Abstract
The conversion of what has been interpreted as “normal brain aging” to Alzheimer’s disease (AD) via transition states, i.e., preclinical AD and mild cognitive impairment, appears to be a continuous process caused primarily by aging-dependent accumulation of amyloid β peptide (Aβ) in the brain. This notion however gives us a hope that, by manipulating the Aβ levels in the brain, we may be able not only to prevent and cure the disease but also to partially control some very significant aspects of brain aging. Aβ is constantly produced from its precursor and immediately catabolized under normal conditions, whereas dysmetabolism of Aβ seems to lead to pathological deposition upon aging. We have focused our attention on elucidation of the unresolved mechanism of Aβ catabolism in the brain. In this review, I describe a new approach to prevent AD development by reducing Aβ burdens in aging brains through up-regulation of the catabolic mechanism involving neprilysin that can degrade both monomeric and oligomeric forms of Aβ. The strategy of combining presymptomatic diagnosis with preventive medicine seems to be the most pragmatic in both medical and socioeconomical terms.
Keywords: Alzheimer’s disease (AD), mild cognitive impairment (MCI), preclinical AD, amyloid β peptide (Aβ), metabolism, neprilysin, secretase
1. Introduction
Aging is a major risk factor for most of the dementing disorders predominantly represented by Alzheimer’s disease (AD). The estimated number of AD patients in the world is approximately 50 million and is increasing as the world population ages. More than one out of every two people older than 85 years suffer from either dementia or mild cognitive impairment (MCI), a prodrome of dementia.1,2) This fact was not considered a major threat to the society until a few decades ago when average life expectancy could be well below 70 years, even in the developed countries. Today, however, the average life expectancy in most developed countries is either close to or beyond 80 years. This indicates that dementia is becoming an ultimate form of the manifestation of brain aging. In other words, the chances of the general population being affected by the AD neuropathology sometime later in life are likely to be greater than 50% unless the disease becomes preventable and curable. Symbolically, China, with a population of more than a billion people, presently exhibits the highest rate of increase in the number of AD patients. A recent epidemiological study in Japan indicates that, of those persons over 65 years of age, 10% suffer from dementia and 7% from MCI.3)
Of particular note is that two of the major neuropathological hallmarks of AD, senile plaques and neurofibrillary tangles, take place with the aging of the human brain many years prior to the disease onset.4–6) This suggests that aging is the predominant risk factor for AD, and that a large number of people entering an old age and manifesting these neuropathological structures in their brains are likely to be in a presymptomatic stage of MCI and AD, termed “preclinical AD”.7) In this respect, it is most important that a preventive medicine combined with presymptomatic diagnosis allows a substantial portion of aged people to escape from the scourge of dementing syndromes. To achieve this goal, we need to clarify the primary cause(s) of AD development and the molecular mechanisms underling the neuropathological cascade.
2. Etiology of AD
Pathological and patho-biochemical studies mostly up to 1990 established the chronology of the major pathological events of AD, at least in the neocortex, and identified the molecular components contributing to the pathological structures. Senile plaques, present extracellularly, consist mainly of amyloid β peptide (Aβ), while neurofibrillary tangles (NFTs), present intracellularly, contain primarily tau protein. The research community seems to be reaching a consensus that these pathological structures as they appear may not be the direct causes of AD symptoms, but rather, that the processes, not fully identified yet, that is represented by the pathology, such as synaptotoxic Aβ oligomer formation, may be essential in the pathogenesis.8)
In any case, the temporal distance of decades between the cause and effect has been the most challenging factor in AD research. Even the most aggressive form of autosomal dominant familial AD takes more than 20 years before the disease onset. Besides, the chronology of pathological events alone does not establish any cause-and-effect relationship. AD research thus resembles archaeology in that researchers must collect a large body of consistent circumstantial evidence in order to form a consensus. The observations of pathological structures such as senile plaques and neurofibrillary tangles in AD brains, for instance, have always engendered arguments as to whether these structures represent pathologically essential and significant pathways or just by-products or consequences of something else that is essential in the process.
In this respect, identification of the familial AD- and tauopathy (FTDP-17: frontotemporal dementia with Parkinsonism liked to chromosome 17)-causing gene mutations and analyses of their phenotypes in the 1990s have played a major, indispensable role in resolving the etiology of AD.6,9–12) Consensus has now been reached in that the decades-long cascade leading to dementia is initiated by the accumulation of Aβ in the brain and that tauopathy is likely to play a major role in the neurodegenerative processes downstream of Aβ deposition although there may possibly exist a tauopathy-independent pathway(s). Thus, the 1990s thus may be called the “decade of familial AD”. The next 10 years of the 2000s have witnessed some advancement in the understanding of the cause-and-effect relationship of sporadic AD, which represents the vast majority of all AD cases,13) but the mechanisms still remain largely elusive.
More than 200 mutations that cause familial AD have been identified14) in the three genes encoding the amyloid precursor protein (APP), presenilin 1 and presenilin 2 proteins, all of which are involved in Aβ generation as indicated by red arrows in Fig. 1. A number of studies in the 1990s described the molecular phenotypes caused by these mutations. Some papers examined the effects of the mutations on Aβ production, while others examined effects on cytoskeletal abnormalities involving tau protein, cell death or apoptosis, endoplasmic reticulum stresses, etc. These studies have established a consensus that the only phenotype commonly shared by essentially all the mutations in vitro (in cell culture), in vivo (in transgenic and knock-in (KI) mice), and in humans carrying these mutations is the increased production of a specific species of Aβ, Aβ42, which is much more hydrophobic and thus more apt to oligomerize and polymerize than the other major species, Aβ40. These results have strongly suggested that Aβ42 is the primary pathogenic agent in the AD cascade.10,12) Typically, most of the presenilin 1 mutations, accounting for more than 70% of all familial AD mutations thus far identified, cause very aggressive presenile Aβ amyoidosis in humans by increasing the steady-state Aβ42 level approximately 1.5-fold, as detected in the brains of mutant presenilin 1 transgenic or KI mice.15,16)
Figure 1.
Generation of Aβ from its precursor, APP. APP is first cleaved by β- or α-secretase, generating a C-terminal fragment (C99 or C83), which then is cleaved by γ-secretase to generate Aβ. The major species generated are Aβ40 and Aβ42. The latter is more hydrophobic and more apt to aggregate and thus is considered to be primarily pathogenic, consistent with the phenotype of the major familial AD-causing mutations. The red arrows indicate where in these processes the major familial AD mutations exert their effects. Atypical mutations (see text) are indicated by blue arrows.
Atypical mutations in the intra-Aβ sequences of APP, such as the Dutch, Flemish, Italian and Arctic mutations, have also been identified (indicated by blue arrows in Fig. 1). Most of these mutations result in hemorrhages or strokes caused by unusually severe cerebral amyloid angiopathy (CAA) that also accompanies the pre-senile parenchymal Aβ deposition. These atypical mutations are generally believed to cause Aβ accumulation by augmenting aggregation or protofibril formation.17–19) Interestingly, the pathological phenotypes of these mutations are not fully identical. Yamamoto et al.20) have pointed out that the difference among the closely located mutations may be caused by differential interactions with different ganglioside species. Additionally, we found that these mutations also cause Aβ to be more resistant to the degradation catalyzed by a physiologically relevant peptidase, neprilysin, described in Section 4.21) An intra-Aβ mutation, the Tottori mutation that seems to cause typical AD, has recently been identified.22) This mutation also seems to render Aβ more resistant to neprilysin-catalyzed proteolysis without altering its aggregation properties (Tsubuki et al., unpublished observations). Therefore, the intra-Aβ sequence mutations may exert dual pathogenic effects associated not only with aggregation/protofibril formation but also with one major aspect of metabolism, proteolytic degradation.
These autosomal dominant familial AD mutations together account for less than 1% of all AD cases.13) Sporadic AD accounts for most cases of AD, and the etiology of sporadic AD remains much more elusive than that of familial AD. However, sporadic AD is essentially indistinguishable from familial AD in both pathological and neurological terms. This suggests that we need to understand why Aβ accumulates in sporadic AD. From this, inhibition of Aβ accumulation in the brain is likely to contribute to, at least in part, the prevention of both familial AD and sporadic AD.
Consistent with the extremely high prevalence of sporadic AD in the aged population, most clinically “normal” people start accumulating Aβ in their brains between the ages 40 and 80 years.23–25) When Aβ reaches levels approximately 10,000-times greater than normal, the disease seems to arise; preclinical AD and MCI represent the transition states from “normal aging” to AD development. Carriers of the apolipoprotein E4 genotype, the predominant genetic risk factor for AD thus far confirmed universally,26) begin accumulating Aβ at an earlier age than non-carriers.25) These facts further indicate that accumulation of Aβ is also a primary cause for “Sporadic AD” and that inhibition of Aβ accumulation would at least provide a preventive measure. Once it becomes possible to manipulate Aβ levels in the brain, such medical treatment after AD development, before MCI appearance, and even before preclinical AD would relate to “postsymptomatic therapy”, “presymptomatic prevention” and “aging control (at least in part)” respectively. For presymptomatic prevention to be possible, we need first to establish reliable measures for presymptomatic diagnosis (see Section 6).
3. Aβ metabolism: three major targets
Aβ is a physiological peptide secreted from neurons under normal conditions both in vitro and in vivo.27–29) Aβ is generated from APP by sequential limited proteolysis conducted by β- and γ-secretases (Fig. 1). As stated in the previous section, most of the “Familial AD” mutations in APP and presenilin (a γ-secretase catalytic component) genes result in overproduction of Aβ42. Aβ does not appear to play a major physiologically important role; the apparent role of APP processing by the α- and β-secretases is the release of soluble forms of APP, or APPs, which are known for their neuroprotective and neurotrophic functions.30) Further to this, splice variants containing the insert sequences corresponding to the Kunitz-type protease inhibitor (KPI) domain have been identified as protease Nexin II, an endogenous inhibitor against a group of serine proteases including thrombin.31) APP has also been reported to possess a domain that inhibits matrix metalloproteinases.32) The other possible function of APP processing may be the release of a cytoplasmic fragment (APP intracellular domain: AICD), generated by γ-secretase, which may translocate to the cellular nucleus and play a physiological regulatory role in transcription in a manner similar to the cleavage of Notch by γ-secretase activity.33) Alternatively, Kim et al.34) indicated that AICD could be neurotoxic.
Thus far, several substrates for β-secretase and a number of substrates for γ-secretase other than APP have been identified.33,35–38) Of all of the known substrates, the fragment that corresponds to Aβ in APP does not seem to possess any biological role. Therefore, at present it is most likely that Aβ is simply an unwanted by-product of APP processing. Discovery of the β- and γ-secretase substrates calls for precaution concerning the anti-Aβ production strategy targeted at the secretases: chronic and over-inhibition of the secretases is likely to cause adverse side-effects. Inhibitors specific to β-secretase appear to be more suitable in terms of clinical applicability because the phenotype of β-secretase deficiency is apparently milder,39) whereas that of γ-secretase deficiency is lethal.33) De Strooper and colleagues (Dominguez et al.40)) demonstrated that β-secretase deficiency causes partial lethality in neonatal mice using two independent knockout lines, indicating that β-secretase inhibition could also provoke a side effect. There however still remains a hope that we may be able to produce a medication that selectively inhibits the generation of Aβ42 without altering metabolism of APP or other γ-secretase substrates.33)
In any case, APP processing takes place constitutively in the brains of both young and old, and, at least in the brains of young and healthy individuals, no Aβ deposition takes place. Taken together, these observations clearly indicate that, under normal conditions, Aβ is constantly anabolized and then rapidly catalyzed before it can be deposited. This catabolism can take place inside the brain or in the circulatory system after transport out of the brain. The kinetic relationships between the three metabolic processes of anabolism, catabolism and transport are schematized in Fig. 2. (Aβ40 is left out for the sake of simplicity.)
Figure 2.
Kinetic relationships between production, degradation within the brain and transport out of the brain. The steady-state Aβ (Aβ42) level in the brain, [Aβ] ([Aβ42]), is primarily a function of the APP level, [APP], the rate constants for production, [K1], in-brain degradation, [K2] and out-of-brain transport, [K3]. See Section 3 for details.
K1, K2, and, K3 are the rate constants for production, in-parenchyma degradation, and out-of-brain transport of Aβ, respectively. Under the assumptions that the kinetics of the reactions can essentially be analyzed linearly, that these rate constants are independent of each other, and that these processes exist in a dynamic steady-state equilibrium (see previous reviews for details regarding these assumptions41,42)), the relationship between the amounts of Aβ42 and APP, represented as [Aβ42] and [APP], respectively, can be expressed by the following equation:
 |
[1] |
Formula [1] is consistent with the phenotypes of almost all the familial AD mutations in APP and presenilin 1 genes; K1 is approximately 1.5-fold greater than that in normal controls, meaning that [Aβ42] also becomes 1.5-fold greater. It is also consistent with one of the phenotypes of Down’s syndrome caused by trisomy of chromosome 21 carrying the APP gene; [APP] is 1.5-fold greater than in normal controls and [Aβ42] also becomes 1.5-fold greater.
Therefore, an increase in the rate constant for production (K1) or decreases in the rate constants for in-parenchyma degradation (K2) and out-of-brain transport (K3) can elevate [Aβ42] and thus be causal for pathological Aβ deposition, or oligomer formation. This logic also indicates that down-regulation of K1 or up-regulation of K2 and K3 can reduce Aβ deposition in the brain. Note that the activation of α-secretase(s) would also contribute to reducing K1.36,43) Taken together, these three major targets provide theoretical possibilities for anti-Aβ therapy.
There has been some discussion regarding the relative importance of K2 (in-parenchyma degradation) versus K3 (out-of-brain transport) in Aβ clearance.44) If K2 is excessively greater than K3, Formula [1] would tend towards [Aβ42] ≈ K1/K2 × [APP], whereas, if K2 is excessively smaller than K3, it would be [Aβ42] ≈ K1/K3 × [APP]. We assume that the rate constants are such that K2 > K3 and thus [Aβ42] ≈ K1/K2 × [APP]. The basis of this reasoning is as follows: (i) Brains are rich sources of various peptidases, which can proteolyze Aβ as long as they are accessible to the substrate; (ii) Because the amounts of Aβ in the brain and in the CSF or plasma are poorly correlated with each other in AD patients,45,46) there does not seem to exist an effective transport-dependent mechanism that clears Aβ out of the brain, at least in humans under the pathological condition where Aβ levels in the brain are 1,000–10,000 times greater than in normal brains;23) (iii) If reduced efficiency of transport out of the brain to the circulatory system was a major cause of the pathological Aβ deposition, one would expect vascular amyloid deposition to arise earlier than parenchymal deposition, but the reality is generally the other way around, particularly in humans; (iv) Human brains are approximately 1,000 times larger than mouse brains in size. Therefore, the advantage of in-parenchyma degradation over transport-dependent clearance is likely to be even greater in humans than in mice, particularly if the major transport pathway is via the interstitial fluid (ISF) and from there to the cerebrospinal fluid (CSF) and plasma. It should be noted that the primary function of proteolysis is the recycling of amino acids; therefore in-parenchyma degradation is more economical from a metabolic (energy consumption) point of view than out-of-brain transport, especially in the case of humans.
The importance of the transport mechanism has however been experimentally highlighted by the Aβ vaccination approach.47,48) Interestingly, DeMattos et al.49) demonstrated that intravenous injection of anti-Aβ antibody into APP transgenic mice induces rapid elevation of Aβ levels in the plasma in a manner correlating to Aβ burdens in the brain, implying the presence of active mechanism(s) that transport Aβ from within the brain to the circulatory system. Even so, the molecular and cellular mechanisms underlying Aβ vaccination are still elusive and whether or not the observations made by DeMattos et al.49) for the mouse model can be extrapolated to humans remains to be determined. Nevertheless, the Aβ vaccination is a strong candidate approach to reduce Aβ burdens since a study using non-human primates50) provided data that support the results of Schenk et al.47) In our view, passive immunization using humanized monoclonal anti-Aβ antibody, rather than active immunization using Aβ or Aβ derivatives, seems to be more promising and safer because the former is likely to generate consistent effects while the latter may result in unexpected side-effects51) if used for several years. Passive immunization would however be a costly medication requiring a large amount of purified monoclonal antibody without any antigenicity. An antibody to an amino-terminal truncated form of Aβ starting from pyroglutamate at position 3 may provide an interesting medical treatment because this form of Aβ is present in human brains only under pathological conditions.52,53)
4. Identification and characterization of Aβ-degrading mechanisms
More than a decade ago, we hypothesized that down-regulation of Aβ degradation may be a primary relevant cause for Aβ accumulation in sporadic AD.41,54,55) This hypothesis was made on the basis that there is little evidence supporting the up-regulation of Aβ generation upon aging prior to Aβ deposition in the brain, that sporadic AD patients seem to accumulate less Aβ42 compared to Aβ40 than familial AD patients56) and because aging generally accompanies down-regulation rather than up-regulation of enzyme activities (except for those associated with inflammation and other pathological processes).
In order to test this working hypothesis, we first set out to identify the major in vivo Aβ-degrading enzyme in the brain. Whereas the mechanism of Aβ generation had been examined in depth using molecular and cellular biological approaches,36,43,57,58) the mechanism of Aβ degradation remained elusive. This was because not only the cellular topology but also the complex structural organization of the brain tissue composed of various types of cells needed to be taken into consideration in analyzing Aβ degradation taking place in the brain. Therefore, we started our series of degradation studies by establishing a novel in vivo experimental paradigm in which we injected synthetic internally multi-radio-labeled Aβ1–42 into the hippocampus of anesthetized live rats and analyzed the degradation process using high-pressure liquid chromatography directly connected to a flow-type scintillation counter.55) Experiments using a panel of more than 20 peptidase inhibitors highlighted that a neutral endopeptidase family member, similar or identical to neprilysin, appears to play a major role in the Aβ1–42 catabolism because thiorphan, a well-characterized neutral endopeptidase inhibitor,59) was the most potent inhibitor. In accordance with this, short-term and long-term infusions of thiorphan into the rat hippocampus resulted in biochemical and pathological accumulation of endogenous Aβ, respectively. Dolev and Michaelson60) recently demonstrated that thiorphan infusion induces Aβ accumulation in an apolipoprotein E genotype-dependent manner, consistent with the human pathology.25,26)
The next task was to identify the major responsible Aβ-degrading peptidase among members of the neutral endopeptidase family. We made efforts to determine the molecular identity by using biochemical and molecular biological approaches and, consequently, predicted that neprilysin is likely to be the primary candidate.61,62) We subsequently confirmed our prediction by examining the degradation of radio-labeled Aβ in neprilysin-knockout (KO) mouse brains using their wild-type littermates as positive controls.63) Neprilysin is a type II membrane-associated peptidase whose active site faces the lumen or extracellular side of membranes59,64,65) (Fig. 3). This topology is suited for the degradation of extracytoplasmic peptides such as Aβ. Using immunofluorescence microscopy, we confirmed that neprilysin is essentially exclusively expressed in neurons, not in glia, and that the peptidase, after synthesis in the soma, is axonally transported to presynaptic terminals,66) presumably in a manner similar to the way APP is transported. Therefore, presynaptic terminals and nearby intracellular (lumen-side) locations are likely to be the sites of Aβ degradation by neprilysin.67)
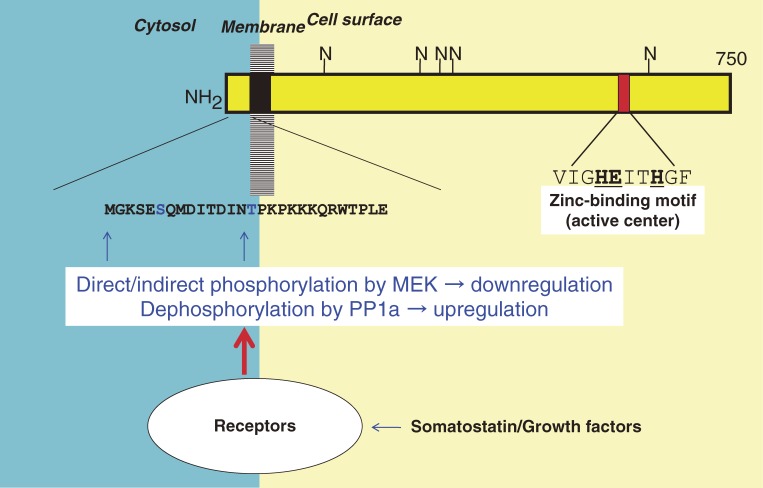
Figure 3.
Schematic structure and cellular topology of neprilysin. Neprilysin is a type II membrane-associated peptidase whose active site faces the lumen or extracellular side of membranes. The enzyme requires the presence of zinc for proper functioning. N: N-glycosylation sites. The glycosylation is reported also to be required for the enzymatic activity to be fully expressed.137)
Based on the above observations, we examined the ability of neprilysin-KO mouse brains to degrade Aβ in vivo and quantified the endogenous Aβ levels in the brains.63) Due presumably to redundancy in the neutral endopeptidase family,59,65,68) the KO mice show normal characteristics in relation to reproduction, development, and adult anatomy to the best of our knowledge.69) The ability to degrade the radiolabeled Aβ was significantly reduced in the KO mouse brains. Consistently, both the endogenous Aβ40 and Aβ42 levels were elevated approximately 2-fold, in a manner comparable to or even greater than what has been described in familial AD-causing mutant presenilin transgenic or knock-in mice.15,16) More importantly, the elevation of Aβ levels was inversely correlated with the gene dose of neprilysin and thus with the enzyme activity.63,70) These observations suggest that even partial loss of neprilysin expression/activity causes the elevation of Aβ1–42 levels and thus could induce Aβ amyloidosis on a long-term basis in a manner similar to that of familal AD-causing gene mutations. These results also indicate that the rate constant for the in-parenchyma degradation of Aβ42 by neprilysin accounts for about 50% of total clearance activity, or K2 + K3 in Formula [1]. Therefore, as it is improbable that K2 is negligibly smaller than K3, K2 is consequently likely to be even greater in human brains for the reasons stated in the previous section.
In a similar manner, some of the other Aβ-degrading enzyme candidates were examined by a reverse genetic approach, i.e., by measuring the Aβ levels in the brains of KO-mice (Table 1). To our knowledge, neprilysin seems to be the dominant peptidase regulating the steady-state level of the primarily pathogenic Aβ species, Aβ42, as do the pathogenic familial AD presenilin mutations. The 2-fold increase in the Aβ levels in neprilysin-KO mouse brains indicates that neprilysin accounts for approximately 50% of total clearance mechanism (K2 + K3 in Formula [1]). Endothelin-converting enzymes (ECEs) are interesting because they resemble neprilysin in structure, belonging to the same family of proteases (M13 family),70) and because they degrade Aβ in acidic intracellular compartments.71) Notably, not only neprilysin but also a number of other Aβ-degrading enzyme candidates, including ECEs and insulin-degrading enzyme (IDE) (Table 1), are zinc-requiring enzymes. Further to this, all the α-secretase candidates, a disintegrin and metalloproteinase (ADAMs) 9, 10 and 17,36,43) which could contribute to reduction of Aβ synthesis, also require zinc for their proteolytic activities. Therefore, although too much zinc would be obviously harmful,72) a heightened zinc deficiency is likely a negative risk factor for AD.
Table 1.
Aβ-degrading enzyme candidates: Effect of genetic deficiency on Aβ levels in the brain
| Mice | Aβ42 | Aβ40 | References |
|---|---|---|---|
| Presenilin 1-KI3 (positive control) | 1.5-fold increase | Not significant | Nakano et al.16) |
| Neprilysin-KO(−/−) | 2-fold | 2-fold | Iwata et al.63) |
| Neprilysin-KO(+/−) | 1.5-fold | 1.5-fold | Iwata et al.63) |
| ECE 2-KO (−/−) | 1.3-fold | 1.3-fold | Eckman et al.71) |
| ECE 2-KO (+/−) | 1.2-fold | 1.2-fold | Eckman et al.71) |
| ECE 1-KO (+/−) | 1.3-fold | 1.3-fold | Eckman et al.71) |
| IDE-KO (−/−) | Not significant | 1.1- to 1.2-fold | Farris et al.94) |
| IDE-KO (−/−)1 | 1.4-fold | 1.6-fold | Miller et al.133) |
| tPA-KO (−/−) | Not significant | Not significant | unpublished data by Iwata et al. |
| uPA-KO (−/−) | Not significant | Not significant | Ertekin-Taner et al.134) |
| ACE-KO (−/−) | Not significant | Not significant | unpublished data by Takaki et al. |
| Plasmin (−/−)2 | Not significant | Not significant | Tucker et al.135) |
KO: (gene) knock-out; KI: (gene) knock-in; tPA: tissue-type plasminogen activator; uPA: urokinase-type plasminogen activator; ACE: angiotensin-converting enzyme. 1Only water-soluble Aβ, which accounts for less than 30% of total Aβ was quantified. 2Plasminogen-KO mice were examined as plasmin-KO mice. 3The data from mutant presenilin 1-KI mice, which show typical pathogenic alterations in Aβ levels, leading to accelerated Aβ accumulation in the brain, are presented as a positive control. Quantification was performed using an identical enzyme-linked immunosorbent assay.136) The mice were from 8 to 10 weeks of age.
McGeer and colleagues reported that neprilysin mRNA levels are significantly reduced in the brain areas vulnerable to Aβ pathology in preclinical AD patients at a relatively early stage (Braak stage II) as compared to age-matched normal controls.73,74) These observations are consistent with our hypothesis. Nevertheless, as it was still unclear whether reduction of neprilysin expression or activity precedes Aβ pathology during the course of aging, we thus examined the effect of aging on the expression/activity of brain neprilyisn using two methods: (i) A biochemical assay to measure neutral endopeptidase activity in the brain (The advantage of this is that it is more quantitative than the latter, whereas the disadvantage is that the method fails to provide information regarding the local or spatial activity/expression of neprilysin).; (ii) An immunofluorescence detection method using an anti-neprilysin antibody.66) Representative immunofluorescence results are shown in Fig. 4. The selective reduction of neprilysin expression upon aging is apparent in the polymorphic cellular layer, inner molecular layer, and outer molecular layer of the dentate gyrus and also in the stratum lucidum of the CA3 sector of the hippocampus.75) The differences were also proven to be statistically significant by quantitative image analyses. The results of the enzymatic quantification were also consistent with these observations; we observed a statistically significant reduction of approximately 10% of the enzyme activity per year in the entire hippocampus for two years and a 10% reduction in the neocortex in two years. If the 1.5-fold increase of Aβ42, caused by the pathogenic mutations in the presenilin gene, is truly sufficient for the development of early-onset familial AD, then a 1% reduction of neprilysin activity per year, leading to 50% reduction of neprilysin activity at the age of 50 and thus about a 1.5-fold elevation of the Aβ levels in the brain, would be sufficient to be causative of late-onset AD in humans.
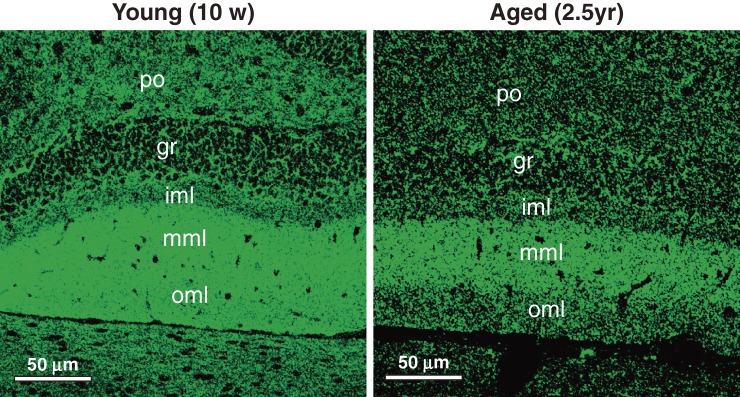
Figure 4.
Aging-dependent reduction of neprilysin in polymorphic cell layer, inner molecular layer and outer molecular layer of the dentate gyrus. The selective reduction of neprilysin expression is apparent in the polymorphic cellular, inner molecular layer and outer molecular layer of the dentate gyrus. See Iwata et al.75) for further details. Reproduced with permission from John Wiley & Sons, Inc.
Since then there have been an overwhelming number of reports indicating that neprilysin expression/activity declines with aging and in AD.76–81) An exception is a report by Miners et al.,79) showing that neprilysin enzyme activity increasesd with aging. This paper however suffers from two major weaknesses. First, the authors used tissues from Brodmann area 6 for their analysis. This area, composed of the premotor cortex and the supplementary motor area, is much less affected by Aβ pathology than such regions as temporal cortex and hippocampus and thus is inappropriate for observing representative aging-dependent changes of neprilysin expression/activity in brain. Second, the immunocapture-enzyme assay used by the authors lacks appropriate controls. We thus analyzed WT, heterozygous and homozygous neprilysin-KO, and aged mice using Western blotting, immunocapture assay and immunocapture-activity assay. The immunocapture-activity assay exhibited very poor linearity (Saito and Saido, unpublished observation) and therefore is only qualitative. This probably accounts for the discrepancy between the neprilysin protein contents and enzyme activities in the Miners’ paper.
The immunofluorescence observations (Fig. 4) indicate that the areas where we see selective aging-induced reduction of neprilysin expression correspond to the terminal zones of mossy fibers and perforant path, which suggest that local Aβ concentrations are particularly elevated at the presynaptic locations originally projecting from the entorhinal cortex. It is notable that the entorhinal cortex is the region where the initial neurodegeneration takes place in AD brains.82) Indeed, Mèlanie et al.83) reported that not only parenchymal Aβ amyloidosis but also amyloid angiopathy correlates inversely with neprilysin levels in control and AD patients. Several reports also describe the possible role of neprilysin in inhibiting Aβ accumulation in the brain.66,84–87) Other reports have associated neprilysin gene polymorphisms with the incidence of AD as summarized elsewhere,67) although the aging dependent decline of neprilysin activity seems to be a natural process.75,88)
Our findings also indicate that regulation of neprilysin activity in a manner specific to brain regions that are vulnerable to Aβ deposition could provide an effective strategy to reduce Aβ burdens in the brain. The advantages of utilizing neprilysin activity for the purpose of regulating brain Aβ levels were discussed previously.70,89) A relatively straightforward approach in experimental terms, but not necessarily in clinical terms, is the application of gene therapy. Indeed, we demonstrated using an in vitro paradigm that overexpression of neprilysin, but not of an inactivated mutant form, in primary cultured neurons caused by the Sindbis virus leads to clearance of both the extracellular and cell-associated Aβ40 and Aβ42 forms.90) We also succeeded in regulating Aβ levels in vivo and in reducing Aβ burdens in APP transgenic mice by expressing neprilysin using adeno-associated virus.87) Similar results were also reported by the groups of Hersh85) and of Masliah.91,92) These approaches however require neurosurgery and thus are quite invasive. In this respect, the success in global brain delivery of neprilysin gene by intravascular administration of AAV vector in mice93) stands as a major breakthrough in the field of gene therapy.
Selkoe and colleagues demonstrated that transgenic overexpression of neprilysin and IDE results in a reduction of the Aβ pathology in APP transgenic mice.86) For a number of reasons, the effect of IDE is likely to have been mediated by alteration of APP processing by increased insulin levels rather than by the direct proteolysis of Aβ. First, insulin is a better substrate than Aβ for IDE,94) and insulin signaling regulates neuronal APP processing and trafficking.95,96) Also, IDE expression failed to reduce Aβ levels in a Drosophila model overexpressing Aβ42 (not APP), whereas neprilysin expression significantly decreased Aβ levels and inhibited Aβ42-induced neurodegeneration.97) The advantages of neprilysin utilization over IDE utilization for the purpose of reducing Aβ levels in the brain are the following: (i) IDE is primarily a cytsolic protein capable of degrading the APP intracellular domain in vivo far better than any known substrates94) and thus is unlikely to have direct access to Aβ unless the cells are permeabilized; (ii) Neprilysin degrades extracytoplasmic peptides particularly at synapses66,87,98,99) where Aβ may cause neuronal dysfunction; (iii) The in vivo effect of neprilysin on brain Aβ seems to be unexpectedly selective because neprilysin deficiency does not seem to alter the levels of “neprilysin substrate” neuropeptides such as enkephalin, cholecystokinin, neuropeptide Y, substance P and somatostatin in the brain (Iwata & Saido, unpublished data); (iv) Neprilysin can degrade Aβ oligomers, which can impair neuronal plasticity,100) both in tubes101) and in vivo102) whereas IDE can degrade only Aβ monomers.103)
The ability of neprilysin to degrade Aβ oligomer provides an important implication about the spatial relationship between the cause and effect in AD etiology. Even under normal conditions, hippocampus exhibits the highest levels of Aβ in the presence or absence of neprilysin.63) Because neprilysin deficiency increases the quantity of synaptotoxic Aβ oligomers,102) oligomer formation is likely primary in AD pathogenesis while plaque formation secondary.
Since the gene therapy approach, which requires surgical procedures, is not yet a realistic approach for clinical application to humans, we have sought a pharmacological means to selectively up-regulate brain neprilysin activity as a new therapeutic candidate.104) A rationale for this strategy was based on the fact that at least two cell type-specific ligands capable of up-regulating neprilysin activity have been identified: opioids for monocytes105) and substance P for bone marrow cells106) as part of negative feedback mechanism. It is notable that receptors for these ligands are G protein-coupled receptors (GPCRs). We found that somatostatin (SST) regulates the metabolism of Aβ peptide in the brain via the modulation of proteolytic degradation catalyzed by neprilysin: Among various effector candidates, only SST up-regulated neprilysin activity in primary cortical neurons; A genetic deficiency of SST altered hippocampal neprilysin activity/localization and increased the quantity of a hydrophobic 42mer form of Aβ, Aβ42, in a manner similar to presenilin gene mutations that cause familial AD.99) These results indicated that SST receptor(s) now emerge as pharmacological target candidates for the prevention and treatment of AD (Fig. 3).67)
Thus far, five SST receptor subtypes have been identified, all of which are GPCRs,107) the most suitable pharmacological target category of proteins in the history of pharmaceutical science. Among the five subtypes, types two and four may serve as primary candidate targets because they are relatively potently expressed in the neocortex and hippocampus.107,108) Synthesis of blood brain barrier-permeable agonists that can distinguish between different receptor subtypes, which should not be an impossible task in modern medicinal chemistry,107) would make it possible to achieve a medical application to our findings. Alternatively, the use of an “anti-dementia” compound such as FK960 that elevates hippocampal SST levels109) in brain may provide another effective approach. One potential benefit of harnessing neprilysin activity by agonizing SST receptor(s) among other Aβ-reducing strategies is that, if used conservatively, it is unlikely to be accompanied by major adverse side effects.
The expression of SST in the brain is known to decline with age in various mammals, including rodents, apes and humans.110,111) In human brains, SST mRNA is one of approximately 50 transcripts, the expression of which significantly declines after the age of 40, among approximately 11,000 transcripts examined.111) This indicates that the aging-dependent reduction of SST expression in the brain is a biologically specific and universal process. A prominent decrease in SST also represents a pathological characteristic of AD.112) These facts, combined with our observations that SST regulates neuronal neprilysin activity, led us to propose the following scenario for the etiology of sporadic AD development.113) First, the aging-dependent reduction of SST causes a decrease of neprilysin activity, which then causes the steady-state Aβ levels in brain to increase. Chronic elevation of the Aβ levels may result in further downregulation of SST levels,112) oxidative inactivation of neprilysin,76) increased expression of APP and β-secretase because APP is a stress-responsive protein114) and because expression both APP and β-secretase have been reported to increase in the relatively downstream cascade of AD development.74,115) These events form a vicious circle leading to a catastrophic accumulation of Aβ in the brain.23–25) Expression of cortistatin, a recently discovered SST-like neuropeptide that can activate SST receptors,107) may also decline upon aging. If this hypothesis turns out to be true, we will not only be able to understand the etiology of sporadic AD but will also have identified a primary strategic target for the prevention and treatment of AD.
5. Proposal for cocktail therapy strategy
Currently, four major, distinct strategies to reduce Aβ burdens in brain have been proposed: β-secretase inhibition,57) γ-secretase inhibition,58) Aβ vaccination,51,116) and enhanced degradation of Aβ.42,85–87,104) Each strategy, if practiced too extensively, is likely to cause chronic adverse effects. For instance, inhibition of β- and γ-secretases will interfere with proteolytic processing of other secretase substrates such as sialyltransferase35) and Notch,33,36) respectively, possibly resulting in perturbation of the immune system. The side effect of Aβ vaccination has already been reviewed.51)
However, if medications based on these strategies are used at relatively low doses or in a mild fashion so that Aβ is downregulated by just 30% in each case, then the side effects will probably become negligibly small. Still, if such medications are combined as a cocktail (putting vaccination aside), a very selective and potent effect on the Aβ level can be expected; a combination of β-secretase inhibitor, γ-secretase inhibitor and neprilysin activator, for instance, at doses that would lower the Aβ levels by 30% each would bring down Aβ to 34% (= 70% × 70% × 70%) or down by 66%, while other substrates down only to 70% (Fig. 5). Indeed, Asai et al.117) have shown that a cocktail of secretase inhibitors affects Aβ levels in an additive manner. Because any medications used to prevent or treat AD would be administered to patients for a long period of time, i.e., a few years or more, we propose that seeking both safety and effectiveness in this cocktail therapy approach would be given high priority as a potential near-future strategy to combat AD. The successful history of the cocktail therapy in the conquest of human immunodeficiency virus118) supports this proposal. This strategy may be extended to the targeting of other downstream pathological processes, such as inflammation,119) tauopathy120) and calpain activation121–124) to achieve maximum effects.
Figure 5.
Proposal for a cocktail therapy strategy targeted at three distinct aspects of Aβ metabolism. The inhibition of secretases or upregulation of Aβ degradation to the extent that they independently lower Aβ levels by 30% is unlikely to generate adverse side effects by influencing the metabolism of substrates other than APP and Aβ. When used in combined fashion, however, they would achieve a selective 62% decrease of the Aβ level. Substrates subjected to processing by the target molecule are presented in the circles. NSAIDS: non-steroidal anti-inflammatory drugs; PSGL-1: P-selectin glycoprotein ligand 1; ST-6Gal: sialyltransferease that produces a sialylα2,6galactose residue.
6. Future perspectives
One currently unresolved issue in AD research is the elucidation of the precise mechanism(s) by which Aβ deposition causes subsequent pathological effects, i.e., tauopathy, dysfunction and degeneration of neurons. Unraveling this mystery will open more opportunities for therapeutic interventions at various time points between Aβ deposition and neurodegeneration. The accelerating effect of excessive amounts of Aβ on tauopathy demonstrated in mouse models,125,126) although potentially problematic as stated above, may indicate the presence of an unknown phenomenon that relays pathological signals from the former to the latter. However, perhaps the most important and fundamental question yet to be answered is why Aβ is deposited in sporadic AD which accounts for 99% or more of all AD cases. It should be noted that the number of sporadic AD patients will grow as the average life expectancy increases, whereas the number of familial AD patients will simply remain proportional to the total population. Although our hypothesis described in Section 4 is one of the relevant possibilities, other possibilities also need to be pursued.
Recent studies indicate that virtually all humans start to accumulate Aβ in the brain upon aging.5,23–25) This suggests that Aβ deposition is an important factor that could determine the life span of our brain. It provides us with the hope that a large portion of AD cases can be prevented if we can make the brain life span longer than the body life span by reducing Aβ deposition (Fig. 6). The other way of looking at brain aging and Alzheimer’s disease is that aging causes abnormal proteolysis, resulting in abnormal deposition of proteins followed by neuronal dysfunction and degeneration as schematized in Fig. 7. The importance of proteolysis in AD pathogenesis is obvious because both generation (Fig. 1) and degradation (Table 1) of Aβ are performed by proteases and because proteases such as calpain127) and caspase128,129) may be involved in the downstream pathological cascade. The importance of proteolysis has been highlighted in other neurodegenerative disorders including Parkinson’s disease as well; loss-of-function mutations associated with juvenile Parkinsonism have been identified in the gene encoding Parkin,130) which functions as an E3 ubiquitin ligase involved in the proteosome-dependent proteolysis.131) Although Parkin mutation carriers generally do not display the neuropathology of Lewy bodies composed of α-synuclein,132) it is possible that impairment of proteolytic α-synuclein metabolism due to as yet unidentified mechanism(s) could be a cause of Lewy body formation in most cases of sporadic Parkinson’s disease. It is also true that the mechanism that accounts for normal tau metabolism has not yet been identified; tau metabolism is difficult to analyze because tau exists in two forms, microtubule-bound and unbound, in neurons. Identification of protease(s) involved in normal tau metabolism may lead to elucidation of the mechanism of tauopathy formation and contribute to the development of a more relevant AD mouse model than is provided presently by transgenic mice.
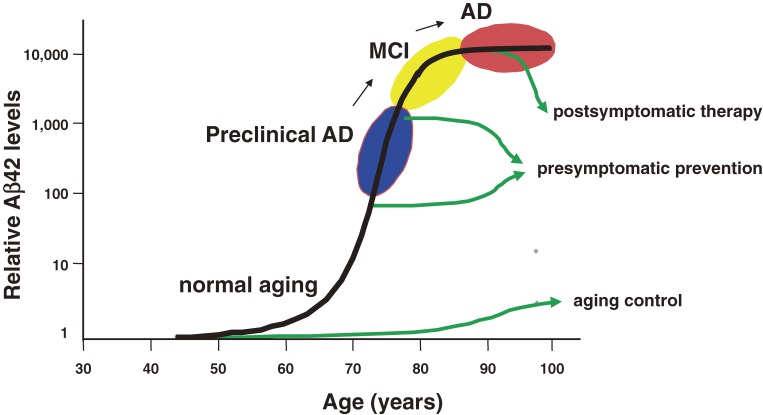
Figure 6.
Relationship between Aβ deposition and normal aging, preclinical AD, MCI and AD. Treatment after the onset, during preclinical AD and normal aging would be postsymptomatic therapy, presymptomatic prevention and aging control.
Figure 7.
Brain aging, proteolysis, protein deposition, neuronal dysfunction/degeneration and dementia. See Section 6.
Figure 7 also indicates that there are a number of potential therapeutic targets for the prevention and treatment of dementing disorders. If it becomes possible to pre-diagnose the preclinical AD, a pre-symptomatic intervention can be initiated that includes the Aβ-reducing strategies described in Fig. 5. Because the conversion of “normal aging” to AD via preclinical AD and MCI appears to be a continuous process caused primarily by the gradual acceleration of Aβ accumulation, we may even be able to partially control some very significant aspects of brain aging by maintaining low Aβ levels throughout our lives (Figs. 2 and 6). Recently, Mawuenyega and colleagues observed decreased clearance rather than increased production of Aβ in sporadic AD patients,138) suggesting up-regulation of Aβ degradation may indeed be an effective preventive measure. We also need to learn a lesson from what has happened to the Aβ vaccination.51) We need to be patient and cautious in bringing the knowledge obtained from experimental science to clinical applications; we should move from mice to humans only after confirming effectiveness and safety of a given approach in non-human primates.
Acknowledgements
The authors thank all the present and past members of the Laboratory for Proteolytic Neuroscience, RIKEN Brain Science Institute, for their participation in the work described herein. In particular, Nobuhisa Iwata, Makoto Higuchi, Takashi Saito, Satoshi Tsubuki, Keiro Shirotani, Yoshie Takaki, Misaki Sekiguchi, Kaori Watanabe, Yukio Matsuba and Emi Hosoki have made indispensable contributions to this research from the founding of our laboratory. We also thank our collaborators, including Maho Morishima-Kawashima & Yasuo Ihara (University of Tokyo, School of Medicine), Craig Gerard & his colleagues (Harvard Medical School), Masatoshi Takeda & his colleagues (Osaka University, School of Medicine), Takashi Iwatsubo & his colleagues (University of Tokyo, Graduate School of Pharmaceutical Sciences), Hiroshi Kiyama & his colleagues (Nagoya University, Graduate School of Medicine), Keiya Ozawa & his colleagues (Jichi Medical School) and Ute Hochgeschwenger (Oklahoma Medical Research Foundation). Our work have been supported by a number of research grants from RIKEN BSI, the Ministry of Education, Culture, Sports, Science and Technology, Special Coordination Funds for promoting Science and Technology from the STA, the Ministry of Health, Labor and Welfare of Japan and Takeda Chemical Industries.
Profile
Takaomi C. Saido was born in 1959 and started his research career in 1988 with studies on the biological and pathological roles of calpain, calcium-activated neutral protease, at Tokyo Metropolitan Institute of Medical Science. One of the pioneering works has been to devise antibodies that distinguish proteolytic products from their intact precursors. In particular, antibodies specific to calpain-cleaved spectrin have been used by a number of researches all over the world. He further applied this technique to processing of amyloid precursor protein (APP) and amyloid β peptide (Aβ) that are closely associated with pathogenesis of Alzheimer’s disease (AD). He discovered that ischemic conditions accelerates amyloidogenic processing of APP and that the major Aβ species that accumulates in aged and AD brains starts with pyroglutame (pE) at position 3. Aβ3(pE)–42 is now considered to be one of the most toxic Aβ species. He then moved to RIKEN Brain Science Institute to start a new laboratory as a laboratory head in 1977 to further develop the research on AD and calpain. As a milestone work in the AD research field, he and colleagues have discovered that a neutral endopeptidase, neprilysisn, is a major Aβ-degrading enzyme in brain. This finding has led to successful gene therapy of AD using mouse models. Preclinical and clinical trials are expected to bring this gene therapy to reality for prevention and treatment of AD in the near future. Other findings at RIKEN include the following: (1) In vivo detection of Aβ plaques by MRI; (2) Regulation of neprilysin activity by somatostatin; (3) Deterioration of Aβ pathology by calpain hyperactivation; (4) Generation of single APP locus knockin mouse models that overproduce Aβ without overexpressing APP. He was awarded Young Investigator’s Award from the Japanese Biochemical Society in 1995 and Tokizane Award from the Japan Neuroscience Society in 2007. He serves as a board member for Japan Society for Dementia Research.
References
- 1).Flicker C., Ferris S.H., Reisberg B. (1991) Mild cognitive impairment in the elderly: predictors of dementia. Neurology 41, 1006–1009 [DOI] [PubMed] [Google Scholar]
- 2).Golomb J., Kluger A., Ferris S.H. (2000) Mild cognitive impairment: identifying and treating the earliest stages of Alzheimer’s disease. Neurosci. News 3, 46–53 [Google Scholar]
- 3).Asada, T. (2003) Study on the risk factors and preventive interventions of dementing disorders. Kokateki Iryogijutsuno Kakuritsu Suishin Rinshoukenkyuujigyoou, Ministry of Health, Labor and Welfare of Japan. 1–5. [Google Scholar]
- 4).Braak H., Braak E. (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 [DOI] [PubMed] [Google Scholar]
- 5).Funato H., Enya M., Yoshimura M., Morishima-Kawashima M., Ihara Y. (1999) Presence of sodium dodecyl sulfate-stable amyloid β-protein dimers in the hippocampus CA1 not exhibiting neurofibrillary tangle formation. Am. J. Pathol. 155, 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C., Marcus D.S., Cairns N.J., Xie X., Blazey T.M., Holtzman D.M., Santacruz A., Buckles V., Oliver A., Moulder K., Aisen P.S., Ghetti B., Klunk W.E., McDade E., Martins R.N., Masters C.L., Mayeux R., Ringman J.M., Rossor M.N., Schofield P.R., Sperling R.A., Salloway S., Morris J.C., Dominantly Inherited Alzheimer Network (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Chong M.S., Sahadevan S. (2005) Preclinical Alzheimer’s disease: diagnosis and prediction of progression. Lancet Neurol. 4, 576–579 [DOI] [PubMed] [Google Scholar]
- 8).Masters C.L., Selkoe D.J. (2012) Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect Med. doi:10.1101/cshperspect.a006262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Kwon J. (2002) http://www.alzforum.org/res/com/mut/tau/default.asp
- 10).Hardy J., Selkoe D.J. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 11).Higuchi M., Lee V.M., Trojanowski J.Q. (2002) Tau and axonopathy in neurodegenerative disorders. Neuromolecular Med. 2, 131–150 [DOI] [PubMed] [Google Scholar]
- 12).Forman M.S., Trojanowski J.Q., Lee V.M. (2004) Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat. Med. 10, 1055–1063 [DOI] [PubMed] [Google Scholar]
- 13).Campion D., Dumanchin C., Hannequin D., Dubois B., Belliard S., Puel M., Thomas-Anterion C., Michon A., Martin C., Charbonnier F., Raux G., Camuzat A., Penet C., Mesnage V., Martinez M., Clerget-Darpoux F., Brice A., Frebourg T. (1999) Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 65, 664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Hardy J. (2012) http://www.alzforum.org/res/com/mut/pre/default.asp
- 15).Duff K., Eckman C., Zehr C., Yu X., Prada C.M., Perez-tur J., Hutton M., Buee L., Harigaya Y., Yager D., Morgan D., Gordon M.N., Holcomb L., Refolo L., Zenk B., Hardy J., Younkin S. (1996) Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature 383, 710–713 [DOI] [PubMed] [Google Scholar]
- 16).Nakano Y., Kondoh G., Kudo T., Imaizumi K., Kato M., Miyazaki J., Tohyama M., Takeda J., Takeda M. (1999) Accumulation of murine amyloidβ42 in a gene-dosage-dependent manner in PS1 ‘knock-in’ mice. Eur. J. Neurosci. 11, 2577–2581 [DOI] [PubMed] [Google Scholar]
- 17).Demeester N., Mertens C., Caster H., Goethals M., Vandekerckhove J., Rosseneu M., Labeur C. (2001) Comparison of the aggregation properties, secondary structure and apoptotic effects of wild-type, Flemish and Dutch N-terminally truncated amyloid β peptides. Eur. J. Neurosci. 13, 2015–2024 [DOI] [PubMed] [Google Scholar]
- 18).Nilsberth C., Westlind-Danielsson A., Eckman C.B., Condron M.M., Axelman K., Forsell C., Stenh C., Luthman J., Teplow D.B., Younkin S.G., Naslund J., Lannfelt L. (2001) The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci. 4, 887–893 [DOI] [PubMed] [Google Scholar]
- 19).Murakami K., Irie K., Morimoto A., Ohigashi H., Shindo M., Nagao M., Shimizu T., Shirasawa T. (2002) Synthesis, aggregation, neurotoxicity, and secondary structure of various Aβ1–42 mutants of familial Alzheimer’s disease at positions 21–23. Biochem. Biophys. Res. Commun. 294, 5–10 [DOI] [PubMed] [Google Scholar]
- 20).Yamamoto N., Matsuzaki K., Yanagisawa K. (2005) Cross-seeding of wild-type and hereditary variant-type amyloid beta-proteins in the presence of gangliosides. J. Neurochem. 95, 1167–1176 [DOI] [PubMed] [Google Scholar]
- 21).Tsubuki S., Takaki Y., Saido T.C. (2003) Dutch, Flemish, Italian, and Arctic mutations of APP and resistance of Aβ to physiologically relevant proteolytic degradation. Lancet 361, 1957–1958 [DOI] [PubMed] [Google Scholar]
- 22).Wakutani Y., Watanabe K., Adachi Y., Wada-Isoe K., Urakami K., Ninomiya H., Saido T.C., Hashimoto T., Iwatsubo T., Nakashima K. (2004) Novel amyloid precursor protein gene missense mutation (D678N) in probable familial Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 75, 1039–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Funato H., Yoshimura M., Kusui K., Tamaoka A., Ishikawa K., Ohkoshi N., Namekata K., Okeda R., Ihara Y. (1998) Quantitation of amyloid β-protein (Aβ) in the cortex during aging and in Alzheimer’s disease. Am. J. Pathol. 152, 1633–1640 [PMC free article] [PubMed] [Google Scholar]
- 24).Wang J., Dickson D.W., Trojanowski J.Q., Lee V.M. (1999) The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 158, 328–337 [DOI] [PubMed] [Google Scholar]
- 25).Morishima-Kawashima M., Oshima N., Ogata H., Yamaguchi H., Yoshimura M., Sugihara S., Ihara Y. (2000) Effect of apolipoprotein E allele e4 on the initial phase of amyloid β-protein accumulation in the human brain. Am. J. Pathol. 157, 2093–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Saunders A.M., Trowers M.K., Shimkets R.A., Blakemore S., Crowther D.J., Mansfield T.A., Wallace D.M., Strittmatter W.J., Roses A.D. (2000) The role of apolipoprotein E in Alzheimer’s disease: pharmacogenomic target selection. Biochim. Biophys. Acta 1502, 85–94 [DOI] [PubMed] [Google Scholar]
- 27).Shoji M., Golde T.E., Ghiso J., Cheung T.T., Estus S., Shaffer L.M., Cai X.D., McKay D.M., Tintner R., Frangione B., Younkin S.G. (1992) Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science 258, 126–129 [DOI] [PubMed] [Google Scholar]
- 28).Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C., McCormack R., Wolfert R., Selkoe D., Lieberburg I., Schenk D. (1992) Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature 359, 325–327 [DOI] [PubMed] [Google Scholar]
- 29).van Gool W.A., Schenk D.B., Bolhuis P.A. (1994) Concentrations of amyloid-β protein in cerebrospinal fluid increase with age in patients free from neurodegenerative disease. Neurosci. Lett. 172, 122–124 [DOI] [PubMed] [Google Scholar]
- 30).Mattson M.P. (1997) Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77, 1081–1132 [DOI] [PubMed] [Google Scholar]
- 31).Oltersdorf T., Fritz L.C., Schenk D.B., Lieberburg I., Johnson-Wood K.L., Beattie E.C., Ward P.J., Blacher R.W., Dovey H.F., Sinha S. (1989) The secreted form of the Alzheimer’s amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature 341, 144–147 [DOI] [PubMed] [Google Scholar]
- 32).Miyazaki K., Hasegawa M., Funahashi K., Umeda M. (1993) A metalloproteinase inhibitor domain in Alzheimer amyloid protein precursor. Nature 362, 839–841 [DOI] [PubMed] [Google Scholar]
- 33).Koo E.H., Kopan R. (2004) Potential role of presenilin-regulated signaling pathways in sporadic neurodegeneration. Nat. Med. 10 (Suppl.), S26–S33 [DOI] [PubMed] [Google Scholar]
- 34).Kim H.S., Kim E.M., Lee J.P., Park C.H., Kim S., Seo J.H., Chang K.A., Yu E., Jeong S.J., Chong Y.H., Suh Y.H. (2003) C-terminal fragments of amyloid precursor protein exert neurotoxicity by inducing glycogen synthase kinase-3β expression. FASEB J. 17, 1951–1953 [DOI] [PubMed] [Google Scholar]
- 35).Kitazume S., Tachida Y., Oka R., Shirotani K., Saido T.C., Hashimoto Y. (2001) Alzheimer’s β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a golgi-resident sialyltransferease. Proc. Natl. Acad. Sci. U.S.A. 98, 13554–13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Hartmann, D. (2003) Functional roles of APP secretases. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 49–60. [Google Scholar]
- 37).Lichtenthaler S.F., Dominguez D., Westmeyer G.G., Reiss K., Haass C., Saftig P., De Strooper B., Seed B. (2003) The cell adhesion protein p-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J. Biol. Chem. 278, 48713–48719 [DOI] [PubMed] [Google Scholar]
- 38).Li Q., Südhof T.C. (2004) Cleavage of amyloid-β precursor protein and amyloid-β precursor-like protein by BACE1. J. Biol. Chem. 279, 10542–10550 [DOI] [PubMed] [Google Scholar]
- 39).Cai H., Wang Y., McCarthy D., Wen H., Borchelt D.R., Price D.L., Wong P.C. (2001) BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 4, 233–234 [DOI] [PubMed] [Google Scholar]
- 40).Dominguez D., Tournoy J., Hartmann D., Huth T., Cryns K., Deforce S., Serneels L., Camacho I.E., Marjaux E., Craessaerts K., Roebroek A.J., Schwake M., D’Hooge R., Bach P., Kalinke U., Moechars D., Alzheimer C., Reiss K., Saftig P., De Strooper B. (2005) Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J. Biol. Chem. 280, 30797–30806 [DOI] [PubMed] [Google Scholar]
- 41).Saido T.C. (1998) Alzheimer’s disease as proteolytic disorders: anabolism and catabolism of β-amyloid. Neurobiol. Aging 19, S69–S75 [DOI] [PubMed] [Google Scholar]
- 42).Saido, T.C. (2003) Overview-Aβ metabolism: from Alzheimer research to brain aging control. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 1–16. [Google Scholar]
- 43).Ishiura, S., Asai, M., Hattori, C., Hotoda, N., Szabo, B., Sasagawa, N. and Tanuma, S. (2003) APP α-secretase, a novel target for Alzheimer drug therapy. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 27–32. [Google Scholar]
- 44).Zlokovic B.V., Yamada S., Holtzman D., Ghiso J., Frangione B. (2000) Clearance of amyloid β-peptide from brain: transport or metabolism? Nat. Med. 6, 718; [DOI] [PubMed] [Google Scholar]; Iwata N., Tsubuki S., Hama E., Takaki Y., Shirotani K., Saido T.C. (2000) Reply to: ‘Clearance of amyloid β-peptide from brain: transport or metabolism?’ Nat. Med. 6, 718–719 [Google Scholar]
- 45).Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T.D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. (1996) Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 2, 864–870 [DOI] [PubMed] [Google Scholar]
- 46).Vanderstichele H., Van Kerschaver E., Hesse C., Davidsson P., Buyse M.A., Andreasen N., Minthon L., Wallin A., Blennow K., Vanmechelen E. (2000) Standardization of measurement of β-amyloid(1–42) in cerebrospinal fluid and plasma. Amyloid 7, 245–258 [DOI] [PubMed] [Google Scholar]
- 47).Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Liao Z., Lieberburg I., Motter R., Mutter L., Soriano F., Shopp G., Vasquez N., Vandevert C., Walker S., Wogulis M., Yednock T., Games D., Seubert P. (1999) Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 [DOI] [PubMed] [Google Scholar]
- 48).Zlokovic, B.V. and Frangione, B. (2003) Transport-clearance hypothesis for Alzheimer’s disease and potential therapeutic implications. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 117–125. [Google Scholar]
- 49).DeMattos R.B., Bales K.R., Cummins D.J., Paul S.M., Holtzman D.M. (2002) Brain to plasma amyloid-β efflux: A measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science 295, 2264–2267 [DOI] [PubMed] [Google Scholar]
- 50).Lemere C.A., Beierschmitt A., Iglesias M., Spooner E.T., Bloom J.K., Leverone J.F., Zheng J.B., Seabrook T.J., Louard D., Li D., Selkoe D.J., Palmour R.M., Ervin F.R. (2004) Alzheimer’s disease Aβ vaccine reduces central nervous system Aβ levels in a non-human primate, the Caribbean vervet. Am. J. Pathol. 165, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Schenk D. (2003) Amyloid-β immunotherapy for Alzheimer’s disease. The end of the beginning. Nat. Rev. Neurosci. 3, 824–828 [DOI] [PubMed] [Google Scholar]
- 52).Saido T.C., Iwatsubo T., Mann D.M., Shimada H., Ihara Y., Kawashima S. (1995) Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron 14, 457–466 [DOI] [PubMed] [Google Scholar]
- 53).Kawarabayashi T., Younkin L.H., Saido T.C., Shoji M., Ashe K.H., Younkin S.G. (2001) Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 21, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Saido T.C., Yamao-Harigaya W., Iwatsubo T., Kawashima S. (1996) Amino- and carboxyl-terminal heterogeneity of β-amyloid peptides deposited in human brain. Neurosci. Lett. 215, 173–176 [DOI] [PubMed] [Google Scholar]
- 55).Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., Kawashima-Morishima M., Lee H.J., Hama E., Sekine-Aizawa Y., Saido T.C. (2000) Identification of the major Aβ1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat. Med. 6, 143–150 [DOI] [PubMed] [Google Scholar]
- 56).Lemere C.A., Lopera F., Kosik K.S., Lendon C.L., Ossa J., Saido T.C., Yamaguchi H., Ruiz A., Martinez A., Madrigal L., Hincapie L., Arango L. J.C., Anthony D.C., Koo E.H., Goate A.M., Selkoe D.J., Arango J.C. (1996) The E280A Presenilin 1 mutation leads to a distinct Alzheimer’s disease phenotype: Increased Aβ42 deposition and severe cerebellar pathology. Nat. Med. 2, 1146–1150 [DOI] [PubMed] [Google Scholar]
- 57).Citron, M. (2003) β-Secretase: Progress and open questions. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 17–25. [Google Scholar]
- 58).Wolfe, M. (2003) γ-Secretase and presenilin. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 33–47. [Google Scholar]
- 59).Turner, A.J. (2004) Neprilysin. In Handbook of Proteolytic Enzymes (eds. Barrett, A.J., Rawlings, N.D., Woessner, J.F.). Academic Press, London, pp. 419–426. [Google Scholar]
- 60).Dolev I., Michaelson D.M. (2004) A nontransgenic mouse model shows inducible amyloid-β (Aβ) peptide deposition and elucidates the role of apolipoprotein E in the amyloid cascade. Proc. Natl. Acad. Sci. U.S.A. 101, 13909–13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Takaki Y., Iwata N., Tsubuki S., Taniguchi S., Toyoshima S., Lu B., Gerard N.P., Gerard C., Lee H.J., Shirotani K., Saido T.C. (2000) Biochemical identification of the neutral endopeptidase family member responsible for the catabolism of amyloid β peptide in the brain. J. Biochem. 128, 897–902 [DOI] [PubMed] [Google Scholar]
- 62).Shirotani K., Tsubuki S., Iwata N., Takaki Y., Harigaya W., Maruyama K., Kiryu-Seo S., Kiyama H., Iwata H., Tomita T., Iwatsubo T., Saido T.C. (2001) Neprilysin degrades both amyloid β peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J. Biol. Chem. 276, 21895–21901 [DOI] [PubMed] [Google Scholar]
- 63).Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., Gerard C., Hama E., Lee H.J., Saido T.C. (2001) Metabolic regulation of brain Aβ by neprilysin. Science 292, 1550–1552 [DOI] [PubMed] [Google Scholar]
- 64).Roques B.P., Noble F., Dauge V., Fournie-Zaluski M.C., Beaumont A. (1993) Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 45, 87–146 [PubMed] [Google Scholar]
- 65).Turner A.J., Isaac R.E., Coates D. (2001) The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays 23, 261–269 [DOI] [PubMed] [Google Scholar]
- 66).Fukami S., Watanabe K., Iwata N., Haraoka J., Lu B., Gerard N.P., Gerard C., Fraser P., Westaway D., St. George-Hyslop P., Saido T.C. (2002) Aβ-degrading endopeptidase, neprilysin, in mouse brain: synaptic and axonal localization inversely correlating with Aβ pathology. Neurosci. Res. 43, 39–56 [DOI] [PubMed] [Google Scholar]
- 67).Iwata N., Higuchi M., Saido T.C. (2005) Metabolism of amyloid-β peptide and Alzheimer’s disease. Pharmacol. Ther. 108, 129–148 [DOI] [PubMed] [Google Scholar]
- 68).Kiryu-Seo S., Sasaki M., Yokohama H., Nakagomi S., Hirayama T., Aoki S., Wada K., Kiyama H. (2000) Damage-induced neuronal endopeptidase (DINE) is a unique metallopeptidase expressed in response to neuronal damage and activates superoxide scavengers. Proc. Natl. Acad. Sci. U.S.A. 97, 4345–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Lu B., Gerard N.P., Kolakowski L.F., Jr., Bozza M., Zurakowski D., Finco O., Carroll M.C., Gerard C. (1995) Neutral endopeptidase modulation of septic shock. J. Exp. Med. 181, 2271–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Saido, T.C. and Nakahara, H. (2003) Proteolytic degradation of Aβ by neprilysin and other peptidases. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 61–80. [Google Scholar]
- 71).Eckman, E.A. and Eckman, C.B. (2003) Aβ degradation by endothelin-converting enzymes. In Aβ Metabolism and Alzheimer’s Disease (ed. Saido, T.C.). Landes Bioscience, Georgetown, pp. 81–94. [Google Scholar]
- 72).Cherny R.A., Atwood C.S., Xilinas M.E., Gray D.N., Jones W.D., McLean C.A., Barnham K.J., Volitakis I., Fraser F.W., Kim Y., Huang X., Goldstein L.E., Moir R.D., Lim J.T., Beyreuther K., Zheng H., Tanzi R.E., Masters C.L., Bush A.I. (2001) Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30, 665–676 [DOI] [PubMed] [Google Scholar]
- 73).Yasojima K., Akiyama H., McGeer E.G., McGeer P.L. (2001a) Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of β-amyloid peptide. Neurosci. Lett. 297, 97–100 [DOI] [PubMed] [Google Scholar]
- 74).Yasojima K., McGeer E.G., McGeer P.L. (2001b) Relationship between β amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 919, 115–121 [DOI] [PubMed] [Google Scholar]
- 75).Iwata N., Takaki Y., Fukami S., Tsubuki S., Saido T.C. (2002) Region-specific reduction of Aβ-degrading endopeptidase, neprilysin, in mouse hippocampus upon aging. J. Neurosci. Res. 70, 493–500 [DOI] [PubMed] [Google Scholar]
- 76).Wang D.S., Iwata N., Hama E., Saido T.C., Dickson D.W. (2003) Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 310, 236–241 [DOI] [PubMed] [Google Scholar]
- 77).Wang D.S., Lipton R.B., Katz M.J., Davies P., Buschke H., Kuslansky G., Verghese J., Younkin S.G., Eckman C., Dickson D.W. (2005) Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J. Neuropathol. Exp. Neurol. 64, 378–385 [DOI] [PubMed] [Google Scholar]
- 78).Russo R., Borghi R., Markesbery W., Tabaton M., Piccini A. (2005) Neprylisin decreases uniformly in Alzheimer’s disease and in normal aging. FEBS Lett. 579, 6027–6030 [DOI] [PubMed] [Google Scholar]
- 79).Miners J.S., van Helmond Z., Kehoe P.G., Love S. (2010) Changes with age in the activities of β-secretase and the Aβ-degrading enzymes neprilysin, insulin-degrading enzyme and angiotensin-converting enzyme. Brain Pathol. 20, 794–802, doi:10.1111/j.1750-3639.2010.00375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Hellström-Lindahl E., Ravid R., Nordberg A. (2008) Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with Aβ levels. Neurobiol. Aging 29, 210–221 [DOI] [PubMed] [Google Scholar]
- 81).Wang S., Wang R., Chen L., Bennett D.A., Dickson D.W., Wang D.S. (2010) Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer’s brain. J. Neurochem. 115, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Gómez-Isla T., Price J.L., McKeel D.W., Jr., Morris J.C., Growdon J.H., Hyman B.T. (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 16, 4491–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Mèlanie C., Robitalle Y., DesGroseillers L., Boileau G., Marcinkiewicz M. (2002) Declining expression of neprilysin in Alzheimer disease vasculature: possible involvment in cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 61, 849–856 [DOI] [PubMed] [Google Scholar]
- 84).Akiyama H., Kondo H., Ikeda K., Kato M., McGeer P.L. (2001) Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid β-protein (Aβ) deposition. Brain Res. 902, 277–281 [DOI] [PubMed] [Google Scholar]
- 85).Marr R.A., Rockenstein E., Mukherjee A., Kindy M.S., Hersh L.B., Gage F.H., Verma I.M., Masliah E. (2003) Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J. Neurosci. 23, 1992–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Leissring M.A., Farris W., Chang A.Y., Walsh D.M., Wu X., Sun X., Frosch M.P., Selkoe D.J. (2003) Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093 [DOI] [PubMed] [Google Scholar]
- 87).Iwata N., Mizukami H., Shirotani K., Takaki Y., Muramatsu S., Lu B., Gerard N.P., Gerard C., Ozawa K., Saido T.C. (2004) Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-β peptide in mouse brain. J. Neurosci. 24, 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Caccamo A., Oddo S., Sugarman M.C., Akbari Y., LaFerla F. (2005) Age- and region-dependent alterations in Aβ-degrading enzymes: implications for Aβ-induced disorders. Neurobiol. Aging 26, 645–654 [DOI] [PubMed] [Google Scholar]
- 89).Saido T.C. (2000) Degradation of amyloid-β peptide: a key to Alzheimer pathogeneis, prevention, and therapy. NeuroSci. News 5, 52–62 [Google Scholar]
- 90).Hama E., Shirotani K., Masumoto H., Sekine-Aizawa Y., Aizawa H., Saido T.C. (2001) Clearance of extracellular and cell-associated amyloid β peptide through viral expression of neprilysin in primary neurons. J. Biochem. 130, 721–726 [DOI] [PubMed] [Google Scholar]
- 91).Marr R.A., Rockenstein E., Mukherjee A., Kindy M.S., Hersh L.B., Gage F.H., Verma I.M., Masliah E. (2003) Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J. Neurosci. 23, 1992–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Spencer B., Marr R.A., Rockenstein E., Crews L., Adame A., Potkar R., Patrick C., Gage F.H., Verma I.M., Masliah E. (2008) Long-term neprilysin gene transfer is associated with reduced levels of intracellular Aβ and behavioral improvement in APP transgenic mice. BMC Neurosci. 9, 109, doi:10.1186/1471-2202-9-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Iwata N., Sekiguchi M., Hattori Y., Takahashi A., Asai M., Ji B., Higuchi M., Staufenbiel M., Muramatsu S., Saido T.C. (2013) Global brain delivery of neprilysin gene by intravascular administration of AAV vector in mice. Sci. Rep. 3, 1472, doi:10.1038/srep01472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E.A., Frosch M.P., Eckman C.B., Tanzi R.E., Selkoe D.J., Guenette S. (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. U.S.A. 100, 4162–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Gasparini L., Gouras G.K., Wang R., Gross R.S., Beal M.F., Greengard P., Xu H. (2001) Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. J. Neurosci. 21, 2561–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Solano D.C., Sironi M., Bonfini C., Solerte S.B., Govoni S., Racchi M. (2000) Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J. 14, 1015–1022 [DOI] [PubMed] [Google Scholar]
- 97).Finelli A., Kelkar A., Song H.-J., Yang H., Konsolaki M. (2004) A model for studying Alzheimer’s Aβ42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. 26, 365–375 [DOI] [PubMed] [Google Scholar]
- 98).Hama E., Shirotani K., Iwata N., Saido T.C. (2004) Effects of neprilysin chimeric proteins targeted to subcellular compartments on amyloid β peptide clearance in primary neurons. J. Biol. Chem. 279, 30259–30264 [DOI] [PubMed] [Google Scholar]
- 99).Saito T., Iwata N., Tsubuki S., Takaki Y., Takano J., Huang S.M., Suemoto T., Higuchi M., Saido T.C. (2005) Somatostatin regulates brain amyloid β peptide, Aβ42, via modulation of proteolytic degradation. Nat. Med. 11, 434–439 [DOI] [PubMed] [Google Scholar]
- 100).Cleary J.P., Walsh D.M., Hofmeister J.J., Shankar G.M., Kuskowski M.A., Selkoe D.J., Ashe K.H. (2004) Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 8, 79–84 [DOI] [PubMed] [Google Scholar]
- 101).Kanemitsu H., Tomiyama T., Mori H. (2003) Human neprilysin is capable of degrading amyloid β peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci. Lett. 350, 113–116 [DOI] [PubMed] [Google Scholar]
- 102).Huang S.M., Mouri A., Kokubo H., Nakajima R., Suemoto T., Higuchi M., Staufenbiel M., Noda Y., Yamaguchi H., Nabeshima T., Saido T.C., Iwata N. (2006) Neprilysin-sensitive synapse-associated amyloid-β peptide oligomers impair neuronal plasticity and cognitive function. J. Biol. Chem. 281, 17941–17951 [DOI] [PubMed] [Google Scholar]
- 103).Morelli L., Llovera R., Gonzalez S.A., Affranchino J.L., Prelli F., Frangione B., Ghiso J., Castano E.M. (2003) Differential degradation of amyloid β genetic variants associated with hereditary dementia or stroke by insulin-degrading enzyme. J. Biol. Chem. 278, 23221–23226 [DOI] [PubMed] [Google Scholar]
- 104).Saito T., Takaki Y., Iwata N., Trojanowski J., Saido T.C. (2003) Perspectives: Alzheimer’s disease, neuropeptides, neuropeptidase, and amyloid-β peptide metabolism. SAGE-KE, doi:10.1126/sageke.2003.3.pe1 [DOI] [PubMed] [Google Scholar]
- 105).Wang T.-L., Chang H., Hung C.-R., Tseng Y.-Z. (1998) Morphine preconditioning attenuates neutrophil activation in rat models of myocardial infarction. Cardiovasc. Res. 40, 557–563 [DOI] [PubMed] [Google Scholar]
- 106).Joshi D.D., Dang A., Yadav P., Qian J., Bandari P.S., Chen K., Donnelly R., Castro T., Gascon P., Haider A., Rameshwar P. (2001) Negative feedback on the effect of stem cell factor on hematopoiesis is partly mediated through neutral endopeptidase activity on substance P: a combined functional and proteomic study. Blood 98, 2697–2706 [DOI] [PubMed] [Google Scholar]
- 107).Moller L.N., Stidsen C.E., Hartmann B., Holst J.J. (2003) Somatostatin receptors. Biochim. Biophys. Acta 1616, 1–84 [DOI] [PubMed] [Google Scholar]
- 108).Bruno J.F., Xu Y., Song J., Berelowitz M. (1992) Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc. Natl. Acad. Sci. U.S.A. 89, 11151–11155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109).Doggrell S.A. (2004) The potential of activation of somatostatinergic neurotransmission with FK960 in Alzheimer’s disease. Expert Opin. Investig. Drugs 13, 69–72 [DOI] [PubMed] [Google Scholar]
- 110).Hayashi M., Yamashita A., Shimizu K. (1997) Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNA during aging. Brain Res. 749, 283–289 [DOI] [PubMed] [Google Scholar]
- 111).Lu T., Pan Y., Kao S.Y., Li C., Kohane I., Chan J., Yankner B.A. (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 [DOI] [PubMed] [Google Scholar]
- 112).Davies P., Katzman R., Terry R.D. (1980) Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer’s disease and Alzheimer senile dementia. Nature 288, 279–280 [DOI] [PubMed] [Google Scholar]
- 113).Hama E., Saido T.C. (2005) Etiology of sporadic Alzheimer’s disease: somatostatin, neprilysin, and amyloid β peptide. Med. Hypotheses 65, 498–500 [DOI] [PubMed] [Google Scholar]
- 114).Storey E., Capprai R. (1999) The amyloid precursor protein of Alzheimer’s disease and the Aβ peptide. Neuropathol. Appl. Neurobiol. 25, 81–97 [DOI] [PubMed] [Google Scholar]
- 115).Li R., Lindholm K., Yang L.B., Yue X., Citron M., Yan R., Beach T., Sue L., Sabbagh M., Cai H., Wong P., Price D., Shen Y. (2004) Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. U.S.A. 101, 3632–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Lemere C.A., Spooner E.T., Leverone J.F., Mori C., Iglesias M., Bloom J.K., Seabrook T.J. (2003) Amyloid-β immunization in Alzheimer’s disease transgenic mouse models and wildtype mice. Neurochem. Res. 28, 1017–1027 [DOI] [PubMed] [Google Scholar]
- 117).Asai M., Iwata N., Tomita T., Iwatsubo T., Ishiura S., Saido T.C., Maruyama K. (2010) Efficient four-drug cocktail therapy targeting amyloid-β peptide for Alzheimer’s disease. J. Neurosci. Res. 88, 3588–3597 [DOI] [PubMed] [Google Scholar]
- 118).Flexner C. (2000) Dual protease inhibitor therapy in HIV-infected patients: pharmacologic rationale and clinical benefits. Annu. Rev. Pharmacol. Toxicol. 40, 649–674 [DOI] [PubMed] [Google Scholar]
- 119).Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. (2000) Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120).Cairns N.J., Lee V.M., Trojanowski J.Q. (2004) The cytoskeleton in neurodegenerative diseases. J. Pathol. 204, 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121).Saito K., Elce J.S., Hamos J.E., Nixon R.A. (1993) Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc. Natl. Acad. Sci. U.S.A. 90, 2628–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Higuchi M., Tomioka M., Takano J., Shirotani K., Iwata N., Masumoto H., Maki M., Itohara S., Saido T.C. (2005) Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice over-expressing their specific inhibitors. J. Biol. Chem. 280, 15229–15237 [DOI] [PubMed] [Google Scholar]
- 123).Takano J., Tomioka M., Tsubuki T., Higuchi M., Iwata N., Itohara S., Maki M., Saido T.C. (2005) Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways: evidence from calpastatin-mutant mice. J. Biol. Chem. 280, 16175–16184 [DOI] [PubMed] [Google Scholar]
- 124).Higuchi M., Iwata N., Matsuba Y., Takano J., Suemoto T., Maeda J., Ji B., Ono M., Staufenbiel M., Suhara T., Saido T.C. (2012) Mechanistic involvement of the calpain-calpastatin system in Alzheimer neuropathology. FASEB J. 26, 1204–1217 [DOI] [PubMed] [Google Scholar]
- 125).Lewis J., Dickson D.W., Lin W.L., Chisholm L., Corral A., Jones G., Yen S.H., Sahara N., Skipper L., Yager D., Eckman C., Hardy J., Hutton M., McGowan E. (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 [DOI] [PubMed] [Google Scholar]
- 126).Götz J., Chen F., van Dorpe J., Nitsch R.M. (2001) Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ 42 fibrils. Science 293, 1491–1495 [DOI] [PubMed] [Google Scholar]
- 127).Nixon R.A. (2003) The calpains in aging and aging-related diseases. Ageing Res. Rev. 2, 407–418 [DOI] [PubMed] [Google Scholar]
- 128).Cribbs D.H., Poon W.W., Rissman R.A., Blurton-Jones M. (2004) Caspase-mediated degeneration in Alzheimer’s disease. Am. J. Pathol. 165, 353–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129).Roth K.A. (2001) Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J. Neuropathol. Exp. Neurol. 60, 829–838 [DOI] [PubMed] [Google Scholar]
- 130).Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 [DOI] [PubMed] [Google Scholar]
- 131).Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., Suzuki T. (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 25, 302–305 [DOI] [PubMed] [Google Scholar]
- 132).Goldberg M.S., Fleming S.M., Palacino J.J., Cepeda C., Lam H.A., Bhatnagar A., Meloni E.G., Wu N., Ackerson L.C., Klapstein G.J., Gajendiran M., Roth B.L., Chesselet M.F., Maidment N.T., Levine M.S., Shen J. (2003) Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 278, 43628–43635 [DOI] [PubMed] [Google Scholar]
- 133).Miller B.C., Eckman E.A., Sambamurti K., Dobbs N., Chow K.M., Eckman C.B., Hersh L.B., Thiele D.L. (2003) Amyloid-β peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc. Natl. Acad. Sci. U.S.A. 100, 6221–6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Ertekin-Taner N., Ronald J., Feuk L., Prince J., Tucker M., Younkin L., Hella M., Jain S., Hackett A., Scanlin L., Kelly J., Kihiko-Ehman M., Neltner M., Hersh L., Kindy M., Markesbery W., Hutton M., de Andrade M., Petersen R.C., Graff-Radford N., Estus S., Brookes A.J., Younkin S.G. (2005) Elevated amyloid β protein (Aβ42) and late onset Alzheimer’s disease are associated with single nucleotide polymorphisms in the urokinase-type plasminogen activator gene. Hum. Mol. Genet. 14, 447–460 [DOI] [PubMed] [Google Scholar]
- 135).Tucker H.M., Simpson J., Kihiko-Ehmann M., Younkin L.H., McGillis J.P., Younkin S.G., Degen J.L., Estus S. (2004) Plasmin deficiency does not alter endogenous murine amyloid β levels in mice. Neurosci. Lett. 368, 285–289 [DOI] [PubMed] [Google Scholar]
- 136).Suzuki N., Cheung T.T., Cai X.D., Odaka A., Otvos L., Jr., Eckman C., Golde T.E., Younkin S.G. (1994) An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science 264, 1336–1340 [DOI] [PubMed] [Google Scholar]
- 137).Lafrance M.H., Vezina C., Wang Q., Boileau G., Crine P., Lemay G. (1994) Role of glycosylation in transport and enzymic activity of neutral endopeptidase-24.11. Biochem. J. 302, 451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Mawuenyega K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C., Yarasheski K.E., Bateman R.J. (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]