Abstract
Background
The relationship between surgical margin and local recurrence (LR) in osteosarcoma patients with poor responses to chemotherapy is unclear. Moreover, the incidences of LR according to three different resection planes (bone, soft tissue, and perineurovascular) are not commonly known.
Methods
We evaluated the incidence of LR in three areas. To assess whether there is a role of surgical margin on LR in patients resistant to preoperative chemotherapy, we designed a case (35 patients with LR) and control (70 patients without LR) study. Controls were matched for age, location, initial tumor volume, and tumor volume change during preoperative chemotherapy.
Results
LR occurred at the soft tissues in 18 cases (51.4%), at the perineurovascular tissues in 11 cases (31.4%), and at the bones in six cases (17.2%). The proportion of inadequate perineurovascular margin was higher in the case group than in the control group (p = 0.01). Within case-control group (105 patients), a correlation between each margin status and LR at corresponding area was found in the bone (p < 0.001) and perineurovascular area (p = 0.001).
Conclusions
LR is most common in soft tissues. In patients showing similar unfavorable responses to chemotherapy, the losses of perineurovascular fat plane on preoperative magnetic resonance imaging may be a valuable finding in predicting LR.
Keywords: Osteosarcoma, Local recurrence, Surgical margin
It is generally accepted that osteosarcoma patients with local recurrence (LR) show a worse survival rate than those with only metastatic diseases.1-5) Known predicting factors for LR are limb salvages with inadequate surgical margin and poor histologic responses to preoperative chemotherapy.2,6,7) However, LR is considered an expression rather than a factor of poor prognosis for tumors with an aggressive biologic behavior.8)
Recently, we reported that an increase in tumor volume (TV) during preoperative chemotherapy is related with poor histologic responses and it also is a novel predictor of LR in extremity osteosarcoma.9) Furthermore, subsequently performed cohort and case-control survival analysis between patients with LR and without LR suggested that LR has only a small effect on survivals among patients showing resistance to chemotherapy.10) When considering the worse survival rates of poor responders, paradoxically, the limb salvage would be a preferred option. However, the role of surgical margin of the development of LR in patients showing resistance to chemotherapy is not clear. Additionally, the incidence of LR according to three types of resection margin (bone, soft tissue, and perineurovascular) is not being addressed. Therefore, we subdivided the location of LR into three areas. To assess whether there is a role of surgical margin on LR in patients resistant to preoperative chemotherapy, we designed a case (35 patients with LR) and control (70 patients without LR) study. Control group was matched to the LR group for age, initial tumor size, location, and TV change during preoperative chemotherapy.
We questioned 1) the prevalence of LR in these three areas, 2) whether there is a difference in surgical margins between case and control group, and 3) additionally, whether there is a relationship between surgical margin status in these areas and LR at corresponding area in the case-control group.
METHODS
Patient Selection
To accomplish the objectives of this study, we performed three analyses. The data of 98 osteosarcoma with LR and 778 patients without LR registered from March 1990 to March 2009 at our institute were extracted from computerized archives. In the first analysis, we identified 35 patients with LR (cases). Sixty-three of 98 patients who met the following criteria were excluded: 1) stage III at presentation (19 patients), 2) a history of an inadvertent procedure, including open reduction and internal fixation at a referral hospital (16 patients), 3) an axial location (13 patients), 4) primary tumor excision at other institute (six patients), 5) no neoadjuvant chemotherapy due to initial misdiagnosis (three patients), 6) LR development at more than 5 years from primary tumor excision (three patients), 7) surface osteosarcoma (two patients), and 8) a pathologic fracture with diagnostic delay at presentation (one patient). These exclusions left 35 localized extremity osteosarcoma patients that developed LR after limb salvage surgery. LRs were screened according to symptomatology or plain radiographic or bone scans. LR locations were categorized as bones, soft tissues, or perineurovascular, and were confirmed using plain-radiographs and magnetic resonance (MR)/computed tomographic (CT) images. Mean follow-up of cases was 42.5 months (range, 9 to 195 months).
In the second analysis (case-control study), we selected 70 out of 410 patients who met our inclusion criteria as the control group. Control group members were matched with cases in terms of age, tumor size at presentation, tumor location, and TV change after chemotherapy. All controls and cases received the same chemotherapeutic protocol initially. The mean follow-up of controls was 66.4 months (range, 9 to 211 months). Three hundred sixty-eight out of 778 patients that met the following criteria were excluded: 1) histologic responses to preoperative chemotherapy was not available (84 patients), 2) have not completed our treatment protocol (82 patients), 3) history of an intralesional procedure (54 patients), 4) follow-up period of less than 36 months (52 patients), 5) low grade or atypical osteosarcoma (50 patients), 6) an axial location (33 patients), 7) unavailable data on preoperative volume change (10 patients), and 8) death not related to the disease (three patients).
All patients underwent staging studies, preoperative chemotherapy, surgery, and postoperative chemotherapy, and were followed as previously described.11) The factors assessed in the present study were: age, gender, primary tumor location, pathologic subtype, pathologic fracture, TV at presentation, pre- and postchemotherapeutic TV change, surgical margin, location of LR, histologic response to preoperative chemotherapy, and metastasis-free duration. TVs were calculated using MR images, as described by Gobel et al.12) using the formula: TV = 0.53 × tumor length × tumor width × tumor depth. TV changes due to chemotherapy are expressed as tumor volume ratios (TVRs), which were calculated by dividing TV post-chemotherapy by TV prechemotherapy. Patients were assigned to three groups based on TVRs. Specifically, TVRs of < 0.95 were classified as "decreased," TVRs between 0.95 and 1.05 were classified as "stable," and TVRs of > 1.05 were classified as "increased".13) Surgical margins were evaluated from three areas (bones, soft tissues, and perineurovascular) of each patient. Bone and soft tissue margins were determined using pathologic specimens whereas neurovascular margins were determined by preoperative magnetic resonance imaging (MRI). An adequate margin was defined as a tumor free margin of ≥ 2 cm for bone, the existence of a normal soft tissue cuff enveloping the tumor for soft tissue, and intact perineurovascular fat plane on MRI for neurovascular margin (Fig. 1). Conversely, inadequate margins were defined as a tumor-free margin of < 2 cm for bones, an exposed tumor pseudo-capsule for soft tissues, and a neurovascular bundle abutting (loss of fat plane) a tumor by MRI. However, patients who showed neurovascular encasements (1 in patient with LR and 5 in control) on preoperative MRI were categorized as having adequate margins through vessel graft (1) or amputation (5). Tumor-free bone margins were measured using pathologic specimens and minimal thickness of normal tissue planes between tumors and neurovascular bundles were measured by MRI. In 11 cases, a close soft tissue margin was defined as < 1 cm of normal tissue, and was regarded as adequate. The presence of metastasis was determined by routine plain chest radiography, computed tomography, and bone scan. This study was approved by our Institutional Review Board.
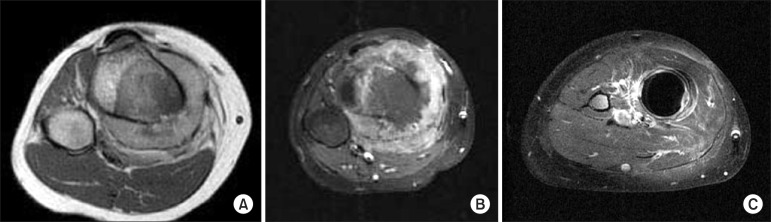
Fig. 1.
(A) Prechemotherapy T1 magnetic resonance imaging shows visible normal tissue plane between posterior tibial vessel and extra-osseous tumor mass. (B) After chemotherapy, the tumor volume increased and normal tissue plane between tumor and neurovascular bundle disappeared (inadequate margin in neurovascular area). (C) At 9 months postoperatively, local recurrence developed around inadequate margin of perineurovascular area.
Fisher exact chi-square test and the Student t -test were used to identify differences between patients in the case and control groups in terms of clinical characteristics. For the 105 patients in the case-control group (third analysis), the correlation between surgical margin status in three resection planes and LR at corresponding area was being assessed. Analyses were performed using SPSS ver. 13.0 (SPSS Inc, Chicago, IL, USA), and p < 0.05 were considered significant.
RESULTS
The LR rate in this study is 7.8% (35/445). The LR rate of upper and lower extremity in the whole cohort were 16.3% (8/49) and 6.8% (27/396) respectively. LRs occurred in soft tissues of 18 cases (51.4%), in perineurovascular tissues of 11 cases (31.4%) and in bones of 6 cases (17.2%). The clinico-pathologic characteristics of the 35 LR patients are summarized in Table 1. Perineurovascular LR patients had largest initial TV while bone LR patients had the smallest. Most patients (5/7) with a pathologic fracture developed LR in the soft tissues. More than half of the patients that developed LR in perineurovascular or soft tissues had preceding or synchronous (within 8 weeks of LR) metastasis. In terms of final outcome, patients with LR in bones showed a higher survival chance (67%) than patients with LR in soft or perineurovascular tissues (24%). LRs in bones tend to develop later (> 1 year from resection) than the LR in soft or perineurovascular tissues (p = 0.086).
Table 1.
Characteristics of 35 Patients According to the Location of Local Recurrences (LRs)
Comparison of the clinicopathologic characteristics between the 35 cases and 70 controls are summarized in Table 2. As expected, the cases and controls were well matched for age, initial TV, TVR, and location. The mean TVRs of cases and controls were 1.73 (range, 0.7 to 4.12) and 1.68 (range, 0.8 to 4.33), respectively. No significant difference was observed between the two groups in terms of bones (p = 0.29) and soft tissues (p = 0.3) surgical margin and the proportion of good responders to preoperative chemotherapy (p = 0.25). The proportion of inadequate perineurovascular margin was low in control groups (p = 0.01). On the final follow-up, 31 of the 35 cases (88.6%) and 54 of 70 control group (77.1%) developed metastasis (p = 0.19).
Table 2.
Comparison of Clinicopathologic Characteristics of 35 Patients with Local Recurrences and 70 Control Patients
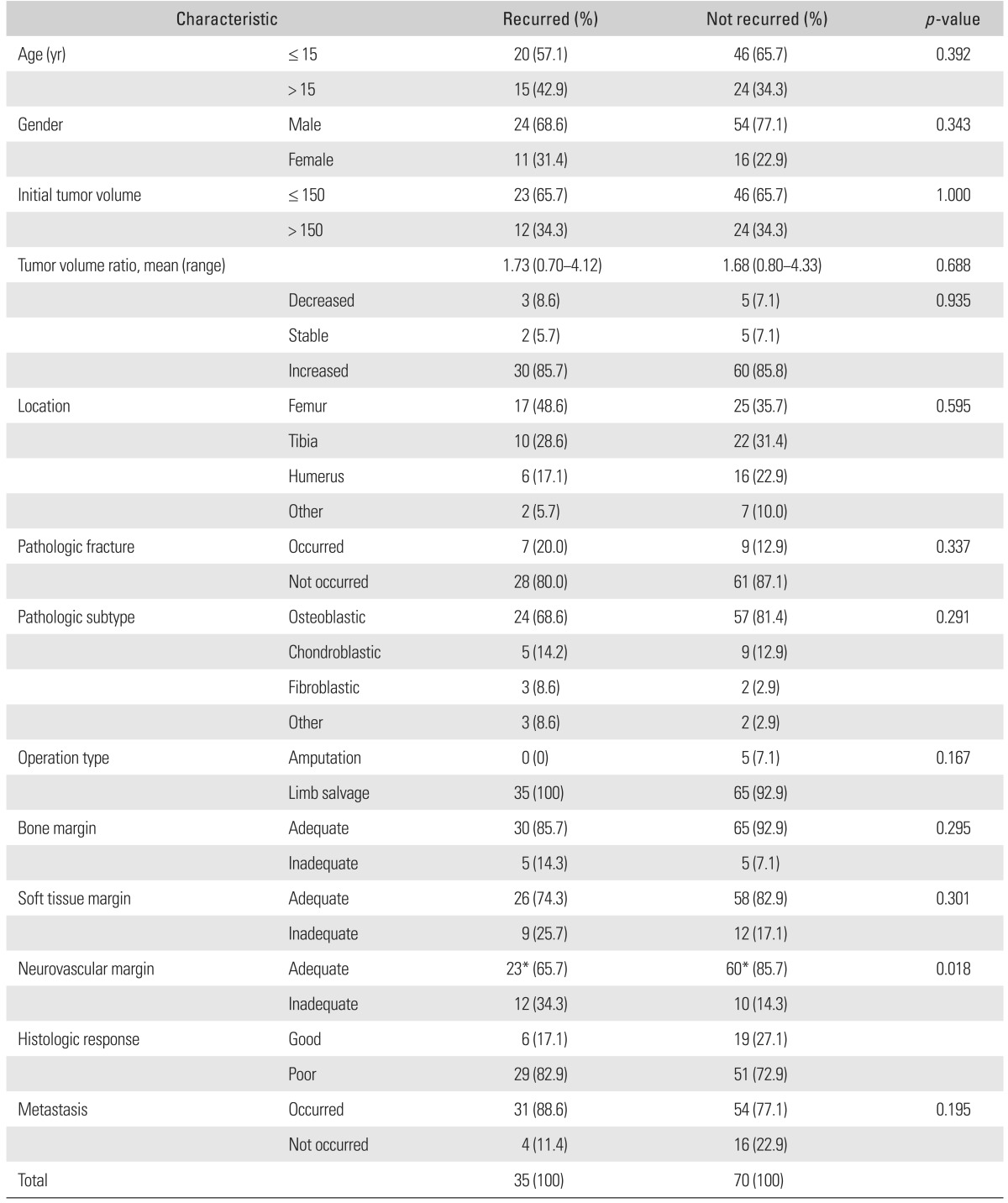
*One of 23 in local recurrence group and 5 of 60 patients in control group showed neurovascular encasement on preoperative magnetic resonance imaging and were treated with vessel graft (1) and amputation (5), respectively.
Within case-control group, poor surgical margins at the bone (p < 0.001) and perineurovascular areas (p = 0.001) were related with LR at corresponding site (Table 3, Fig. 2). On the other hand, LR in the soft tissue was not related to the adequacy of surgical margin (Fig. 3). Regarding bone margins, 95 patients (90.4%) were adequate (mean margin width, 4.2 cm; range, 2 to 10 cm) and the other 10 patients (9.6%) were inadequate (mean margin width, 0.8 cm; range, 0 to 1.7 cm). For soft tissue margins, 84 patients (80%) were adequate and 21 patients (20%) were marginal. LRs developed in 14 of 84 patients (16%) adequate soft tissue margin. The number of LR according to the type of soft tissues enveloping the tumor are 1 of 13 fascia (7.7%), 1 of 8 fat (12.5%), and 12 of 63 muscle tissue (19%). On the preoperative MRI, a normal perineurovascular fat plane (mean distance to tumor, 4.2 mm; range, 1 to 20 mm) was interposed in 77 patients (73.3%) and in the remaining 28 patients (26.7%) neurovascular tissues are either abutted (22 patients, 20.9%) or encased (6 patients, 5.8%) the tumor.
Table 3.
Correlations between Surgical Margin Status in Three Resection Planes and Local Recurrences at Corresponding Areas of 105 Case-Control Patients
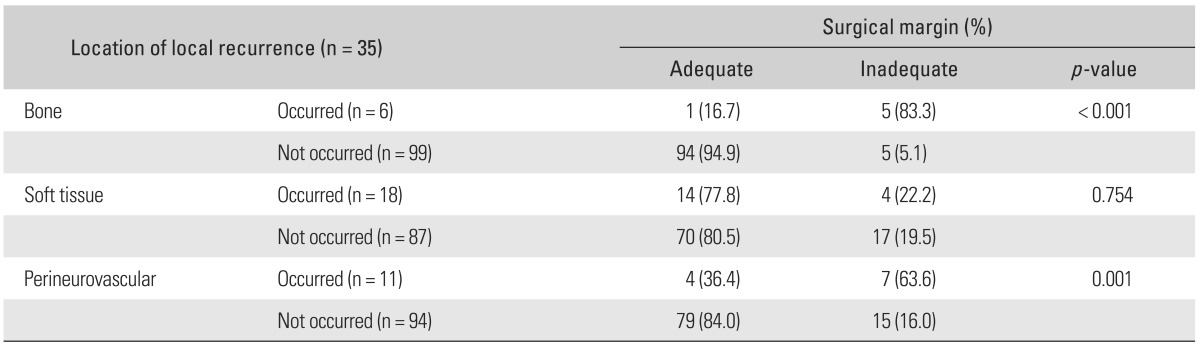
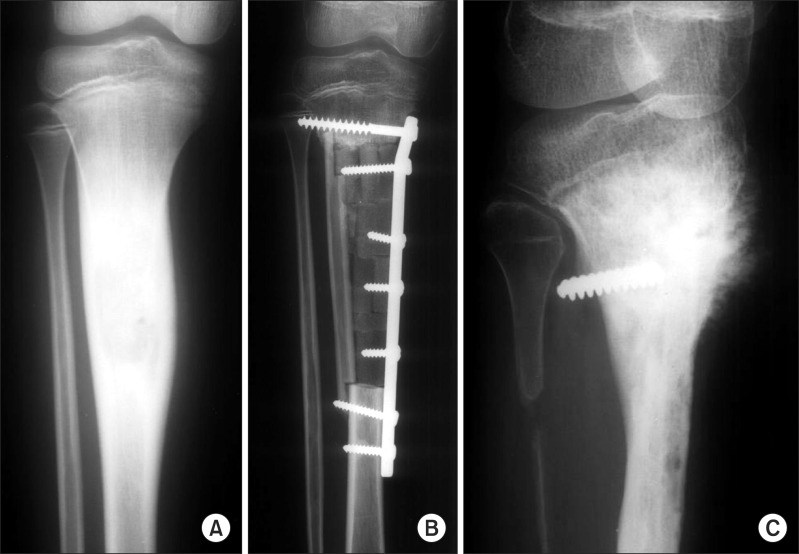
Fig. 2.
(A) Initial X-ray shows diaphyseal osteosarcoma of tibia. (B) Intercalary resection resulted in marginal resection at proximal osteotomy site. (C) Local recurrence developed in the area with inadequate bone margins 57 months postoperatively.
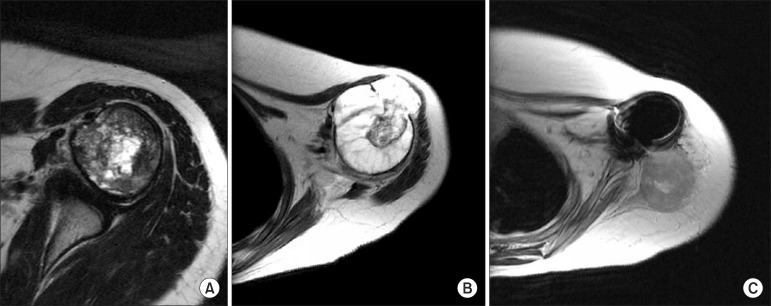
Fig. 3.
(A) Prechemotherapy T2 magnetic resonance imaging of proximal humeral osteosarcoma shows intra-compartmental lesion. (B) The tumor showed extra-compartmental disruption on antero-lateral side after chemotherapy. (C) Although the whole deltoid muscle layer was excised with tumor, recurrence developed in widely resected soft tissue areas 36 months postoperatively.
DISCUSSION
Although orthopedic oncologist should spare no efforts to prevent the LR, reports suggest a small or no survival gains with mutilating procedure in poor responders to chemotherapy.10,14) This may suggest limb salvages with close margin would be a favorable option for the patients showing resistance to chemotherapy. Yet, if possible, the LR should be avoided even in poor responders. However, patients showing resistance to chemotherapy do not often develop LR after marginal resection; moreover, for patients with LR, the actual location of LR is not necessarily around the weakest margin. Therefore, we analyzed the surgical margins of bone, soft tissue, and perineurovascular tissues separately and performed case-control study consisting mainly of poor responders to chemotherapy. Loss of perineurovascular fat plane on preoperative MRI was a valuable factor in predicting LR in patients with poor responses to chemotherapy. Though the neurovascular margin seems to be the most vulnerable area for the development of LR, our study indicates that LR is most common in the soft tissues.
In the present study, although our pathologist examined the surgical specimen, the adequacy of neurovascular surgical margin was determined by MRI which constitutes a study limitation. The reason why we used MRI finding in neurovascular area is that the intact perineurovascular fat plane will be a last barrier before sheathing on the neurovascular side. Moreover, this intactness of fat plane cannot be easily evaluated after fixing the specimen for microscopic examinations. Similarly, the status of soft tissue margin was debatable with a margin of < 1 cm; however, this would be an innate problem of Enneking system in which the adequacy of margin is not determined by a numeral distance from tumor.
The rate of LR in our study was within reported ranges. The proportion of inadequate margin in LR cases showed relatively wide variations across the studies. Nevertheless, more than a half of LR patients responded poorly to preoperative chemotherapy.15,16) Thus, eventually, the fates of patient with LR were similar (Table 4).
Table 4.
Comparisons with Previous Studies
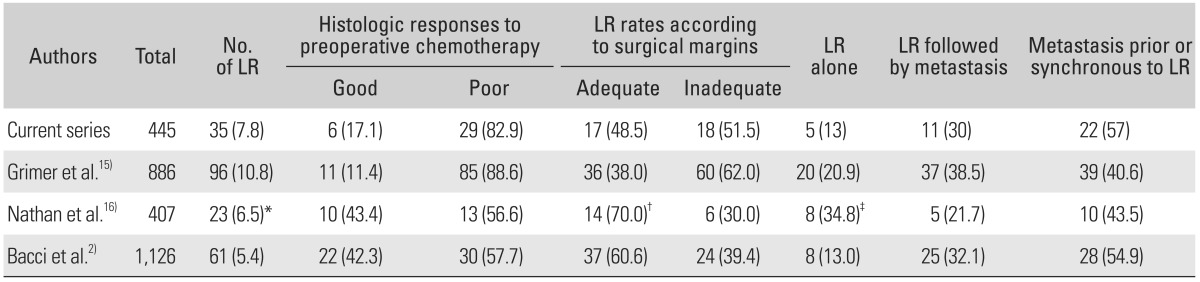
Values are presented as number (%).
LR: local recurrence.
*Five of 23 cases are axial site. †Criteria of surgical margin is positive versus negative. ‡Four patients had metastasis at presentation.
LR is a conjoined effect of surgical margin and histologic response. However, we hypothesized, in patients showing resistance to chemotherapy, LR would be a consequence to local metastasis (tumor aggressiveness) rather than the inadequacy of surgical margin. Therefore, we assumed that there would be no differences in the surgical margin between case and control group of patients. Nevertheless, our analysis between surgical margin and LR showed contradictory findings according to the three resection planes.
The presence or absence of neurovascular involvement by a tumor is an important factor when deciding on a limb salvage procedure. MRI can demonstrate gross encasement of a vessel readily, but it usually cannot differentiate mere contact, adherence or subtle invasion. When no tissue plane is evident between the tumor mass and the vessels, MRI is interpreted as showing invasions of the vessel; however, in most cases, the vessel dissection is possible at surgery.17)
Therefore, surgeons hesitate to consider this finding as an indication of amputation or vessel graft. Yet, in our case-control study, the only difference between patients with LR and without LR was the integrity of tissue plane between mass and neurovascular bundle. In an extensive review by Kawaguchi et al.18) on the tumor barrier effects shown by different biologic structures, the vessel sheath was classified as a thin barrier. The loss of a normal tissue plane probably means that the barrier has been disrupted, and thus, it represents an important component of inducing the risk of LR.
Regarding the bone margin, the issue is how close we can approach tumor to save a nearby joint or the host bone stock to improve longevity of implants. Although most surgeons accept bone margin of 2 cm, reports suggest that a margin as narrow as 6 mm of tumor-free bone does not increase the LR rate.19-23) However, in our study which mainly consists of poor responders, a significant increase in the rate of bone recurrences with < 2 cm bone margin suggests narrow margins of < 2 cm may be hazardous. Nonetheless, because most patients with bone margins > 2 cm did not develop bone recurrences, even for poor respondents, the regional tumor spread mechanisms in the bone did not seem to be unusual.
As for bone margins, the soft tissue surgical margins can be extended freely. Therefore, conceptually, the inadequate amount of soft tissues resected would be a main culprit for LR in this area. However, our data show no relations between the adequacy of soft tissue margin and LR in corresponding area. We experienced LR located away from initial tumor or in patients who underwent whole muscle compartment resections. This suggests, in the soft tissues, poor responders might have different local tumor spread mechanisms from that of good responders. It could be more dependent on vascular or lymphatic pathways rather than through direct infiltrations.
In conclusion, of the three areas examined, the LR in osteosarcoma was found to be most prevalent in soft tissues. In patients showing similar unfavorable responses to preoperative chemotherapy, the loss of perineurovascular fat plane on preoperative MRI is a notable finding predicting LR. Furthermore, a bone margin of less than 2 cm is still risky. However, the LR in soft tissue is not predictable using surgical margins as presently defined.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Cancer Center Hospital research competence promotion program (50247-2012).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88–92. doi: 10.1302/0301-620x.84b1.12211. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Forni C, Longhi A, et al. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96(2):118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 3.Chi SN, Conklin LS, Qin J, et al. The patterns of relapse in osteosarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2004;42(1):46–51. doi: 10.1002/pbc.10420. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Galindo C, Shah N, McCarville MB, et al. Outcome after local recurrence of osteosarcoma: the St. Jude Children's Research Hospital experience (1970-2000) Cancer. 2004;100(9):1928–1935. doi: 10.1002/cncr.20214. [DOI] [PubMed] [Google Scholar]
- 5.Weeden S, Grimer RJ, Cannon SR, Taminiau AH, Uscinska BM European Osteosarcoma Intergroup. The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer. 2001;37(1):39–46. doi: 10.1016/s0959-8049(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 6.Picci P, Sangiorgi L, Bahamonde L, et al. Risk factors for local recurrences after limb-salvage surgery for high-grade osteosarcoma of the extremities. Ann Oncol. 1997;8(9):899–903. doi: 10.1023/a:1008230801849. [DOI] [PubMed] [Google Scholar]
- 7.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12(12):2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 8.Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: the experience of a single institution with 44 patients. Cancer. 2006;106(12):2701–2706. doi: 10.1002/cncr.21937. [DOI] [PubMed] [Google Scholar]
- 9.Song WS, Jeon DG, Kong CB, et al. Tumor volume increase during preoperative chemotherapy as a novel predictor of local recurrence in extremity osteosarcoma. Ann Surg Oncol. 2011;18(6):1710–1716. doi: 10.1245/s10434-010-1536-8. [DOI] [PubMed] [Google Scholar]
- 10.Kong CB, Song WS, Cho WH, Oh JM, Jeon DG. Local recurrence has only a small effect on survival in high-risk extremity osteosarcoma. Clin Orthop Relat Res. 2012;470(5):1482–1490. doi: 10.1007/s11999-011-2137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MS, Cho WH, Song WS, Lee SY, Jeon DG. Time dependency of prognostic factors in patients with stage II osteosarcomas. Clin Orthop Relat Res. 2007;463:157–165. doi: 10.1097/BLO.0b013e318142b27d. [DOI] [PubMed] [Google Scholar]
- 12.Göbel V, Jurgens H, Etspuler G, et al. Prognostic significance of tumor volume in localized Ewing's sarcoma of bone in children and adolescents. J Cancer Res Clin Oncol. 1987;113(2):187–191. doi: 10.1007/BF00391442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holscher HC, Bloem JL, Vanel D, et al. Osteosarcoma: chemotherapy-induced changes at MR imaging. Radiology. 1992;182(3):839–844. doi: 10.1148/radiology.182.3.1535905. [DOI] [PubMed] [Google Scholar]
- 14.Grimer RJ, Taminiau AM, Cannon SR Surgical Subcommittee of the European Osteosarcoma Intergroup. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br. 2002;84(3):395–400. doi: 10.1302/0301-620x.84b3.12019. [DOI] [PubMed] [Google Scholar]
- 15.Grimer RJ, Sommerville S, Warnock D, et al. Management and outcome after local recurrence of osteosarcoma. Eur J Cancer. 2005;41(4):578–583. doi: 10.1016/j.ejca.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Nathan SS, Gorlick R, Bukata S, et al. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107(7):1607–1616. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- 17.Panicek DM, Hilton S, Schwartz LH. Assessment of neurovascular involvement by malignant musculoskeletal tumors. Sarcoma. 1997;1(1):61–63. doi: 10.1080/13577149778506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;(419):165–172. doi: 10.1097/00003086-200402000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Andreou D, Bielack SS, Carrle D, et al. The influence of tumor- and treatment-related factors on the development of local recurrence in osteosarcoma after adequate surgery: an analysis of 1355 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Ann Oncol. 2011;22(5):1228–1235. doi: 10.1093/annonc/mdq589. [DOI] [PubMed] [Google Scholar]
- 20.Deijkers RL, Bloem RM, Kroon HM, Van Lent JB, Brand R, Taminiau AH. Epidiaphyseal versus other intercalary allografts for tumors of the lower limb. Clin Orthop Relat Res. 2005;439:151–160. doi: 10.1097/00003086-200510000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Kumta SM, Chow TC, Griffith J, Li CK, Kew J, Leung PC. Classifying the location of osteosarcoma with reference to the epiphyseal plate helps determine the optimal skeletal resection in limb salvage procedures. Arch Orthop Trauma Surg. 1999;119(5-6):327–331. doi: 10.1007/s004020050420. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Wang Z, Guo Z, Chen GJ, Yang M, Pei GX. Irregular osteotomy in limb salvage for juxta-articular osteosarcoma under computer-assisted navigation. J Surg Oncol. 2012;106(4):411–416. doi: 10.1002/jso.23105. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchiya H, Abdel-Wanis ME, Tomita K. Biological reconstruction after excision of juxta-articular osteosarcoma around the knee: a new classification system. Anticancer Res. 2006;26(1B):447–453. [PubMed] [Google Scholar]