Caucasian; CD, Crohn’s disease; UC, ulcerative colitis; 6-MP, 6-mercaptopurine; NA, not available.
H*, the patient whose transformed lymphocytes are the most resistant to 6-MP; K*, the patient whose transformed lymphocytes are the most sensitive to 6-MP.
Chemicals
Unless specified, all chemicals used in this study are from Sigma-Aldrich (St Louis, MO, USA).
Transport assay
Cell counts were first determined using a hemacytometer. A standard curve was then created for each cell line by correlating cell counts with optical density (OD) at 600 nm on a Beckman DU-640 spectrophotometer (Beckman Coulter, Brea, CA, USA). Subsequent cell counting was done by measuring OD at 600 nm. The transport assay was done in 150 μL volume (1 × 106 cells). 14C-radiolabeled 6-MP (Moravek Biochemicals, Brea, CA, USA) was dissolved in water with 1 mol/L sodium hydroxide (pH 11) at a concentration of 3.8 μg/mL (125 mCi/mL). This solution was then added to each cell aliquot to make a final drug concentration of 0.05 μg/mL (33 μmol/L or 0.24 μCi of isotope per reaction). In previous study with human T lymphocytes, culture with AZA concentrations of 5 μmol/L led to an intracellular 6-thioguanine level of 168 pmol/mg DNA by HPLC at day 5.11 These concentrations were comparable to those reported in leukocytes of patients with Crohn’s disease (CD) receiving long-term AZA or 6-MP treatment (100–2 305 pmol/mg DNA).6 After the 14C-radiolabeled 6-MP was added, the cells were incubated at 37°C. The addition of drug at time 0 was done on ice. Each reaction was stopped using 1 mL ice-cold phosphate-buffered saline (PBS, pH 7.4) over time, as indicated. The cells were immediately centrifuged at 4°C for 4 min at 3000 × g and the cell pellets were washed thrice with ice-cold PBS. The pellet was resuspended in radioimmunoprecipitation assay (RIPA) buffer, pH 7.5 (150 mmol/L sodium chloride [NaCl], 14 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], 1% Triton X-100, 1% dexoycholate, 0.1% sodium dodecylsulfate [SDS], 10 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L dithiothreitol and 1 mmol/L sodium vanadate) and transferred to a scintillation vial. Scintillation fluid was added for solubilization and the samples were counted on a Beckman scintillation counter (Beckman Coulter, Brea, CA, USA). At least two independent experiments were done for each cell line, with each experiment done in triplicate.
Differentiating simple diffusion from carrier-mediated transport
The transport assay was run according to the above protocol at 0°C for 0 (control) and 60 min (to assess simple diffusion) and at 37°C for 60 min (to assess carrier-mediated transport). Each assay was done at least in triplicate.
Competitive inhibition of 6-MP transport
The transport assay was done in 150 μL volume (1 × 106 cells). Non-radiolabeled 6-MP was added to each reaction at a final concentration of 5 μg/mL. 14C-radiolabeled 6-MP was then added to each reaction at a concentration of 0.05 μg/mL (100-fold less drug). Control samples were done with the addition of an equal volume of water (pH 11) in place of the non-radiolabeled drug, in order to maintain pH and volume consistency. The transport assay was performed, as above, at 37°C for 60 min. Each assay was done at least in triplicate.
Determining 6-MP transport under sodium-free conditions
The transport assay was done in 150 μL volume (1 × 106 cells). Cells from each line were washed thrice in buffer warmed to 37°C, either sodium-containing HEPES buffer, pH 7.4 (5 mmol/L HEPES, 135 mmol/L NaCl, 5 mmol/L potassium chloride [KCl], 3.33 mmol/L monosodium phosphate, 0.83 mmol/L disodium phosphate, 1 mmol/L calcium chloride [CaCl2], 1 mmol/L magnesium chloride [MgCl2] and 10 mmol/L glucose) or sodium-free HEPES buffer, pH 7.2 (5 mmol/L HEPES, 140 mmol/L N-methyl-D-glutamine, 5 mmol/L monopotassium phosphate, 1 mmol/L CaCl2, 1 mmol/L MgCl2 and 10 mmol/L glucose). The cells were then resuspended in the respective buffer at a volume of 150 μL and 14C-radiolabeled 6-MP was added to each tube at a final concentration of 0.05 μg/mL. The cells were incubated at 37°C for 60 min and the reaction was then stopped by adding 1 mL ice-cold PBS. The cells were immediately centrifuged and washed thrice with ice-cold PBS. The pellet was resuspended in RIPA buffer (pH 7.5) and transferred to a scintillation vial. Scintillation fluid was added for solubilization and the samples were counted on a scintillation counter. Each assay was done at least in triplicate.
Colorimetric cell proliferation methyl thiazolyl tetrazolium (MTT) assay to determine cell viability after culture with 6-MP
The MTT assay (Roche Applied Science, Indianapolis, IN: USA) is a standard colorimetric assay to determine cell proliferation and viability. This assay has also been used for the measurement of cytotoxicity.22,23 The MTT assay was performed on lines B, D, F, H, J, K and L. Cells were plated at equal numbers (3 × 105 cells) in 6-well plates with the addition of varying concentrations of 6-MP (0, 0.001, 0.01, 0.1, 1, 10 μg/mL). The cells were cultured with 6-MP for either 3 or 12 days. The MTT assay was run according to the manufacterer’s instructions. The plate was analyzed for OD at 570 nm using an ELISA reader (Dynex Technologies MRX-TC Revelation Microtiter Plate Reader, Chantilly, VA, USA). Cell proliferation rate was calculated by the following method: OD of test well/OD of control well × 100%.
Total cellular protein profiles and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression after culture of lymphocyte lines with 6-MP, as an indicator of cell susceptiblity to 6-MP cytotoxicity
Lymphocytes (1 × 106 cells/well in 6-well plate) were cultured with 6-MP at various concentrations (0, 0.01, 0.1, 1, 10 and 100 μg/mL) for 72 h. The cells were collected and washed thrice with cold PBS buffer, resuspended and lysed in an equal volume of HEPES buffer, pH 7.4 containing 1% Triton X-100. The protein concentration of the control cells (grown without 6-MP), which had the highest number of cells (and therefore contained highest amount of total proteins), was measured using Bio-Rad Protein Assay solution (Bio-Rad, Hercules, CA, USA). An equal volume of cell lysate from each treatment condition was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and electrically blotted to a nitrocellulose membrane (45 μg of total protein from the control cells) was loaded, using Bio-Rad protein gel systems. The total protein profiles were revealed by Ponceau-S staining. The reduced GAPDH amount was visualized by Western blot using monoclonal antibody against GAPDH (US Biological, Marble-head, MA, USA). The amount of total proteins and GAPDH in each treatment is a reflection of the number of lymphocytes surviving after the 6-MP treatment.
Demonstration of apoptosis by measurement of caspase-3 activity
To determine whether apoptosis was the mechanism for 6-MP cytotoxicity at the concentrations used for our assays, drug-induced apoptosis was analyzed in line K using activation of caspase-3, a key protease that is activated during early apoptosis. A nitrocellulose blot identical to that used in above GAPDH Western blot was used in this assay, prepared from lymphocytes that were cultured with 6-MP at various concentrations (0, 0.01, 0.1, 1, 10 and 100 μg/mL) for 72 h, as described above. The activation of caspase-3 (auto-catalytic processing) was visualized by anti-caspase 3 monoclonal antibodies (BD Pharmingen, San Diego, CA, USA).
Amplification of potential 6-MP transporters by reverse transcriptase polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated from each cell line (2.5 × 106 cells) using Trizol reageant (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was quantified by determining absorbance at 260 nm. SuperScript® III One-Step RT-PCR System with Platinum® Taq (Invitrogen, Carls-bad, CA USA) was used for all PCR reactions as per the manufacturer’s instructions. Transporters and the corresponding primers used for their PCR amplification are listed in Table 2. RT-PCR was performed under the following conditions: cDNA synthesis and pre-denaturation one cycle at 50°C for 30 min and 94°C for 2 min. PCR amplication was performed in 40 cycles: denaturing at 94°C for 15 s, annealing at 60°C for 30 s and extending at 72°C for 1 min with a final extension cycle at 72°C for 7 min. 18S rRNA was used for standardization of RNA input for all PCR reactions.
Table 2.
Primer sequences used for reverse transcription-polymerase chain reaction
| Primers | Forward | Reverse |
|---|---|---|
| ENT-1 | 5′-GCTGGTGTTTCTGATCACTGCC-3′ | 5′-AATGAGGTCCAACTTGGTCTCC-3′ |
| ENT-2 | 5′-TGTTGGTCTTCACAGTCACCC-3′ | 5′-GCCAGGAAGAAGGTCATGAGG-3′ |
| ENT-3 | 5′-CTGGTGAAGAGGAGCTTCCCCAGG-3′ | 5′-GGCTGGTAGTTACAGAGCACG-3′ |
| ENT-4 | 5′-GCACCACGACGTTGTCGCCGGGGACGTCCA-3′ | 5′-TCAGAGGCCTGCGAGGATGGAACCATTGGC-3′ |
| CNT-1 | 5′-TAGCCTTCTTGATGGGTGTGGCGT-3′ | 5′-GTCCATTGCTCTGTCCACAGTGG-3′ |
| CNT-2 | 5′-GTCCATTGCTCTGTCCACAGTGG-3′ | 5′-CCAGTGCCCTCTGGAAATTC-3′ |
| CNT-3 | 5′-GTTCTCTGGGATCACCTGATGGCC-3′ | 5′-GCTATAAATCCAGGGTCAGTC-3′ |
| LRP | 5′-GTCTTCGGGCCTGAGCTGTCG-3′ | 5′-CTTGGCCGTCTCTTGGGGGTCCTT-3′ |
| MDR1 | 5′-TCCAGTTTCCTTTTGGAGGA-3′ | 5′-TGTCGTTTTGTTTCAGGATCA-3′ |
| MRP1 | 5′-TGGTCATCAGCAGCATCGTG-3′ | 5′-GCCTGTATCGACGGACCTGTAA-3′ |
| MRP2 | 5′-ATGCGATACTGTCCACCATG-3′ | 5′-ATCATCATGGGAAGC-3′ |
| MRP3 | 5′-GATACGCTCGCCCACAGTC-3′ | 5′-CAGTTGCCGTGATGTGGCT-3′ |
| MRP4 | 5′-CATTGAAGATCTTCCTG-3′ | 5′-GGTGTTCAATCTGTGTGC-3′ |
| MRP5 | 5′-GGATAACTTCTCAGTGG-3′ | 5′-ATGGCAATGCTCTAAAG-3′ |
| MRP6 | 5′-CTTGCCAGCTACCAACGCT-3′ | 5′-TGCTAATGCCTTGTTGTTGTGC-3′ |
| 18S rRNA | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ |
ENT, equilibrative nucleoside transporter; CNT, concentrative nucleoside transporter; LRP, lung resistance protein; MDR1, multidrug resistant protein 1 or P-glycoprotein; MRP, multidrug resistance-related protein; 18S rRNA, 18S ribosomal RNA.
RESULTS
Intracellular accumulation of 6-MP varied among cell lines derived from different individuals
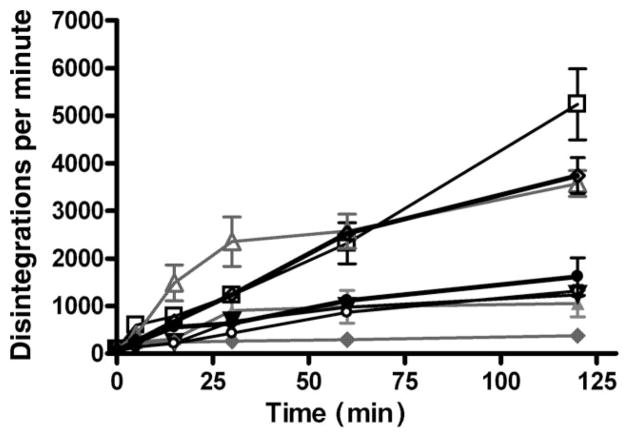
To analyze 6-MP transport among cell lines, a time course for intracellular accumulation of 6-MP at 37°C at 0, 5, 15, 30, 60 and 120 min was determined. The intracellular drug accumulation was variable between cell lines (Fig. 1). A notable increase in the intracellular accumulation of 14C-labeled 6-MP occured at the 15 min timepoint with a continued increase over time. There was reliable variability between the cell lines derived from different individuals. Cells from three patients (patients E, K and L) exhibited the highest rate and accumulation of 6-MP compared with the rest, with a final concentration of at least twice higher, suggesting a negative association between 6-MP uptake efficiency and risk for surgery.
Figure 1.
Transport of 14C-radiolabeled 6-mercaptopurine (6-MP) varies between lymphocytes derived from individuals with inflammatory bowel disease. The lymphocytes were incubated in triplicate samples at 37°C in medium containing 14C-labeled 6-MP at a final concentration of 0.05 μg/mL for 0, 5, 15, 30, 60 and 120 min, respectively. Among all eight cell lines tested from patients (
 ) B, (
) B, (
 ) D, (
) D, (
 ) E, (
) E, (
 ) F, (
) F, (
 ) H, (
) H, (
 ) J, (
) J, (
 ) K and (
) K and (
 ) L, lymphocytes from E, K and L, who did not have any surgery (Table 1), exhibited much higher efficiency in 6-MP uptake than those from patients D, H and J, who had undergone at least one surgery.
) L, lymphocytes from E, K and L, who did not have any surgery (Table 1), exhibited much higher efficiency in 6-MP uptake than those from patients D, H and J, who had undergone at least one surgery.
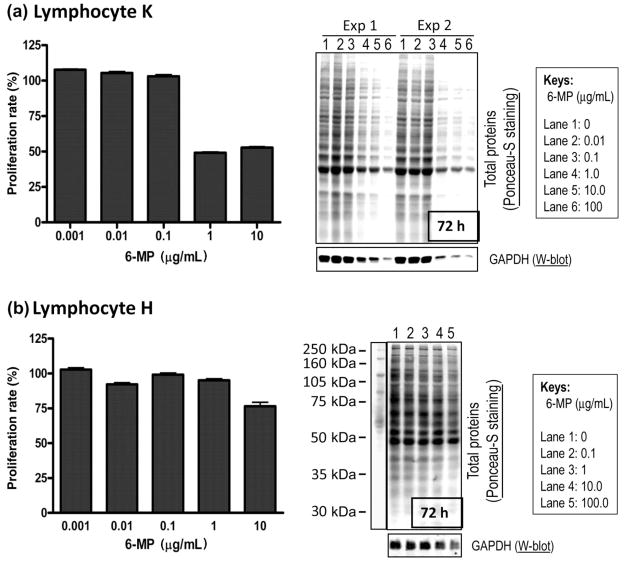
Susceptibility to 6-MP cytotoxicity, as demonstrated by protein analysis and MTT cell proliferation assay, correlates with efficiency of intracellular drug accumulation
After 3 days of incubation with 6-MP, cell growth was minimally affected in any of the cell lines at 0.1 μg/mL or lower. At 1 μg/mL drug concentration, growth was clearly inhibited, as shown by the MTT assay, but the level of inhibition varied among lymphocyte cell lines. Two cell lines, H and K, were of particular interest given that their intracellular accumulation of 6-MP was so divergent (H accumulated 6-MP the least efficiently and K accumulated 6-MP the most efficiently, Fig. 1). Line K was the most susceptible to the cytotoxic effects of 6-MP (Fig. 2a, left panel), whereas H was the least susceptible (Fig. 2b, left panel), as evidenced by a decrease in proliferating cells. At 3 days of incubation with 1 μg/mL 6-MP, there was also a dramatic decrease in total cellular proteins and GAPDH (as a measure of living cell numbers) in cell K (Fig. 2a, right panel), while little change was visible in cell H (Fig. 2b, right panel). These data correlated with the results of the MTT assay. After 12 days of incubation, cytotoxicity was noticable even at 0.01 μg/mL 6-MP in line K (data not shown). Overall, susceptibility to 6-MP toxicity again correlated well with the efficiency of drug accumulation.
Figure 2.
Susceptibility to 6-mercaptopurine (6-MP) toxicity correlates with efficiency of intralymphocyte accumulation in cells derived from individuals with inflammatory bowel disease. Cytotoxicity to 6-MP was measured in two representative cell lines: (a) cell K from patient K with the most efficient drug accumulation and (b) cell H from patient H with the least efficient drug accumulation. Cytotoxicity was determined using two independent approaches: a colorimetric methyl thiazolyl tetrazolium (MTT) assay (a, b, left panels) and the amount of protein expression as a reflection of surviving number of cells (a, b, right panels). Total proteins were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane and visualized by Ponceau-S staining. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was visualized by Western blot using anti-GAPDH antibodies. At 1 μg/mL of 6-MP after 72 h, clear cytotoxicity is observed in cell K by both assays, while cell H shows no significant cytotoxicity until the drug concentration is 10-fold higher, suggesting a positive correlation between drug accumulation and cytotoxicity.
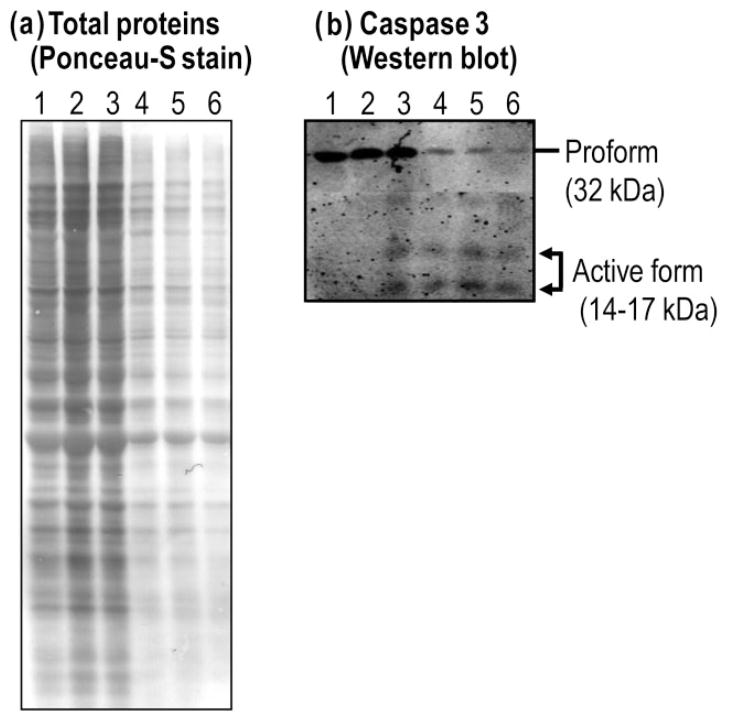
6-MP cytotoxicity occurs, at least in part, as a result of apoptosis
Although the total amount of proteins (as an indicator of cell numbers) was dramatically decreased and cyto-toxicity was markedly increased at 1 μg/mL 6-MP, after 3 days of 6-MP exposure to lymphocytes, there were no visible changes in these parameters at 0.1 μg/mL 6-MP (Fig. 3a). However, the activation (autocatalytic processing) of caspase-3 was visible at 0.1 μg/mL and increased with increasing drug concentration, while the nonproteolytic, full-length and inactive form of caspase-3 decreased gradually (Fig. 3b). These data suggest that the cytotoxicity is at least in part due to apoptosis, and that apoptosis occurs before cytotoxicity can be dectected from the other methods used.
Figure 3.
Cytotoxicity of 6 mercaptopurine (6-MP) occurs through induction of apoptosis. (a) Total protein profiles stained by Ponceau-S. A dramatic decrease of total proteins was seen at 1 μg/mL 6-MP. (b) Activation of caspase-3 was visualized by anti-caspase-3 antibody. Caspase 3 activation was clearly detectable at 0.1 μg/mL 6-MP after 3 days (arrow indicating activated [cleaved] form [14–17 kDa], comparing with the proform [32 kDa]). Lanes 1–6 represent the following concentrations of 6-MP (μg/mL): 0, 0.01, 0.1, 1, 10, 100, respectively.
Intracellular accumulation of 6-MP is achieved by a carrier-mediated transport process, not by simple diffusion
Carrier-mediated transport processes rely on transporters that are inactive at 0°C, whereas passive diffusion occurs over a wider spectrum of temperatures. Thus, to determine whether 6-MP transport occurs via simple diffusion, the intracellular accumulation of 6-MP was examined at 0°C and 37°C. Transport did not occur when samples were maintained on ice, but was facilitated at 37°C (Fig. 4). This suggests that the transport of 6-MP occurs as the result of a carrier-mediated transport process and not by simple diffusion.
Figure 4.
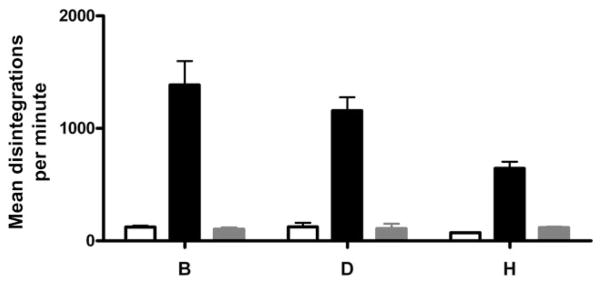
The transport assay was performed on three representative cell lines D, H and K using a final 6-mercaptopurine (6-MP) concentration of 0.05 μg/mL. Assays were done for points (▭) 0 min, (
 ) 60 min on ice (0°C) and (▬) 60 min at 37°C. The reaction was terminated with ice-cold phosphate-buffered saline. Transport/accumulation occurs only at 37°C and not on ice, suggesting that the transport of 6-MP is carrier-mediated, not via simple passive diffusion.
) 60 min on ice (0°C) and (▬) 60 min at 37°C. The reaction was terminated with ice-cold phosphate-buffered saline. Transport/accumulation occurs only at 37°C and not on ice, suggesting that the transport of 6-MP is carrier-mediated, not via simple passive diffusion.
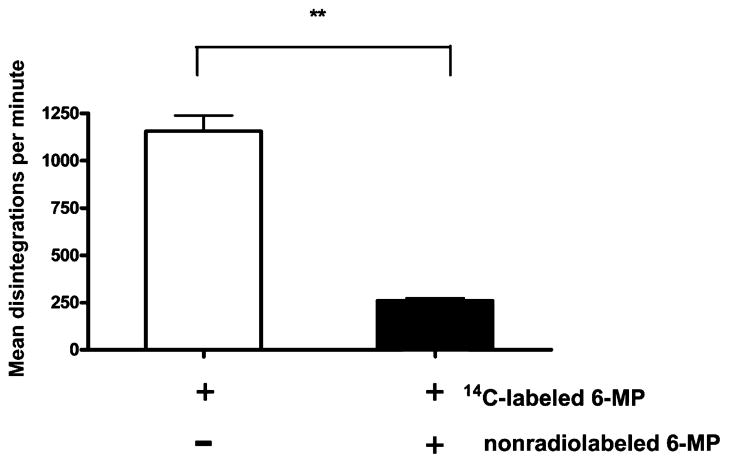
Intracellular accumulation of 14C-labeled 6-MP can be competitevly inhibited by an excess of non-radiolabeled drugs
Knowing that the uptake of 6-MP is mediated by a carrier-mediated transport process, we tested whether the binding of 6-MP to the putative transporter could be saturated. As shown in Figure 5, 6-MP transport was subject to competitive inhibition using a 100-fold concentration of non-radiolabeled 6-MP (5 μg/mL). It is important to note that 6-MP exposure at this concentration for a short period of time (60 min) resulted in no cytotoxicity to the cells, as shown by the MTT assay (data not shown). These findings suggest that the drug accumulation occurs via carrier-mediated transport, as it can be displaced by an excess of non-radiolabeled drug.
Figure 5.
Transport of 6-mercaptopurine (6-MP, 0.05 μg/mL) can be competitively inhibited by an excess of cold (non-radiolabeled) drug. 6-MP transport was performed in the (▬) presence and (▭) absence of 5 μg/mL non-radiolabeled 6-MP for 60 min at 37°C. Transport is inhibited by the excess of non-radiolabeled 6-MP, suggesting that transport requires a carrier that can be saturated by non-radiolabeled drug. **P < 0.01.
Intracellular accumulation of 14C-labeled 6-MP can be partially inhibited by a sodium-free medium
If 6-MP uptake is achieved by one of the CNTs and ENTs, they can be differentiated by their requirement for sodium. CNTs are sodium dependent, while ENTs are sodium independent.14 To determine whether the transport of 6-MP was sodium dependent, the transport assay was performed under sodium-containing and sodium-free conditions, controlled for pH and osmolarity. Transport was partially, though not completely, inhibited in a sodium-free environment using a pH and osmolarity-matched buffer (Fig. 6). From our data, sodium-dependent transport accounted for approximately 50% of intracellular 6-MP accumulation. This suggests that 6-MP transport may involve both sodium-dependent and sodium-independent transporters.
Figure 6.
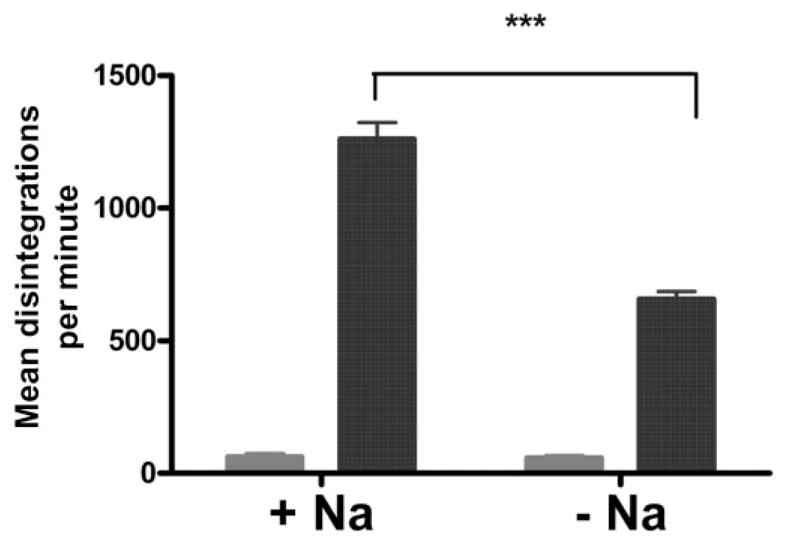
Approximately 50% of 6-mercaptopurine (6-MP) transport is mediated through a sodium-dependent mechanism. The transport of 14C-labeled 6-MP was measured after incubation at 37°C in sodium-containing (+Na) or sodium-free (−Na) 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffers for (
 ) 0 and (
) 0 and (
 ) 60 mins. Transport at 60 min was only partially inhibited in the sodium-free medium, which was controlled for pH and osmolarity. These findings suggest that approximately 50% of drug transport is sodium dependent. ***P < 0.01.
) 60 mins. Transport at 60 min was only partially inhibited in the sodium-free medium, which was controlled for pH and osmolarity. These findings suggest that approximately 50% of drug transport is sodium dependent. ***P < 0.01.
RT-PCR of potential 6-MP transporters
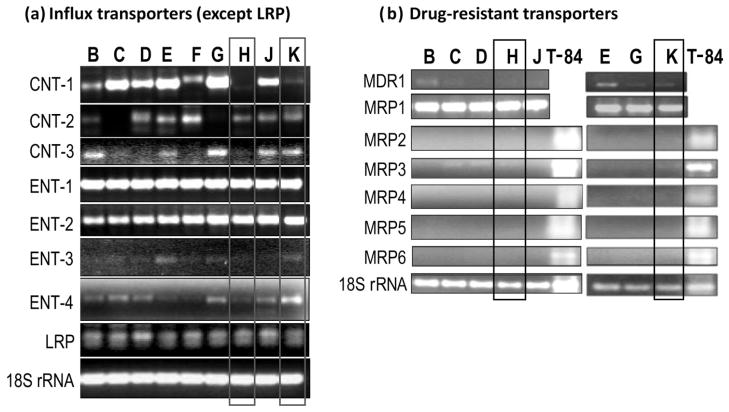
To identify potential 6-MP transporters responsible for the differences in drug transport between lymphocyte lines, RT-PCR was performed to characterize the expression of potential inward and outward nucleoside/nucleobase transporters (Fig. 7). RT-PCR was completed using primers to an array of known nucleoside transporters: ENT-1, ENT-2, ENT-3, ENT-4, CNT-1, CNT-2, CNT-3; multidrug resistance-related protein (MRP) 4 and MRP5. The expression of other potential drug transporters, including lung resistance protein (LRP), multidrug resistant protein (MDR) 1 or P-glycoprotein, MRP1, 2, 3 and MRP6 were also analyzed, though it is not known if they transport nucleo-bases. All seven influx transporters (ENT-1, 2, 3, 4 and CNT-1, 2, 3) were expressed. ENT-1, ENT-2 and LRP were expressed equally across cells from all patients. Of the efflux transporters, MRP2, 4, 5 and 6 did not show detectable expressions in these cell lines. MRP1, MRP3 and MDR1 showed detectable expression, with MRP1 expressing equally among the cell lines tested. Line H, which had the least efficient transport and was the most resistent to cytotoxicity, had low or non-detectable expression of CNT-1, CNT-3, ENT-3 and ENT-4, but not CNT-2. On the other hand, line K, which was the most efficient in drug accumulation and least drug-resistant, expressed relatively higher levels of all influx transporters (except CNT-1), particularly ENT-4. However, no individual transporter showed a clear correlation with susceptibility to 6-MP cytotoxicity or with intracellular drug accumulation. As a loading control for RT-PCR, 18S rRNA was amplified equally across all cell lines. As a positive control, total RNA from colonic cell T-84 cells was included in the PCR experiments when transporter expression was negative (i.e., MRP, the expression of which was not detectable in lymphocytes).
Figure 7.
Drug-resistant cells from patient H exhibit the most pronounced lack or low level of expression of multiple potential nucleoside transporters. Expression of drug transporters was examined using reverse transcription polymerase chain reaction (RT-PCR) in lymphocytes derived from inflammatory bowel disease patients (B–K). (a) Potential uptake (influx) transporters (except lung resistance protein [LRP], considered as an intracellular transporter). (b) Potential drug-resistance (efflux) transporters. While there are similar expressions of equilibrative nucleoside transporter (ENT)-1, ENT-2 and LRP across all cell lines, the expressions of concentrative nucleoside transporter (CNT)-1, -2, -3 and ENT-3, -4 vary substantially between cells. Among drug-resistance transporters, only multidrug resistance-related protein 1 (MRP1), MRP3 and multidrug resistant proteins (MDR-1) was detectable. Importantly, the 6-MP-resistant cells from patient H (panel a, lane H) exhibits the most profound lack or decreased expression of multiple drug influx transporters, including CNT-1, CNT-3, ENT-3 and ENT-4. 18S rRNA was used as a standardized control. Colonic cell T-84 was used as a positive control for MRP2–6.
DISCUSSION
Although 6-MP has been used extensively in the treatment of IBD, the exact mechanism of its entry and exit from target cells has not been elucidated. Moveover, many patients treated with 6-MP do not achieve therapeutic goals, despite 6-TGN metabolite levels (measured in human erythrocytes) being within an acceptable therapeutic range. This study demonstrated that 6-MP is transported into human lymphocytes via a carrier-mediated mechnism of transport, not via simple diffusion. Additionally, transport of 6-MP is at least partially sodium dependent, suggesting that CNTs may be involved in the uptake of this drug. Overall, our results found that specific transporters are likely to be involved in the intracellular accumulation of 6-MP in lymphocytes from IBD patients and that intracellular accumulation of 6-MP directly correlates with susceptibility to cytotoxicity.
Transporters such as MDR1, MRP1–6 act as efflux pumps for cytotoxic drugs and may also affect intracellular drug accumulation. The adenosine triphosphate (ATP) binding cassette transporters, including MDR1 and MRP1–5, are a family of large proteins in membranes that are able to transport a large variety of compounds across membranes against a concentration gradient using ATP hydrolysis. The substrate specificity of some transporters has been well defined and MRP-4 and MRP-5 have the unique ability to transport nucleoside monophosphate analogues.24–26 LRP, also known as major vault protein, is a cytoplasmic ribonucleoprotein complex that has been shown to be overexpressed in several chemoresistant cancer cell lines, including non-small cell lung cancer, U-937 leukemia cells and the colon carcinoma cell line SW-620.27–29 The function of this protein is not clearly understood, though it is hypothesized that it may act by sequestrating active drugs within lysosomes.30,31
Other studies have implicated specific transporters in the intracellular accumulation of 6-MP. Murine COS cells transfected with overexpressed ENT-2 demonstrated a resultant increase in transport of 6-MP.32 In another study using the human T-lymphoblastic cell line MOLT-4, a 6-MP-resistant cell line was developed by prolonged exposure to the drug. This line had elevated expression of ENT-2 and CNT-3. Small interfering RNA knockdown of CNT-3 and ENT-2 resulted in a reduction of 6-MP transport by 47% and 21%, respectively.33 Resistance to 6-MP as well as to other purine and pyrimidine nucleoside and nucleobase analogues has been described in the murine leukemic cell line L1210/VMDRC.06 after transfection with the human MDR1.34
EBV-transformed B lymphocytes were used for this study, as they provided an indefinite number of cells derived from original serum samples, permitting repeat transport studies. Although the use of PBMCs with isolation of T lymphocytes would have been preferable, they can be used only once and do not allow for repetitions of transport and molecular studies. Our study is unique in that it allows the examination and comparison of 6-MP transport capability and expression levels of all potential drug transporters in several lymphocyte cell lines derived from PBMCs of patients with IBD.
Our data suggest that there is inherent variability in intra-lymphocyte uptake of 6-MP between lymphocyte lines derived from different individuals and this transport is correlated with susceptibility to 6-MP cytotoxicity. It is interesting to note that the cell lines which were most efficient in the uptake and accumulation of 6-MP were those isolated from patients E, K and L; none of whom had undergone surgery. On the other hand, the cells that were the least effectient in 6-MP uptake and accumulation and the most resistant to 6-MP cytoxicity were from patient H, who, in spite of being treated with 6-MP, had undergone surgery. This indicates that there is a potential positive correlation between cellular 6-MP accumulation in lymphocytes and treatment response.
We describe the expression of most, if not all, potential 6-MP transporters within human lymphocytes, including seven influx (inward) and seven efflux (outward) nucleoside transporters, plus LRP. The efflux transporters, which are the main contributers to drug resistance, did not appear to contribute to the variability of intracellular drug accumulation between cell lines, since only three of the seven efflux transporters were expressed in lymphocytes, none of which correlated with drug accumulation or cytotoxicity. Rather, expression variation in influx (uptake) transporters may be a key contributer to the difference in the ability of the lymphocytes to accumulate drugs. No single transporter stood out alone in our study as underexpressed or overexpressed to explain a pattern of 6-MP transport and resistance. Perhaps a combination of several transporters handles intracellular 6-MP accumulation and a decrease in several of the transporters contributes to the resistance to the drug. Interestingly, the lymphocyte line H in our study demonstrated the lowest expression of influx transporters, correlating with the least susceptibility to 6-MP cyto-toxicity. In this particular lymphocyte line, the expression of multiple inward transporters was notably low or undetectable, including CNT-1, CNT-3, ENT-3 and ENT-4. Conversely, lymphocyte line K, which exhibited robust transport and an increased susceptibility to 6-MP cytotoxicity, had relatively high levels of expression of almost all (except CNT-1) influx transporters, including CNT-2, CNT-3, ENT-3 and particularly ENT-4.
Despite their ‘therapeutic’ erythrocyte 6-TGN levels, some patients with IBD are refractory to the immuno-modulatory effects of 6-MP.35,36 Other patients are at increased risk for developing severe leukopenia. Though erythrocyte levels may appear optimized, endogenous variation in intra-lymphocyte accumulation of 6-MP may contribute to in vivo differences in responses to 6-MP. In the future, larger and prospective studies are needed to confirm that inherent variation in 6-MP transport specific to lymphocytes exists and to correlate it with clinical response to 6-MP therapy. Further work is also necessary to identify specific 6-MP transporters that contribute to differences in intra-lymphocyte drug accumulation. The identification of such transporters might allow prediction of response to the drug before institution of therapy, or possibly identification of novel therapeutic targets for the management of IBD.
Acknowledgments
This study was approved by National Institutes of Health T32 Grant HD4435 and Broad Medical Research Program (IBD-0119R). We would like to thank the following individuals who have helped in this work: Dr Chuan Hua YANG and Ms Huan WANG for characterizing the lymphocytes and Dr Ted BAYLESS, Maria OLIVA-HEMKER and Mark DONOW-ITZ for their advices and suggestions.
Footnotes
Declaration of personal interests: Carmen CUFFARI has served as a speaker and consultant for Prometheus, Shire, UCB Pharmaceuticals, Nestle, Abbott Laboratories, TAP Pharmaceuticals, Novartis, Roche and Salix Pharmaceuticals. Steven R. BRANT has served as a consultant for UCB Pharmaceuticals and Proctor and Gamble.
Declaration of funding interests: Carmen Cuffari has received research funding from Prometheus, Shire, Roche and Glaxo-Wellcome.
References
- 1.Adler DJ, Korelitz BI. The therapeutic efficacy of 6-mercaptopurine in refractory ulcerative colitis. Am J Gastroenterol. 1990;85:717–22. [PubMed] [Google Scholar]
- 2.Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132–42. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol. 1996;91:423–33. [PubMed] [Google Scholar]
- 4.Korelitz BI, Adler DJ, Mendelsohn RA, Sacknoff AL. Long-term experience with 6-mercaptopurine in the treatment of Crohn’s disease. Am J Gastroenterol. 1993;88:1198–205. [PubMed] [Google Scholar]
- 5.Derijks LJ, Gilissen LP, Hooymans PM, Hommes DW. Review article: thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:715–29. doi: 10.1111/j.1365-2036.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 6.Cuffari C, Seidman EG, Latour S, Théorêt Y. Quantitation of 6-thioguanine in peripheral blood leukocyte DNA in Crohn’s disease patients on maintenance 6-mercaptopurine therapy. Can J Physiol Pharmacol. 1996;74:580–5. [PubMed] [Google Scholar]
- 7.Kurowski V, Iven H. Plasma concentrations and organ distribution of thiopurines after oral application of azathioprine in mice. Cancer Chemother Pharmacol. 1991;28:7–14. doi: 10.1007/BF00684949. [DOI] [PubMed] [Google Scholar]
- 8.Chan GL, Erdmann GR, Gruber SA, Matas AJ, Canafax DM. Azathioprine metabolism: pharmocokinetics of 6-mercaptopurine, 6-thiouric acid and 6-thioguanine nucleotides in renal transplant patients. J Clin Pharmacol. 1990;30:358–63. doi: 10.1002/j.1552-4604.1990.tb03606.x. [DOI] [PubMed] [Google Scholar]
- 9.Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43:329–39. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- 10.Fairchild CR, Maybaum J, Kennedy KA. Concurrent unilateral chromatid damage and DNA strand breakage in response to 6-thioguanine treatment. Biochem Pharmacol. 1986;35:3533–41. doi: 10.1016/0006-2952(86)90623-4. [DOI] [PubMed] [Google Scholar]
- 11.Teide I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ lymphocytes. J Clin Invest. 2003;111:1133–45. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hortelano S, Boscá L. 6-mercaptopurine decreases the Bcl-2/Bax ratio and induces apoptosis in activated splenic B lymphocytes. Mol Pharmacol. 1997;51:414–21. [PubMed] [Google Scholar]
- 13.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci. 2006;27:416–25. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Griffith DA, Jarvis SM. Nucleoside and nucleobase transport systems of mammalian cells. Biochim Biophys Acta. 1996;1286:153–81. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 15.Rose JB, Coe IR. Physiology of nucleoside transporters: back to the future. Physiology (Bethesda) 2008;23:41–8. doi: 10.1152/physiol.00036.2007. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–43. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 17.Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 2004;447:728–34. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- 18.Pastor-Anglada M, Molina-Arcas M, Casado FJ, Bellosillo B, Colomer D, Gil J. Nucleoside transporters in chronic lymphocytic leukaemia. Leukemia. 2004;18:385–93. doi: 10.1038/sj.leu.2403271. [DOI] [PubMed] [Google Scholar]
- 19.Smith KM, Slugoski MD, Loewen SK, et al. The broadly selective human Na+/nucleoside cotransporter (hCNT3) exhibits novel cation-coupled nucleoside transport characteristics. J Biol Chem. 2005;280:25436–49. doi: 10.1074/jbc.M409454200. [DOI] [PubMed] [Google Scholar]
- 20.Penno MB, Pedrotti-Krueger M, Ray T. Cryopreservation of whole blood and isolated lymphocytes for B-cell transformation. J Tissue Cult Meth. 1993;15:43–8. [Google Scholar]
- 21.Penno MB, Pedrotti-Krueger M, Ray T. Cryopreservation of human blood for B-cell immortalization. In: Doyle A, Griffiths JB, editors. Cell and Tissue Culture for Medical Research. New York, NY: John Wiley & Sons; 2000. pp. 179–86. [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Wilson AP. Cytotoxicity and Viability Assays. In: Masters JRW, editor. Animal Cell Culture: A Practical Approach. 3. Oxford: Oxford University Press; 2000. pp. 175–219. [Google Scholar]
- 24.Wijnholds J, Mol CA, van Deemter L, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97:7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wielinga PR, Reid G, Challa EE, et al. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62:1321–31. doi: 10.1124/mol.62.6.1321. [DOI] [PubMed] [Google Scholar]
- 26.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–71. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- 27.Scheper RJ, Broxterman HJ, Scheffer GL, et al. Overexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993;53:1475–9. [PubMed] [Google Scholar]
- 28.Hu Y, Stephen AG, Cao J, et al. A very early induction of major vault protein accompanied by increased drug resistance in U-937 cells. Int J Cancer. 2002;97:149–56. doi: 10.1002/ijc.1590. [DOI] [PubMed] [Google Scholar]
- 29.Kitazono M, Okumura H, Ikeda R, et al. Reversal of LRP-associated drug resistance in colon carcinoma SW-620 cells. Int J Cancer. 2001;91:126–31. doi: 10.1002/1097-0215(20010101)91:1<126::aid-ijc1018>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Mossink MH, van Zon A, Scheper RJ, Sonneveld P, Wiemer EA. Vaults: a ribonucleoprotein particle involved in drug resistance? Oncogene. 2003;22:7458–67. doi: 10.1038/sj.onc.1206947. [DOI] [PubMed] [Google Scholar]
- 31.Herlevsen M, Oxford G, Owens CR, Conaway M, Theodorescu D. Depletion of major vault protein increases doxorubicin sensitivity and nuclear accumulation and disrupts its sequestration in lysosomes. Mol Cancer Ther. 2007;6:1804–13. doi: 10.1158/1535-7163.MCT-06-0372. [DOI] [PubMed] [Google Scholar]
- 32.Nagai K, Nagasawa K, Kihara Y, Okuda H, Fujimoto S. Anticancer nucleobase analogues mercaptopurine and 6-thiouguanine are novel substrates for equilibrative nucleoside transporter 2. Int J Pharm. 2007;333:56–61. doi: 10.1016/j.ijpharm.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 33.Fotoohi AK, Lindqvist M, Peterson C, Albertioni F. Involvement of the concentrative nucleoside transporter 3 and equilibrative nucleoside transporter 2 in the resistance of T-lymphoblastic cell lines to thiopurines. Biochem Biophys Res Commun. 2006;343:208–15. doi: 10.1016/j.bbrc.2006.02.134. [DOI] [PubMed] [Google Scholar]
- 34.Zeng H, Lin ZP, Sartorelli AC. Resistance to purine and pyrimidine nucleoside and nucleobase analogs by the human MDR1 transfected murine leukemic cell line L1210/VMDRC. 06. Biochem Pharmacol. 2004;68:911–21. doi: 10.1016/j.bcp.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Dubinsky MC, Yang H, Hassard PV, et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904–15. doi: 10.1053/gast.2002.32420. [DOI] [PubMed] [Google Scholar]
- 36.Roblin X, Peyrin-Biroulet L, Phelip JM, Nancey S, Flourie B. A 6-thioguanine nucleotide threshold level of 400 pmol/8 × 10(8) erythrocytes predicts azathioprine refractoriness in patients with inflammatory bowel disease and normal TPMT activity. Am J Gastroenterol. 2008;103:3115–22. doi: 10.1111/j.1572-0241.2008.01743.x. [DOI] [PubMed] [Google Scholar]