Abstract
The incidence of prostate cancer in transsexual patients is very low with only few reported cases. Many years before presenting with prostate cancer, these patients receive hormone ablation as a part of their gender therapy. Their disease is already defined as castrate resistant, and the treatment and follow-up of such patients remains a challenge. We report a case of a male-to-female transgender woman who was diagnosed with metastatic prostate cancer, 31 years post-feminization.
Case report
In 1970, a 45-year-old woman underwent male-to-female sex reassignment surgery, including bilateral orchidectomy. Since then she had started feminizing estrogen therapy, which included conjugated estrogen tablets 1.25 mg daily with no other hormone manipulation therapy. She had no documented family history of prostate cancer.
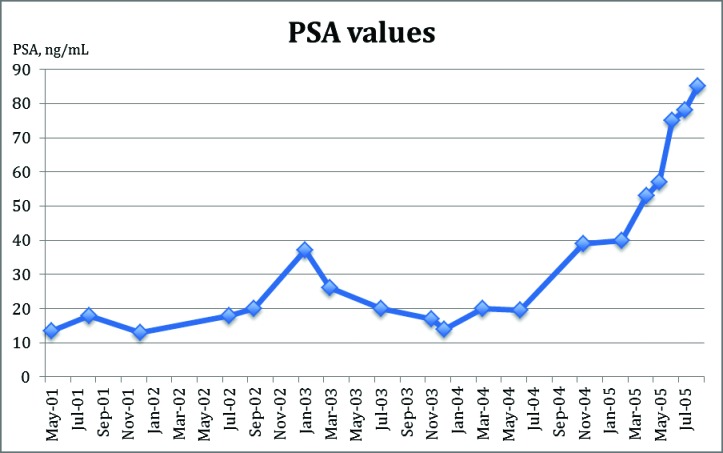
At the age of 75, she presented with obstructive voiding urinary symptoms and was found to have a serum prostate-specific antigen (PSA) level of 13.5 ng/mL; no previous PSA level had been measured (Fig. 1). Her testosterone value was in the castrate range. She underwent a transurethral resection of the prostate and the histology revealed a Gleason 7 prostatic adenocarcinoma. Staging scans did not reveal any evidence of gross metastatic disease. In July 2000, the patient completed a course of external beam radiotherapy (20 fractions of 55.00 Gy) with curative intent. Treatment was well-tolerated. For 18 months following radiotherapy, her PSA values stabilized to about 20 ng/mL. Two years later, her PSA has risen to 37 ng/mL, at which time antiandrogen therapy was initiated (flutamide 250 mg, three times a day) and her estrogen replacement therapy was converted to diethylstilboestrol (1 mg once a day). Four months later, her repeat PSA value decreased to 20 ng/mL. At the end of the same year, diethylstilboestrol had been replaced with ethinyl estradiol (150 mcg once a day), which did not seem to have an effect on future PSA values. At that time a restaging bone scan was done which revealed a suspicion of single metastasis in the proximal femur. She remained asymptomatic. Fourteen months later, her PSA level increased to 40 ng/mL and a repeat bone scan demonstrated significant progression of osseous metastatic disease. At that time palliative chemotherapy was initiated. The patient was treated with mitoxatrone every 21 days and with prednisone 5 mg orally twice daily. She was given 6 cycles of chemotherapy with no toxic side effects. Her PSA initially rose from 53 ng/mL at the start of chemotherapy to 75 at the third cycle and reached plateau at 78 ng/mL after the sixth cycle. Repeated bone and computed tomography scan during chemotherapy showed stable appearances. On August 27, 2005, she was admitted to hospital with general deterioration in health. The next day, the patient died of thromboembolic event.
Fig. 1.
Prostate-specific antigen values during treatment of transgender women with prostate cancer.
Discussion
The development of prostate adenocarcinoma in feminized transgender women is extremely rare. It has been assumed that castration in early life protects against prostate cancer. There are a few case series on castrated Ottoman court eunuchs, who, after 44 years, have small or non-palpable prostates on digital rectal examination, with evidence of atrophy on histological examination.1 It might suggest that the development and the viability of the gland throughout life require the continued presence of androgens. After 8 months of anti-androgen or estrogen therapy, the histological appearance of the prostate reveals low content of malignant epithelial cells, which are only detectible by immunohisto-chemical staining. According to tumour stem cell hypothesis, these cells might contain tumour stem cells which later give rise to recurrent tumours in new environment.2 There are numerous unanswered questions rising with regards to diagnosis and management of prostate cancer in transgender women. Should these patients be offered radical prostatectomy before initiation of estrogen therapy? Or should we closely monitor these patients with routine PSA measurements? One of the unresolved questions is whether the long-term administration of estrogens in male-to-female transsexuals is safe? To our knowledge only 3 cases of prostate cancer in transgender women have been reported.3–5 In these patients, the longest duration between initiation of estrogen therapy and diagnosis was 23 years. Our case is the first reporting the development of metastatic prostate cancer diagnosed 31 years after feminization despite previous radical treatment with curative intent.
In our patient, the initial diagnosis confirmed Gleason 7 prostatic adenocarcinoma on histological samples with no evidence of metastasis on imaging. It is not clear, however, if the same diagnostic and prognostic criteria should apply to prostate glands subjected to previous androgen deprivation. Such specimens are technically difficult for the pathologist to score in the presence of morphologic changes induced by androgen deprivation. One explanation of cancer pathogenesis might be the existence of microscopic cancer lesions before estrogen initiation. If we assume that androgens are necessary for the development of carcinoma then the neoplastic processes must have started before the orchidectomy and were stimulated by prolonged exposure to estrogens in this patient. However, some animal studies suggest that castrate levels of androgens inhibit microscopic lesions to develop to macroscopic ones with potential of metastasizing. Another explanation might be understaging of prostate cancer at initial diagnosis.6
Another interesting aspect in our patient’s case was her moderate response to anti-androgens (flutamide), which may suggest the existence of an active androgen receptor (AR) pathway. Few studies have shown that AR is commonly expressed in prostate cancer with increased levels after androgen deprivation.7–9 If AR were upregulated after a prolonged period of androgen deprivation thus making cancer cells more sensitive to anti-androgens, then we might have expected a longer period of PSA response. In this patient, we could only observe that PSA values plateaued for less than a year while exposed to anti-androgens.
Another interesting aspect in the management of prostate cancer in transgender women is the optimal chemotherapy regimen. In one previously reported case,5 the authors used docetaxel, carboplatin and estramustine as second-line chemotherapy, after previously observed progression on docetaxel chemotherapy, with excellent clinical and biochemical response. In our patient mitoxatrone and pred-nisone was used as first-line palliative therapy with no obvious clinical response. Docetaxel with prednisone therapy is now the standard first-line therapy, and the response rate has been evaluated in large randomized clinical trials.10,11
Conclusion
Currently, new emerging therapies, such as abiraterone, enzalutamide, sipuleucel-T and cabazitaxel, will certainly change the way we treat hormone-resistant prostate cancer. The development of prostate cancer after prolonged exposure to estrogens in transgender women raises questions in the “protective” role of castrate status in cancer pathogenesis.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Wilson JD, Roehrborn C. Long-term consequences of castration in men: lessons from the Skoptzy and the eunuchs of the Chinese and Ottoman courts. J Clin Endocrinol Metab. 1999;84:4324–31. doi: 10.1210/jc.84.12.4324. [DOI] [PubMed] [Google Scholar]
- 2.Maitland NJ, Collins A. A tumour stem cell hypothesis for the origins of prostate cancer. BJU Int. 2005;96:1219–23. doi: 10.1111/j.1464-410X.2005.05744.x. [DOI] [PubMed] [Google Scholar]
- 3.Thurston AV. Carcinoma of the prostate in a transsexual. Br J Urol. 1994;73:217. doi: 10.1111/j.1464-410X.1994.tb07503.x. [DOI] [PubMed] [Google Scholar]
- 4.van Haarst EP, Newling DW, Gooren LJ, et al. Metastatic prostatic carcinoma in a male-to-female transsexual. Br J Urol. 1998;81:776. doi: 10.1046/j.1464-410x.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- 5.Dorff TB, Shazer RL, Nepomuceno EM, et al. Successful treatment of metastatic androgen-independent prostate carcinoma in a transsexual patient. Clin Genitourin Cancer. 2007;5:344–6. doi: 10.3816/CGC.2007.n.016. [DOI] [PubMed] [Google Scholar]
- 6.Tsukamoto S, Akaza H, Onozawa M, et al. A five-alpha reductase inhibitor or an antiandrogen prevents the progression of microscopic prostate carcinoma to macroscopic carcinoma in rats. Cancer. 1998;82:531–7. doi: 10.1002/(SICI)1097-0142(19980201)82:3<531::AID-CNCR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Latil A, Bièche I, Vidaud D, et al. Evaluation of androgen, estrogen (ER alpha and ER beta), and progester-one receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–26. [PubMed] [Google Scholar]
- 8.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 9.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 10.Small EJ, McMillan A, Meyer M, et al. Serum prostate-specific antigen decline as a marker of clinical outcome in hormone-refractory prostate cancer patients: association with progression-free survival, pain end points, and survival. J Clin Oncol. 2001;19:1304–11. doi: 10.1200/JCO.2001.19.5.1304. [DOI] [PubMed] [Google Scholar]
- 11.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]