Abstract
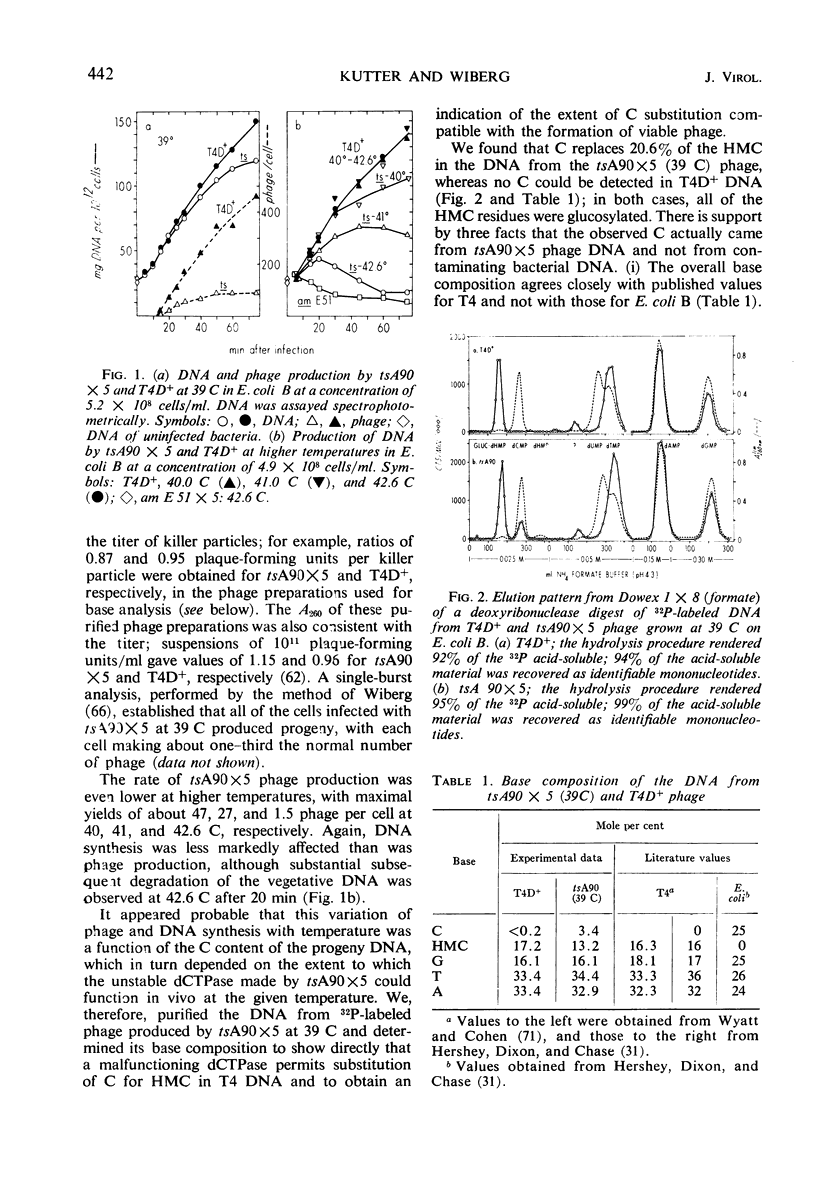
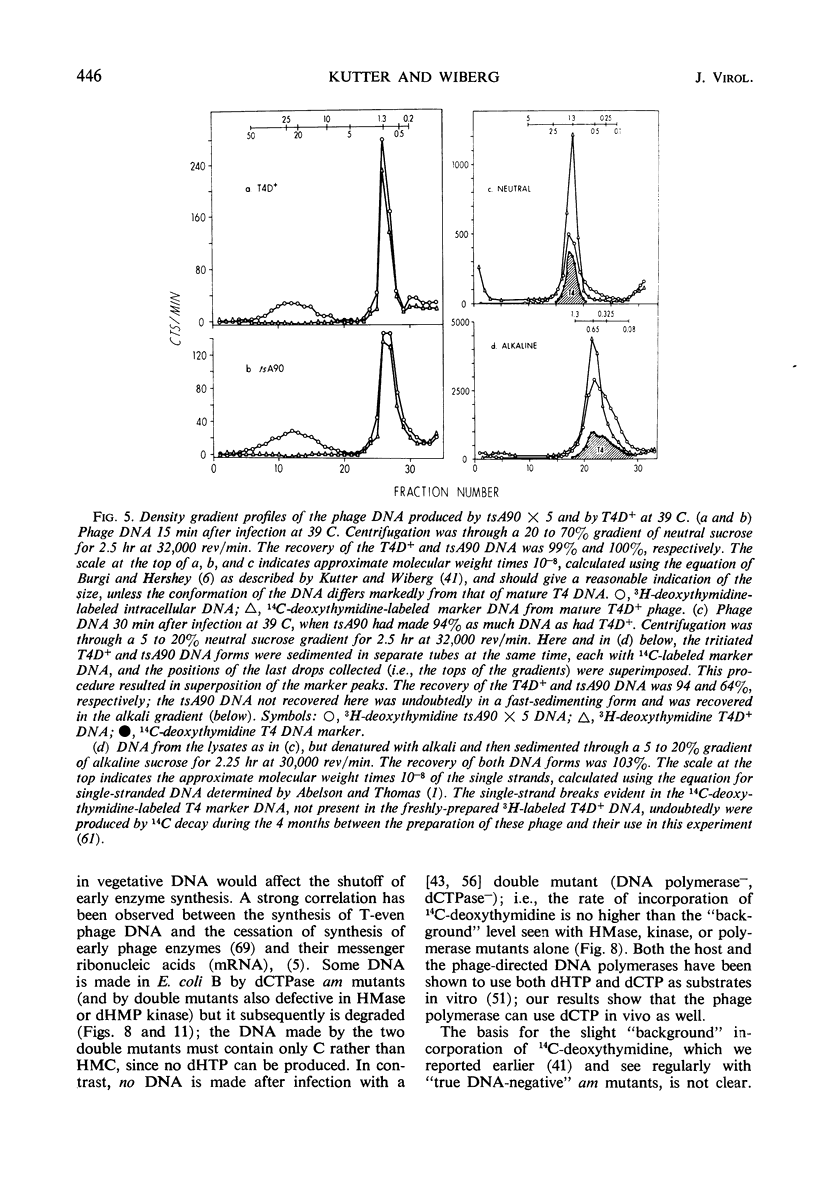
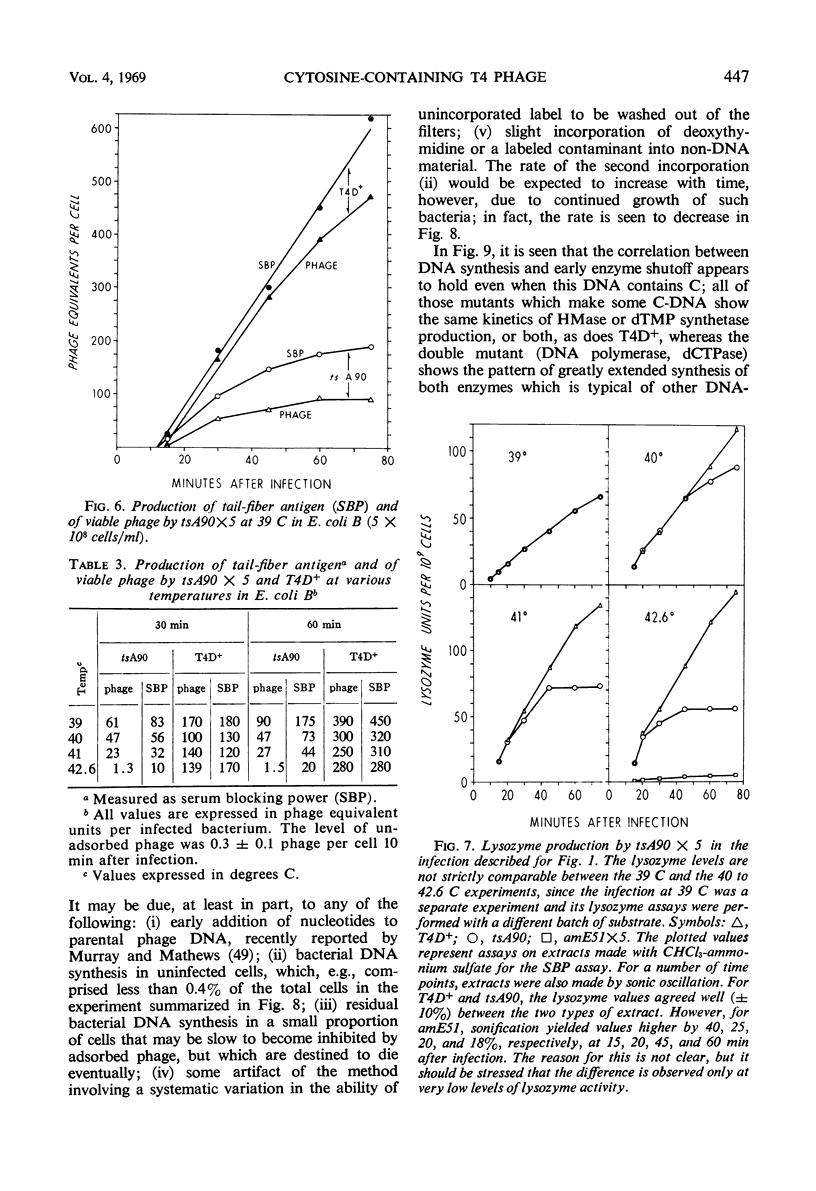
Previous work from this laboratory has shown that the cytosine-containing T4 deoxyribonucleic acid (DNA) made by deoxycytidine triphosphatase (dCTPase) amber mutants is extensively degraded, and that nucleases controlled by genes 46 and 47 participate in this process. In this paper, we examine other consequences of a defective dCTPase. Included are studies of DNA synthesis and phage production, and of the control of both early and late protein synthesis after infection of Escherichia coli B with various T4 mutants defective in genes 56 (dCTPase), 42 (dCMP hydroxymethylase), 1 (deoxynucleotide kinase), 43 (DNA polymerase), 30 (polynucleotide ligase), 46 and 47 (DNA breakdown) or e(lysozyme). By varying the temperature of infection with a temperature-sensitive dCTPase mutant, we have been able to control intracellular dCTPase activity, and thus vary the cytosine content of the phage DNA. We have produced and characterized viable T4 phage in which cytosine replaces 20% of the 5-hydroxymethylcytosine (HMC) in the DNA. We present evidence which suggests that intact, cytosine-containing T4 DNA is much less efficient than is normal T4 DNA in directing the synthesis of tail-fiber antigen. Lysozyme production is much less affected by progressively decreasing dCTPase activity; however, complete substitution of cytosine is correlated with a depression of lysozyme synthesis greater than expected from the defective synthesis of DNA. Low but significant lysozyme synthesis is observed late after infection of E. coli B with T4 amber mutants defective in a number of genes controlling DNA synthesis. The “20% cytosine” T4 phage, once produced, can initiate an apparently normal infection at permissive temperatures; the synthesis of early enzymes, DNA, and phage does not appear to be impaired. Two roles for HMC in T4 DNA have been indicated previously: (i) involvement in host-controlled restriction of the phage, in which glucosylation of the hydroxymethyl group plays a crucial role (16, 29, 53, 58), and (ii) protection of vegetative DNA against phage-controlled nucleases, a protection not dependent on glucosylation (41, 66, 67). A third role is suggested by our present results: transcription of at least some late genes can occur only from HMC-containing DNA and not from cytosine-containing DNA.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI L. E., HAEGGMARK A., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. II. FORMATION AND INTERCONVERSION OF DEOXYURIDINE PHOSPHATES. J Biol Chem. 1963 Oct;238:3407–3413. [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautz E. K., Kasai T., Reilly E., Bautz F. A. Gene-specific mRNA. II. Regulation of mRNA synthesis in E. coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1966 May;55(5):1081–1088. doi: 10.1073/pnas.55.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN W. E., BOLLUM F. J. Chromatography and desalting of nucleotides with ammonium biocarbonate on anion-exchange columns. Biochim Biophys Acta. 1961 Apr 15;48:588–590. doi: 10.1016/0006-3002(61)90058-0. [DOI] [PubMed] [Google Scholar]
- DE MARS R. I. The production of phage-related materials when bacteriophage development in interrupted by proflavine. Virology. 1955 May;1(1):83–99. doi: 10.1016/0042-6822(55)90007-6. [DOI] [PubMed] [Google Scholar]
- DIRKSEN M. L., HUTSON J. C., BUCHANAN J. M. HOST-DEPENDENT SYNTHESIS OF ALTERED DEOXYCYTIDYLATE HYDROXYMETHYLASE AFTER INFECTION OF ESCHERICHIA COLI WITH CERTAIN AMBER MUTANTS OF BACTERIOPHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:507–513. doi: 10.1073/pnas.50.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R. Mutual exclusion between related phages. J Bacteriol. 1952 Feb;63(2):209–217. doi: 10.1128/jb.63.2.209-217.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. The enzymology of virus-infected bacteria. X. A biochemical-genetic study of the deoxynucleotide kinase induced by wild type and amber mutants of phage T4. J Biol Chem. 1967 Jun 25;242(12):2877–2885. [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. Virus-induced acquisition of metabolic function. I. Enzymatic formation of 5-hydroxymethyldeoxycytidylate. J Biol Chem. 1959 Jun;234(6):1501–1506. [PubMed] [Google Scholar]
- FRANKLIN N. C. Serological study of tail structure and function in coliphages T2 and T4. Virology. 1961 Aug;14:417–429. doi: 10.1016/0042-6822(61)90333-6. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- FRENCH R. C., GRAHAM A. F., LESLEY S. M., VAN ROOYEN C. E. The contribution of phosphorus from T2r+ bacteriophage to progeny. J Bacteriol. 1952 Nov;64(5):597–607. doi: 10.1128/jb.64.5.597-607.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKASAWA T. THE COURSE OF INFECTION WITH ABNORMAL BACTERIOPHAGE T4 CONTAINING NON-GLUCOSYLATED DNA ON ESCHERICHIA COLI STRAINS. J Mol Biol. 1964 Aug;9:525–536. doi: 10.1016/s0022-2836(64)80224-2. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. II. The structural gene for polynucleotide ligase in bacteriophage T4. Proc Natl Acad Sci U S A. 1967 Aug;58(2):665–672. doi: 10.1073/pnas.58.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of the replicating DNA in Escherichia coli infected with certain amber mutants of phage T4. J Mol Biol. 1966 Jun;18(1):144–155. doi: 10.1016/s0022-2836(66)80082-7. [DOI] [PubMed] [Google Scholar]
- GREENBERG G. R., SOMERVILLE R. L. Deoxyuridylate kinase activity and deoxyuridinetriphosphatase in Escherichia coli. Proc Natl Acad Sci U S A. 1962 Feb;48:247–257. doi: 10.1073/pnas.48.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg G. R. New dUTPase and dUDPase activites after infection of Escherichia coli by T2 bacteriophage. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1226–1232. doi: 10.1073/pnas.56.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTMAN S., FUKASAWA T. HOST-INDUCED MODIFICATION OF T-EVEN PHAGES DUE TO DEFECTIVE GLUCOSYLATION OF THEIR DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:297–300. doi: 10.1073/pnas.50.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTMAN S. THE FUNCTIONING OF T-EVEN PHAGES WITH UNGLUCOSYLATED DNA IN RESTRICTING ESCHERICHIA COLI HOST CELLS. Virology. 1964 Nov;24:333–348. doi: 10.1016/0042-6822(64)90171-0. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., DIXON J., CHASE M. Nucleic acid economy in bacteria infected with bacteriophage T2. I. Purine and pyrimidine composition. J Gen Physiol. 1953 Jul;36(6):777–789. doi: 10.1085/jgp.36.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., Revel H. R., Luria S. E. Enzyme synthesis directed by nonglucosylated T-even bacteriophages in restrictive hosts. Virology. 1966 Nov;30(3):427–438. doi: 10.1016/0042-6822(66)90120-6. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Levinthal C. Protein synthesis by Escherichia coli infected with bacteriophage T4D. Virology. 1968 Apr;34(4):709–727. doi: 10.1016/0042-6822(68)90092-5. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSSE J., KORNBERG A. Glucosylation of deoxyribonucleic acid. III. alpha- and beta-Glucosyl transferases from T4-infected Escherichia coli. J Biol Chem. 1962 Jun;237:1968–1976. [PubMed] [Google Scholar]
- KOERNER J. F., SMITH M. S., BUCHANAN J. M. Deoxycytidine triphosphatase, an enzyme induced by bacteriophage infection. J Biol Chem. 1960 Sep;235:2691–2697. [PubMed] [Google Scholar]
- KORNBERG S. R., ZIMMERMAN S. B., KORNBERG A. Glucosylation of deoxyribonucleic acid by enzymes from bacteriophage-infected Escherichia coli. J Biol Chem. 1961 May;236:1487–1493. [PubMed] [Google Scholar]
- Kasai T., Bautz E. K., Guha A., Szybalski W. Identification of the transcribing DNA strand for the rII and endolysin genes of coliphage T4. J Mol Biol. 1968 Jun 28;34(3):709–712. doi: 10.1016/0022-2836(68)90192-7. [DOI] [PubMed] [Google Scholar]
- Kasai T., Bautz E. K. Regulation of gene-specific RNA synthesis in bacteriophage T4. J Mol Biol. 1969 May 14;41(3):401–417. doi: 10.1016/0022-2836(69)90284-8. [DOI] [PubMed] [Google Scholar]
- Kornberg A., Zimmerman S. B., Kornberg S. R., Josse J. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. INFLUENCE OF BACTERIOPHAGE T2 ON THE SYNTHETIC PATHWAY IN HOST CELLS. Proc Natl Acad Sci U S A. 1959 Jun;45(6):772–785. doi: 10.1073/pnas.45.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., PRATT E. A. On the structure of the glucosylated hydroxymethylcytosine nucleotides of coliphages T2, T4, and T6. J Biol Chem. 1960 Nov;235:3254–3259. [PubMed] [Google Scholar]
- Lembach K. J., Kuninaka A., Buchanan J. M. The relationship of DNA replication to the control of protein synthesis in protoplasts of T4-infected Escherichia coli B. Proc Natl Acad Sci U S A. 1969 Feb;62(2):446–453. doi: 10.1073/pnas.62.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Mathews C. K., Kessin R. H. Control of bacteriophage-induced enzyme synthesis in cells infected with a temperature-sensitive mutant. J Virol. 1967 Feb;1(1):92–96. doi: 10.1128/jvi.1.1.92-96.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molholt B., Fraser D. Host-controlled restriction of T-even bacteriophages: relation of endonuclease I and T-even-induced nucleases to restriction. J Virol. 1968 Apr;2(4):313–319. doi: 10.1128/jvi.2.4.313-319.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G., Revel H. R. Expression of genes in incomplete T4 genomes. Virology. 1967 Mar;31(3):397–401. doi: 10.1016/0042-6822(67)90218-8. [DOI] [PubMed] [Google Scholar]
- Price A. R., Warner H. R. A structural gene for bacteriophage T4-induced deoxycytidine triphosphate-deoxyuridine triphosphage nucleotidohydrolase. Virology. 1968 Nov;36(3):523–526. doi: 10.1016/0042-6822(68)90183-9. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI M., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. II. SYNTHESIS OF PHAGE-INDUCED RNA AND SEQUENTIAL ENZYME PRODUCTION. J Mol Biol. 1964 May;8:638–659. doi: 10.1016/s0022-2836(64)80114-5. [DOI] [PubMed] [Google Scholar]
- SHEDLOVSKY A., BRENNER S. A CHEMICAL BASIS FOR THE HOST-INDUCED MODIFICATION OF T-EVEN BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1963 Aug;50:300–305. doi: 10.1073/pnas.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., FUERST C. R. Inactivation of bacteriophages by decay of incorporated radioactive phosphorus. J Gen Physiol. 1955 Mar 20;38(4):441–458. doi: 10.1085/jgp.38.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREISINGER G., MUKAI F., DREYER W. J., MILLER B., HORIUCHI S. Mutations affecting the lysozyme of phage T4. Cold Spring Harb Symp Quant Biol. 1961;26:25–30. doi: 10.1101/sqb.1961.026.01.007. [DOI] [PubMed] [Google Scholar]
- SYMONDS N., STACEY K. A., GLOVER S. W., SCHELL J., SILVER S. The chemical basis for a case of host-induced modification in phage T2. Biochem Biophys Res Commun. 1963 Jul 26;12:220–222. doi: 10.1016/0006-291x(63)90193-1. [DOI] [PubMed] [Google Scholar]
- Short E. V., Jr, Warner H. R., Koerner J. F. The effect of cupric ions on the indole reaction for the determination of deoxyribonucleic acid. J Biol Chem. 1968 Jun 25;243(12):3342–3344. [PubMed] [Google Scholar]
- Somerville R., Ebisuzaki K., Greenberg G. R. HYDROXYMETHYLDEOXYCYTIDYLATE KINASE FORMATION AFTER BACTERIOPHAGE INFECTION OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1240–1245. doi: 10.1073/pnas.45.8.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., BUCHANAN J. M. STUDIES ON LABILE DEOXYCYTIDYLATE HYDROXYMETHYLASES FROM ESCHERICHIA COLI B INFECTED WITH TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4. Proc Natl Acad Sci U S A. 1964 Mar;51:421–428. doi: 10.1073/pnas.51.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Deoxyribonucleic acid synthesis in Escherichia coli infected with some deoxyribonucleic acid polymerase-less mutants of bacteriophage T4. Virology. 1966 Jan;28(1):100–107. doi: 10.1016/0042-6822(66)90310-2. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Evidence for a dual role for the bacteriophage T4-induced deoxycytidine triphosphate nucleotidohydrolase. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1233–1240. doi: 10.1073/pnas.56.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S. Amber mutants of bacteriophage T4 defective in deoxycytidine diphosphatase and deoxycytidine triphosphatase. On the role of 5-hydroxymethylcytosine in bacteriophage deoxyribonucleic acid. J Biol Chem. 1967 Dec 25;242(24):5824–5829. [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Henninger M. Attachment of tail fibers in bacteriophage T4 assembly: some properties of the reaction in vitro and its genetic control. J Mol Biol. 1969 Feb 14;39(3):603–618. doi: 10.1016/0022-2836(69)90148-x. [DOI] [PubMed] [Google Scholar]


